1. Introduction
Marine records have provided high-resolution palaeoenvironmental information regarding the climatic changes during the Pleistocene in regional-to-global spatial scale. However, some terrestrial areas have their own ecological peculiarities that prevent the use of marine proxies to infer the environmental condition. The Iberian Peninsula is one of the examples of an area with high ecological diversity, considering the mountainous region and the strong effects of the Mediterranean Sea and Atlantic Ocean (e.g., Sanchez Goñi et al. Reference Sanchez Goñi, Turon, Eynaud and Gendreau2000; Roucoux et al. Reference Roucoux, de Abreu, Shackleton and Tzedakis2005; Fletcher & Sánchez Goñi Reference Fletcher and Sánchez Goñi2008; Álvarez-Lao et al. Reference Álvarez-Lao, Álvarez-Vena, Ballesteros, García and Laplana2020; Álvarez-Vena et al. Reference Álvarez-Vena, Álvarez-Lao, Laplana, Quesada, Rojo, García-Sánchez and Menéndez2021). Particular to the Mediterranean Basin, the effects of global climatic fluctuations during the last glacial period were milder and less contrasting compared to the records from north-western Iberia or northern latitudes, as previous palynological (e.g., Carrión et al. Reference Carrión, Yll, Walker, Legaz, Chaín and López2003; Finlayson et al. Reference Finlayson, Finlayson, Giles Pacheco, Rodriguez Vidal, Carrión and Recio Espejo2008; Burjachs et al. Reference Burjachs, López-García, Allué, Blain, Rivals, Bennàsar and Expósito2012) and small vertebrate studies (e.g., López-García et al. Reference López-García, Blain, Bennàsar and Fernández-García2014b; Fernández-García et al. Reference Fernández-García, López-García and Lorenzo2016, Reference Fernández-García, Royer, López-García, Bennàsar, Goedert, Fourel, Julien, Bañuls-Cardona, Rodríguez-Hidalgo, Vallverdú and Lécuyer2019, Reference Fernández-García, López-García, Royer, Lécuyer, Allué, Burjachs, Chacón, Saladié, Vallverdú and Carbonell2020) have suggested.
Despite becoming one of the crucial periods during human history, the Last Glacial Maximum (LGM) is still highly discussed, regarding the chronology, for instance. There is no a single agreement among scholars about when this event took place. It is highly dependent on the definition and the geographical context discussed. However, the period from 26.5 to 19 ka cal before present (BP) (Clark et al. Reference Clark, Dyke, Shakun, Carlson, Clark, Wohlfarth, Mitrovica, Hostetler and McCabe2009) is commonly used as a timeframe when discussing about the Iberian Peninsula; at least it is consistent for the mountainous region (Cascalheira et al. Reference Cascalheira, Alcaraz-Castaño, Alcolea-González, de Andrés-Herrero, Arrizabalaga, Aura Tortosa, Garcia-Ibaibarriaga and Iriarte-Chiapusso2021). From the cultural development perspective, this period coincides with the Upper Palaeolithic occupations, consisting of the Gravettian, Proto-Solutrean, and Magdalenian cultures. According to Cascalheira et al. (Reference Cascalheira, Alcaraz-Castaño, Alcolea-González, de Andrés-Herrero, Arrizabalaga, Aura Tortosa, Garcia-Ibaibarriaga and Iriarte-Chiapusso2021), the development of those cultures could be linked to the ecogeographical diversity of the region with a pronounced inter-regional variability. However, these authors noted that it is still necessary to do further studies with updated methods to understand and to fill the gaps in the knowledge about the archaeological and palaeoenvironmental diversity in the Iberian Peninsula.
North-eastern Iberia is one of the areas with limited information about archaeological and palaeoenvironmental condition around or during the LGM. Some already known sites from this period are an open-air site of Montlleó in the southern slope of Pyrenees (Lleida), dated to 22.7–22.4 ka cal BP with Solutrean–Badegoulian lithic culture (Fullola et al. Reference Fullola, Mangado, Langlais, De la Torre, Foucher, San Juan, Mercadal, Bicho, Cascalheira and Schmidt2019); and, the Banyoles Lake (Girona), covering the period of the last 30 ka cal BP. Considering this limited record, the small-mammal assemblage from Cudó cave (Tarragona) adds clues to environmental information to fill the gap in this singular spatial and temporal context.
2. Site description
Cudó cave, also known as Aixàviga cave is a natural cavity located in the Prades Mountains (Muntanyes de Prades), a large calcareous mountain massif which belongs to the Catalan Pre-Coastal Range. It is situated at the southern face of Motllats cliff on the elevation of 987 metres above sea level (m.a.s.l.), directly facing the Aixàviga valley where the Glorieta river flows on its base. Specifically, it is located at 41°17′01.5″N 1°04′13.1″E, within the municipality of Mont-ral, in Alt Camp County (Tarragona, Spain). In a straight line, it is roughly 24 km from the modern coastline of Tarragona (Fig. 1) (Vergès Reference Vergès2017).

Figure 1 (A) The location of Cudó cave; (B) the location of Cudó cave in relation to the sites mentioned in this work: (1) Cudó cave; (2) Galls Carboners; (3) Xaragalls cave; (4) Canyars; (5) Colomera cave; (6) Toll cave; (7) Arbreda cave; and (c) current situation in the vicinity of Cudó cave.
The surrounding landscape today shows high variability as a consequence of the abrupt relief changes from high mountain ranges to low plain and by significant hydrological changes, from water springs and streams on the low land to the arid plateau (Fig. 1). These hydrological differences lead to the widely diverse vegetation found within this area, producing a mosaic landscape. The plateau is currently covered with semi-open woodland consisting of Quercus ilex ssp. rotundifolia (holm oak), Pinus sylvestris (Scot's pine), Juniperus communis (common juniper), Buxus sempervirens (common box) as well as shrubs. The slope where Cudó cave is located is covered by a closed woodland which consists of holm oak and Scot's pine on the upper parts and Pinus halepensis (Aleppo pine) on the lower parts. The brushwood consists mainly of boxwood, Viburnum tinus (laurustinus), Rhamnus alaternus (Mediterranean buckthorn) and Arbutus unedo (strawberry tree) also found within this level. The plain at the bottom of the mountains is currently used as a cultivation area (Fig. 1) (López-García et al. Reference López-García, Blain, Bennàsar, Alcover, Bañuls-Cardona, Fernández-García, Fontanals, Martín, Morales, Muñoz, Pedro and Vergés2014a). With those types of vegetation cover, small mammal species with woodland habitat preferences such as Apodemus sylvaticus, Eliomys quercinus, Sciurus vulgaris, Myotis myotis, Myotis blythii, Rhinolophus ferrumequinum and Pipistrellus pipistrellus can be expected to be found; as well as semi-open habitat preferences, such as Crocidura russula. With an agriculture area and water stream in the bottom of the valley, it is also possible to find Arvicola sapidus. On the raptorial birds, it some nocturnal and diurnal raptors such as Athene noctua, Strix aluco, Bubo bubo, Circus cyaneus, Falco tinnunculus and Falco peregrinus (Palomo et al. Reference Palomo, Gisbert and Blanco2007) have been reported. Chionomys nivalis is currently absent from this region as it is exclusively found in several isolated areas of the Iberian Peninsula such as in the mountainous region (Palomo et al. Reference Palomo, Gisbert and Blanco2007).
Archaeological interventions are unknown prior to 2016, and since then, an archaeological excavation has been conducted on this site (Vergès Reference Vergès2017). Two different sectors have been excavated, sector 100 and sector 200, which are situated in the inner and outer part of the cave gallery. From these recent interventions, nine and three stratigraphic levels have been exposed in sector 100 and sector 200, respectively. Among them, levels 107 and 105 of sector 100 are the richest undisturbed levels with archaeological and palaeontological remains, including the small mammal remains.
Radiocarbon dates placed level 107 from 24 to 31 ka cal BP and level 105 from ca. 10 to 15 ka cal BP (Vergès Reference Vergès2017). These dates were obtained from a marine shell of Bittium reticulatum and bone from an unidentified species from level 107 and from charcoal and unidentified bone fragment from level 105 (Table 1). The analysis was conducted using the carbon-14 accelerator mass spectrometry method.
Table 1 Date analysis result on the seven samples from Cudó cave (Vergès Reference Vergès2017).

3. Materials and methods
This work studies the small mammal assemblages from Cudó cave, recovered from excavation campaigns in 2016 and 2017. The remains were identified taxonomically following several published guidelines, among them are: Wilson & Reeder (Reference Wilson and Reeder2005) for the systematics taxonomy; Reumer (Reference Reumer1984) and Furió (Reference Furió2007) for insectivores; Dupuis (Reference Dupuis1986), Sevilla (Reference Sevilla1988), and Galán (Reference Galán2019) for chiropters; Nadachowski (Reference Nadachowski1982) and Gosàlbez (Reference Gosàlbez1987) for Arvicolinae; as well as Chaline (Reference Chaline1972), Chaline et al. (Reference Chaline, Baudvin, Jammot and Girons1974) and Gosàlbez (Reference Gosàlbez1987) for Muridae, Gliridae, and Sciuridae. For practical reasons in recording and analysing, abbreviation of dental remains was used, including incisor (i), canine (c), antemolar (a), premolar (p) and molar (m). Lowercase letter indicates mandibular dentary and uppercase letter indicates maxillar dentary. Numbers are assigned after the letter to indicate the position of the specific tooth. The number starts from the mesial side or anterior side of the mandible or maxilla. The identified remains were all quantified as the number of identified specimens present (NISP). The NISP were grouped into the minimum number of individuals by counting the most represented diagnostic element for each species, considering laterality.
Before performing further analysis, taphonomic alteration were assessed to determine the cause of accumulation. To identify these processes of accumulation, analyses that account for the chemical-induced alterations, which are related to the digestion processes of the predators, were performed following Andrews (Reference Andrews1990) and the last review Fernández-Jalvo et al. (Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016). These previous approaches established four grades of digestion marks for the small mammal remains: light; moderate; heavy; and extreme. Digestion marks on the dental remains are usually easy to identify and distinguished with respect to other taphonomic alterations. The taphonomic analysis on Cudó cave materials was carried out on the taxonomically identified molars from all taxa and also the incisors in the case of rodents. The observation was conducted on the occlusal and lateral surfaces of the molars and incisors. Apart from those four grades, the specimens also were assigned to an ‘absent’ classification for one with no visible digestion traces, and for the teeth with unclear digestion marks due to its preservation state they were classified as ‘null’.
In order to obtain palaeoenvironment information in the vicinity of Cudó cave, several methods were combined in this work. First, the Simpson's index was used to evaluate the evenness of the small mammal community of Cudó cave (Whittaker, Reference Whittaker1972). This analysis was performed in the Paleontological Statistics (PAST) software (version 4.0) (Hammer et al. Reference Hammer, Harper and Ryan2001). Then, it was applied to the habitat weighting method, a quantitative method used for landscape reconstruction based on the habitat preferences of each taxon (Rowe Reference Rowe1956; Evans et al. Reference Evans, Van Couvering and Andrews1981; Gauch Reference Gauch1989), considering a modern atlas of Iberian mammals (Palomo et al. Reference Palomo, Gisbert and Blanco2007). In this method, a fractioned value between 0 and 1 is assigned to each species according to its preferred habitat, where 0 means not preferred and 1 means strongly preferred habitat. The third method was the chorotype classification, a quantitative analysis which aims to climatically characterise the biotic region based on the concept of chorotypes, which considers groups of species with overlapping climatic requirements that determine their current geographical distribution and association (Fattorini Reference Fattorini2015). In this study, four chorotypes were used to infer the past environmental condition within the vicinity of Cudó cave (Sans-Fuentes & Ventura Reference Sans-Fuentes and Ventura2000). The relative abundance of species within each chorotype was considered to quantify the importance of each chorotype in the site area in the past.
In addition, to evaluate the palaeoclimatic conditions, the bioclimatic model (BM) (Hernández Fernández Reference Hernández Fernández2001; Hernández Fernández & Peláez-Campomanes Reference Hernández Fernández and Peláez-Campomanes2003) and mutual ecogeographical range (MER) methods (Blain et al. Reference Blain, Bailon, Cuenca-Bescós, Arsuaga, Bermúdez de Castro and Carbonell2009; López-García et al. Reference López-García, Blain, Cuenca-Bescós, Ruiz-Zapata, Dorado-Valiño, Gil-García, Valdeolmillos, Ortega, Carretero, Arsuaga, de Castro and Carbonell2010; Lyman Reference Lyman2016) were used. The first method uses faunal population, specifically on rodents, which represents climatic zones over the world to infer the climatic condition of the area. In the case of Cudó cave, four climatic zones were considered following the species presented on the assemblages. Those are zone IV, winter rain and summer drought; zone VI, typical temperate; zone VIII, cold temperate; and zone IX, Arctic. From the representation of each climatic typology and using multiple linear regression (Hernández Fernández et al. Reference Hernández Fernández, Álvarez Sierra and Peláez-Campomanes2007), the palaeoclimatic information including mean annual temperature (MAT), mean annual precipitation (MAP), mean temperature for the coldest month and mean temperature of the warmest month could be obtained. Furthermore, on the MER analysis, modern climatic condition (30-years average) of the intersecting area of the species identified from a stratigraphic unit (obtained considering each species’ current distribution in 10 × 10 Universal Transverse Mercator grid) became the basis of palaeoclimatic inferences. Careful consideration was paid on the taxa selection for these two methods. In this analysis, insectivores were included, otherwise, chiropters were not included because of their scarcity and their seasonal migration behaviour. Species with a strong distribution relationship with anthropic activity, in this case Microtus (Iberomys) cabrerae, were also discarded. Sets of recent information were used for modern species’ geographical distributions (Palomo et al. Reference Palomo, Gisbert and Blanco2007), for climatic information (Rivas Martínez Reference Rivas Martínez1987; Agencia Estatal de Meteorología (España) & Instituto de Meteorologia (Portugal) 2011) for the vegetation zone of the Iberian Peninsula, as well as the online sources from the climate-data.org.
4. Results and discussion
4.1. Small mammals of Cudó cave
Small mammals are considered a reliable palaeoenvironmental proxy to reconstruct the climatic and landscape dynamics through time (e.g., López-García et al. Reference López-García, Cuenca-Bescós, Finlayson, Brown and Pacheco2011b; Hadler et al. Reference Hadler, Dias and Bauermann2013; Fernández-García et al. Reference Fernández-García, López-García and Lorenzo2016, Reference Fernández-García, López-García, Bennàsar, Gabucio, Bargalló, Gema Chacón, Saladié, Vallverdú, Vaquero and Carbonell2018; Fernández & Pardiñas Reference Fernández and Pardiñas2018; Baca et al. Reference Baca, Popović, Baca, Lemanik, Doan, Horáček, López-García, Bañuls-Cardona, Pazonyi, Desclaux, Crégut-Bonnoure, Berto, Lenardić, Mie˛kina, Murelaga, Cuenca-Bescós, Krajcarz, Marković, Petculescu, Wilczyński, Knul, Stewart and Nadachowski2020; Avery Reference Avery2022). This is possible because the small mammal species are commonly adapted to a specific habitat, restricted to a relatively small geographical area, sensitive to habitat changes; moreover, they have a short lifespan, high reproduction and evolutionary rate, and can be quickly dispersed over the area (e.g., Chaline et al. Reference Chaline, Baudvin, Jammot and Girons1974; Andrews Reference Andrews1990; Barnosky Reference Barnosky1994; Repenning Reference Repenning2001; Van Dam et al. Reference Van Dam, Abdul Aziz, Sierra, Hilgen, Van Den Hoek Ostende, Lourens, Mein, Van Der Meulen and Pelaez-Campomanes2006).
In total, 1178 remains have been identified in this work, which correspond to a minimum of 502 individuals (Table 2). Those numbers are distributed into 528 taxonomically identified specimens in level 107, which corresponds to a minimum of 264 individuals, and 658 in level 105, which corresponds to a minimum of 238 individuals. From the taxonomic analysis, 14 different taxa have been identified (Table 2; Fig. 2). However, some specimens were unable to be identified into species level, therefore they were grouped at the genus level (cf. Myotis and cf. Pipistrellus) and order level (Chiroptera gen. et sp. indet). These groups, with addition of Microtus gr. arvalis-agrestis are not considered for the environmental reconstruction since they provide little ecological information.

Figure 2 Selected specimens from each taxon recovered from Cudó cave. (1–3) Crocidura russula: (1) anterior fragment of cranium from level 105 in palatal view; (2) anterior fragment of left hemimandible with i–m1 from level 105 in labial view; (3) right hemimandible with a4–m3 from level 105 in labial view; (4) Sorex gr. areneus-coronatus posterior fragment of right hemimandible with m2–m3 from level 107 in labial view; (5–7) Myotis gr. myotis-blythii: 5, fragment of left hemimaxilla with M2–M3 from level 105 in palatal view; (6) left hemimandible with m2–m3 from level 105 in labial view; (7) isolated left P4 in mesial, lingual, and distal view. (8–9) Rhinolophus cf. ferrumequinum: (8) fragment ofleft hemimaxilla with P4 – M3 from level 105 in palatal view; (9) isolated left m1 from level 105 in occlusal and labial view. (10–11) Apodemus sylvaticus: (10) right M1 and M2 from level 105 in occlusal view; (11) left hemimandible with m1–m3 from level 105 in occlusal view; (12) Microtus (Iberomys) cabrerae left m1 from level 105 in occlusal view; (13) Microtus agrestis right m1 from level 105 in occlusal view; (14) Chionomys nivalis left m1 from level 105 in occlusal view; (15) Microtus (Tericola) duodecimcostatus left m1 from level 105 in occlusal view; (16) Microtus arvalis left m1 from level 105 in occlusal view; (17–18) Pipistrellus cf. pipistrellus: 17, left hemimandible with m2–m3 from level 105 in labial view; (18,) right humerus with epiphysis distal from level 105 in anterior, external, posterior and interior views: (19–20) Eliomys quercinus: (19) left hemimandible from level 105 in labial and dorsal views; (20) left M1 from level 105 in occlusal view; (21) Sciurus vulgaris left m1 from level 105 in occlusal view. Scale in 4 mm for insectivores, chiropters and the mandible of Eliomys quercinus, 2 mm for A. sylvaticus and 1 mm for Arvicolinae, molar of Eliomys quercinus, and Sciurus vulgaris. Terminological abbrevations: I = incisor; c = canine; a = antemolar; p = premolar; m = lower molar. Lowercase letter indicates mandibular dentition and uppercase letter indicates maxillary dentition. Numbers situated after the letters constitute the position of the teeth, starting from mesial or anterior side of the mandible or maxilla.
Table 2 Quantification table of the small mammal remains recovered from levels 107 and 105 at Cudó cave and their distributions based on the chorotype and habitat.
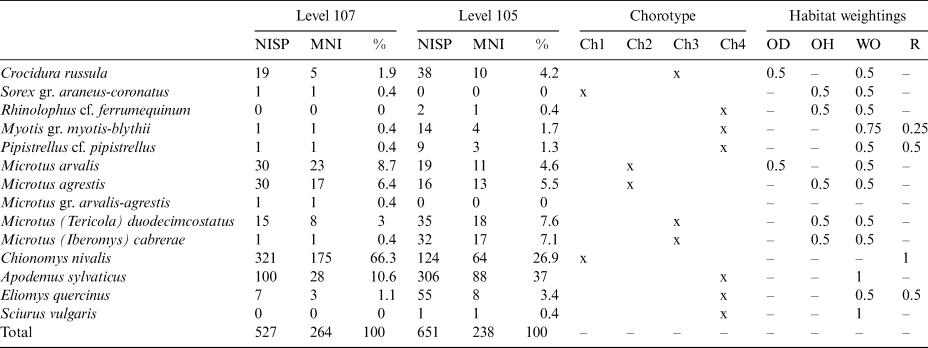
NISP, number of identified specimens present; MNI, minimum number of individuals; Ch, chorotype; OD, open dry; OH, open humid; WO, woodland; R, rocky.
Considering relative abundance of the taxa, in level 107, C. nivalis which appears to be dominating the assemblage, is significantly higher than the other taxa. The taxonomic relative abundances in level 105 are notably different from those in level 107. The species C. nivalis is still highly represented, but it is not dominating the assemblage due to a significant increase of the abundance of A. sylvaticus. Other taxa are relatively well represented in level 105.
All species recovered from Cudó cave correspond to extant species in the Iberian Peninsula. However, in the comparison to the species currently present in the vicinity of the site, not all recovered species can be found today, such as Sorex araneus, Sorex coronatus, Microtus arvalis, Microtus agrestis, M. (I.) cabrerae and C. nivalis. This absence is mainly linked to the open habitat preferences and mid-European environmental requirements of those species which recently cannot be supported by the environment around Cudó cave. The diminishing C. nivalis particularly in the north-eastern Iberian Peninsula is evident after the LGM, with the earliest remains from Colomera cave dated younger than 17 ka BP (López-García & Cuenca-Bescós Reference López-García and Cuenca-Bescós2010; Fernández-García & López-García Reference Fernández-García and López-García2013). In addition, anthropic environmental changes could also be linked to the missing species from the Cudó cave vicinity. The increase of agriculture during the Neolithic period in the mid-Holocene leads to decreasing wet soils, the preferred habitat of M. (I.) cabrerae in this region (López-García et al. Reference López-García, Blain, Morales, Lorenzo, Bañuls-Cardona and Cuenca-Bescós2012).
4.2. The origin of the assemblages
Up to 1131 molars and 349 isolated incisors have been included from both levels in the taphonomic analysis. A wide range of digestion marks are detected on the remains from both levels, covering the digestion degree from light to heavy (Table 3; Fig. 3).

Figure 3 Digestion marks on the Arvicolinae m1 (top row), Muridae molars (second row), insectivores and chiropters (third and fourth row) and isolated incisors of rodents (bottom row). (A) absent; (B) light digestion; (C) moderate digestion; (D) heavy digestion. Scale in 2 mm and 1 mm for detailed view of insectivore digestion marks. Light digested remains are not shown here as they are difficult to distinguish from the non-digested molars.
Table 3 Digestion marks of analysed remains from Cudó cave, differentiating digestion categories reached.
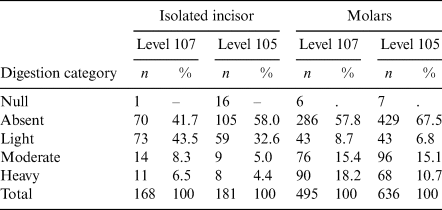
Remains with unclear digestion marks due to poor preservation are considered as ‘null’ and not included in the quantification.
The high abundance of digested remains indicates that predation is the main cause of accumulation in both levels. This is consistent with the common pattern of small mammal remains’ accumulation with predatory activity becoming the most common agent of accumulation (Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016). This general pattern of digested remains with the domination of light digested remains with notable proportion of moderate and high digested remains could point to nocturnal raptors as the main producer group of the assemblages. The probable species contributing, and capable to produce such percentage, are classified as the category 3 predators, including S. aluco, B. bubo, A. noctua and Bubo africanus (Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016).
Associating the accumulation of small mammal remains in Cudó cave with those species of category 3 predators, with the exception of B. africanus, is possible considering their current geographical distribution in the Iberian Peninsula (Sociedad Española de Ornitología/Birdlife 2021). All mentioned species also have similar habitat preferences for dwelling on cliffs, natural crevices or woodland. For the hunting environment, they tend to hunt in open landscapes, such as grassland or woodland margins (Scott et al. Reference Scott, Fernandez-Jalvo and Denys1996). However, their hunting range will vary depending on the landscape and the abundance of their preys.
Among those predators, S. aluco is the most probable species considering the digestion pattern. Compared to B. bubo, S. aluco induces a higher frequency of digested molars and isolated incisors with moderate to high digestion marks. Otherwise, the frequency and intensity of digestion marks are not as high as A. noctua (Andrews Reference Andrews1990; Fernandez-Jalvo & Andrews Reference Fernandez-Jalvo and Andrews1992; Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016). This high proportion of digested remains are observed in both level 107 and 105 of Cudó cave. However, the shifting pattern of digestion marks the degree between these two levels being present. There is an increase of non-digested molars (level 107, 57.8%; level 105 67.5%) and a decrease of heavy-digested molars (level 107, 18.2%; level 105 10.7%). It cannot be barely interpreted as a change of predators involved in the accumulation. Instead, it is linked to the increase of A. sylvaticus remains in the level 105 (Table 4), as it known to have more resistant molar enamel to digestive corrosion (Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016; Avery Reference Avery2022). Therefore, the change of contributing predator can be omitted from the discussion as it implies that the same predators contribute to the accumulation of small mammal remains in both levels.
Table 4 Proportion of digested isolated molars by taxonomic groups of the Cudó cave assemblages.

Strix aluco is considered as an opportunist nocturnal predator that will get pretty much any species present within its hunting range. This predator is known to hunt a relatively equal proportion of rodents or the other prey (Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016). It indicates that the predatory selection is minimum and the prey species represent the environmental condition within their hunting range, which in average is around 0.7 km2 (Andrews Reference Andrews1990; Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016).
4.3. Palaeoenvironment of Cudó cave
There are clear differences between level 107 and level 105 results considering the palaeoenvironmental methods of analysis. From the Simpson's diversity index analysis, level 107 shows a lower index compared to level 105 (Fig. 4). It indicates that level 105 has lower richness and evenness of taxonomic composition than level 105. This signal is consistent with the quantification results, which show C. nivalis dominating the assemblage in level 107, whereas in level 105 A. sylvaticus appears in a considerable proportion (Fig. 4). The increasing diversity index and the change of domination pattern from level 107 to level 105 become a signal of ecosystem improvement, from a less to more favourable ecosystem. The ecosystem could have increased its capacity to support more species linked to an increase on vegetation complexity of arboreal ecosystems (e.g., Williams et al. Reference Williams, Marsh and Winter2002).

Figure 4 Comparison of some ecological indicators extracted from the assemblages of levels 107 and 105 of Cudó cave, including comparison between Chionomys nivalis and Apodemus sylvaticus abundances, Simpson's diversity index score, chorotypes classification, habitat distribution and climatic estimations, expressly with respect to the current climatic conditions surrounding Cudó cave.
The signal of landscape change is supported with the data obtained from the habitat weighting method. In level 107 a habitat with rocky substrate prevails (67.1%) with only a small proportion of woodland habitat (22.1%) (Fig. 4). In contrast, in level 105 there is a predominance of woodland habitat (55.7%) and a smaller proportion of rocky substrate-habitat (29.6%). The increasing proportion of woodland and decreasing rocky substrate in level 105 could be an indicator that there was a climatic amelioration that increased the possibilities of closed vegetation cover to develop. The positive shift is also visible on the proportion of open humid landscape relative to the other types of landscape in level 105. It signals the increase of humidity or precipitation in this area during 15.5–10.2 ka cal BP, consistent with the palynological data extracted from the Banyoles Lake which shows increases on the trees’ taxa after 15 ka BP compared to the period between 31 and 24 ka BP (Pèrez-Obiol & Julià Reference Pèrez-Obiol and Julià1994). However, despite this decreasing proportion, the presence of rocky substrate cannot be ignored as it still has a considerable portion in the landscape (29.6%). It is possible, considering the habitat preference of C. nivalis currently inhabiting the Iberian Peninsula, that they could also live in the bushes or wooded area with stable rocky substrate (Palomo et al. Reference Palomo, Gisbert and Blanco2007).
Moreover, the result displayed from chorotype analysis indicates a consistent trend for this environmental change. The chorotype 1, which corresponds to mid-European faunal taxa, including Sorex gr. araneus-coronatus and C. nivalis, notably dominates the assemblage of level 107 (81.9%). This condition also provides a suitable habitat for chorotype 2 species such as M. arvalis and M. agrestis. Otherwise, in level 105 this domination is shifted to the Mediterranean and generalist species (chorotypes 3 and 4: 63%) (Fig. 4). The increases of chorotypes 3 and 4 taxa and decrease of chorotypes 1 and 2 taxa signal the increase of temperature and humidity (Sans-Fuentes & Ventura Reference Sans-Fuentes and Ventura2000). This could provide better conditions for the flourishing of the woodland habitat and increasing small mammal diversity in level 105.
On the climatic aspect, the BM and MER analysis show detailed information regarding past climatic parameters’ estimations around Cudó cave during the deposition of the remains. In both levels, the results suggest lower mean temperature (level 107, MER −7.2 °C BM −3.3 °C; level 105, MER −4.1 °C BM −3.8 °C) and higher precipitation rates (level 107, MER + 848 mm BM 657.3 mm; level 105, MER + 273 BM + 856.3) compared to the current condition on the area (Fig. 4). However, several differences could be observed when comparing the results of the BM and MER in detail. Both methods follow a qualitative approach based on the presence or absence of the taxa without consideration on the relative abundance. It leads to a significant difference by only a subtle change on the assemblage. By this means, using a different set of data, in case of this work by involving insectivores on the mutual ecogeographical range analysis, can significantly affect the result. Moreover, as both methods were performed using current small mammal distribution, it securely will be affected by the anthropic activities. Even though the distribution of some species (e.g., M. (I.) cabrerae) is already known to be strongly affected by anthropic activities, the impacts on the other species distribution are still difficult to be assessed (López-García et al. Reference López-García, Blain, Morales, Lorenzo, Bañuls-Cardona and Cuenca-Bescós2012; Fernández-García Reference Fernández-García2019).
In spite of that, when the results are compared to other results of this work, such as those derived from the habitat weighting and chorotype analysis, the MER results fit better than those of the BM. In addition, by the comparison to palynological data from the Banyoles Lake where there were increases on the tree taxa after 15 ka BP (Pèrez-Obiol & Julià Reference Pèrez-Obiol and Julià1994), the result from MER analysis also shows better consistency.
4.4. Palaeoenvironment of north-eastern Iberia and the Prades Mountains during the end of Late Glacial
Cudó cave is not the only site providing small mammal assemblage for the palaeoenvironment inference in north-eastern Iberia dated under 35,000 years. There are two other sites situated in very close proximity that could add complementary information about the palaeoenvironment of this region, which are Xaragalls cave and Galls Carboners. In spite of these sites not being exactly contemporaneous to Cudó cave, they provide clue contextual ecological information from the era just before the Cudó cave assemblage formation.
Two important layers from Xaragalls cave, for its chronological proximity to Cudó cave, are C4-dated to 48.2–45.1 ka BP and C3, situated just above layer C4 (López-García et al. Reference López-García, Blain, Bennàsar, Euba, Bañuls, Bischoff, López-Ortega, Saladié, Uzquiano and Vallverdú2011a; Vallverdú et al. Reference Vallverdú, López-García, Blain, Saladié, Uzquiano, Bischoff and Vaquero2012). Palaeoenvironmental inferences from these two layers show domination of woodland covers and mid-European climatic conditions (chorotypes 1 and 2) (López-García et al. Reference López-García, Blain, Bennàsar, Euba, Bañuls, Bischoff, López-Ortega, Saladié, Uzquiano and Vallverdú2011a). From the small mammal analysis, this is indicated by a considerable amount of M. agrestis and also a notably high abundance of A. sylvaticus. Compared to the Cudó cave assemblages, both layer C4 and C3 show a higher proportion of woodland landscape than level 107 and are roughly similar to 105 at Cudó cave. Otherwise, the climatic inference shows that the proportion of species from chorotypes 1 and 2 of Xaragalls cave is lower than in Cudó cave level 107 (Fig. 5). In addition, by the MAT, Xaragalls cave layer C4 was likely warmer than Cudó cave level 107 but still colder than Cudó cave level 105, while Xaragalls cave layer C3 was warmer than Cudó cave level 105 (Fig. 6).

Figure 5 Chorotype and habitat comparison between Cudó levels 107 and 105 with Galls Carboners (López-García et al. Reference López-García, Blain, Bennàsar, Alcover, Bañuls-Cardona, Fernández-García, Fontanals, Martín, Morales, Muñoz, Pedro and Vergés2014a, b) and Xaragalls cave levels C4 and C3 (López-García et al. Reference López-García, Blain, Bennàsar, Euba, Bañuls, Bischoff, López-Ortega, Saladié, Uzquiano and Vallverdú2011a).
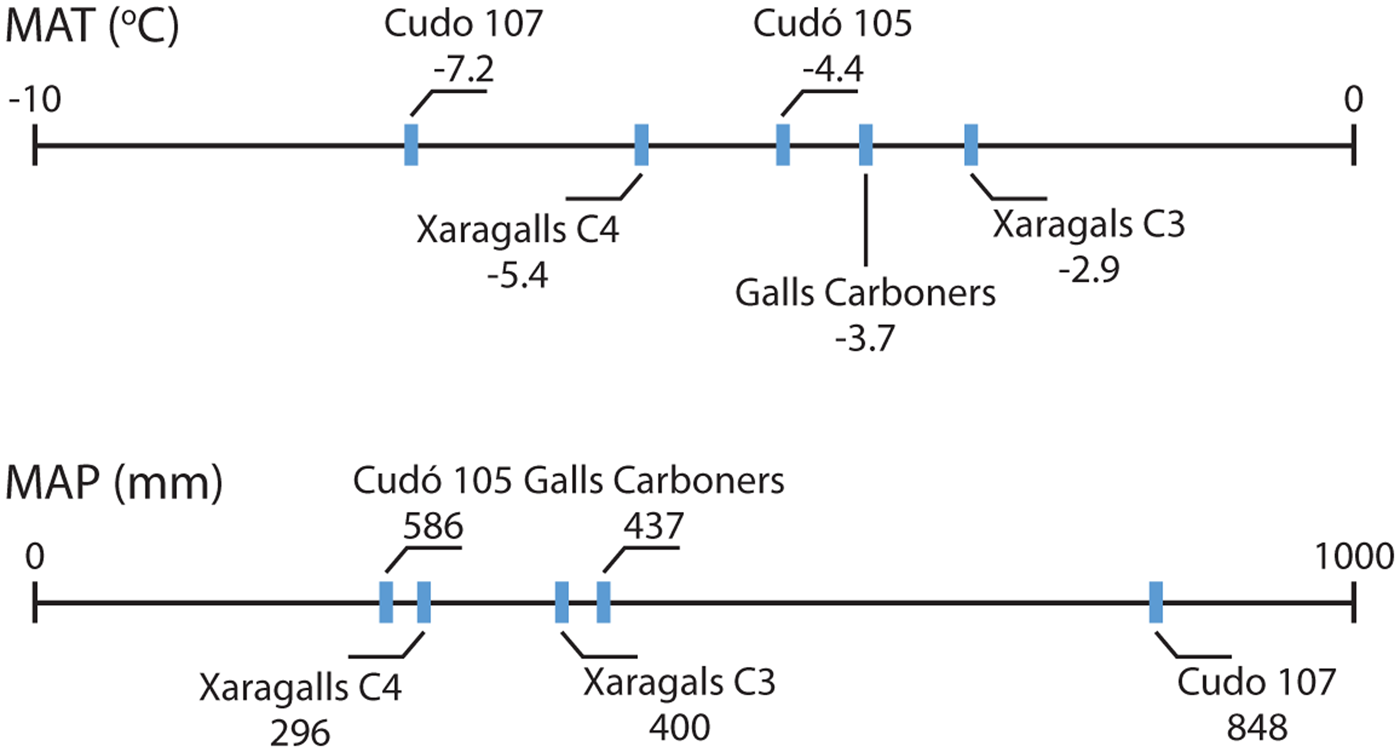
Figure 6 Comparison of the difference of mean annual temperature and mean annual precipitation between Cudó cave levels 107 and 105 with Galls Carboners (López-García et al. Reference López-García, Blain, Bennàsar, Alcover, Bañuls-Cardona, Fernández-García, Fontanals, Martín, Morales, Muñoz, Pedro and Vergés2014a, b) and Xaragalls cave levels C4 and C3 (López-García et al. Reference López-García, Blain, Bennàsar, Euba, Bañuls, Bischoff, López-Ortega, Saladié, Uzquiano and Vallverdú2011a, b). The data are based on mutual ecogeographical range analysis result and in respect to current data from the nearest climatic station of each site.
Moreover, the assemblage from Galls Carboners, dated to ca. 31.3–31.1 ka cal BP (López-García et al. Reference López-García, Blain, Bennàsar, Alcover, Bañuls-Cardona, Fernández-García, Fontanals, Martín, Morales, Muñoz, Pedro and Vergés2014a) could be even more interesting as it is contemporaneous to the early stage of Cudó cave level 107 and they have close geographical proximity. However, the palaeoenvironment inferences show a different pattern than what it is inferred in level 107 from Cudó cave. This level represents an exceptional cold and wet event with the lowest woodland habitat representation and highest proportion of rocky habitat, in contrast to the condition in Galls Carboners which is dominated by woodland. In the species composition, level 107 shows strong dominance of mid-European species (81.9%) with the lowest Mediterranean taxa (17.8%), while in Galls Carboners only 55% of the assemblage composed of mid-European species and 45% of the assemblage are Mediterranean taxa. The big difference also can be seen in the MAT and MAP estimations – level 107 at Cudó cave has lower temperature (−3.5 °C) and significantly higher precipitation (+411 mm) than Galls Carboners (Fig. 6). Furthermore, the signal from Simpson's diversity index shows that Galls Carboners have more diverse and equally represented small mammal species (1–D = 0.83) compared to Cudó cave level 107 (1–D = 0.54). These results indicate that the Galls Carboners palaeoenvironmental condition was more favourable to support diverse small mammal communities.
The difference between the MAT and MAP of Cudó cave level 107 and Galls Carboners is important to be noted since these two sites are located in very close proximity, in similar elevation (around 1000 m.a.s.l.), and chronologically overlapping. Taphonomic biases cannot be rejected as the cause of these differences, although S. aluco is considered the main accumulator of the assemblages from both sites, with a well-known opportunistic hunting behaviour. Further research is recommended to evaluate the real influence. Nevertheless, even if the predatory selection played a major role during the accumulation, the significant difference of mid-European taxa in both assemblages, including the domination of C. nivalis in Cudó cave, become clear evidence of different environmental conditions. Also, the ecological changes between levels 107 and 105 are not accompanied by the change of taphonomic pattern, thus these changes can be significant.
The possible reason is that the Galls Carboners assemblage only correspond to a short chronological period, coincident with the beginning of the Heinrich Event 3 (HE3). Despite that the Heinrich Events are known to have severe climatic conditions, most of these events could be divided into three stages based on the different climatic conditions, which are the early, main, and late stages. The sea surface temperature and palynological records suggest that the climatic condition during the early stage of HE3 was not so severe (see Cacho et al. Reference Cacho, Grimalt, Canals, Sbaffi, Shackleton, Schönfeld and Zahn2001, Reference Cacho, Shackleton, Elderfield, Sierro and Grimalt2006; Fletcher & Sánchez Goñi Reference Fletcher and Sánchez Goñi2008). The less harsh climate maintains the higher proportion of Mediterranean taxa with woodland habitat preferences compared to a colder and dryer climate.
In contrast, level 107 of Cudó cave, which represent the coldest and most humid period, has a chronological range that spans over a longer period, including the whole HE3 and the beginning of Heinrich Event 2 (HE2) and LGM (Fig. 7). The later stage of level 107 might be coincident with the early stage of glaciers’ expansion in the southern latitude (Lewis et al. Reference Lewis, McDonald, Sancho, Peña and Rhodes2009; Moreno et al. Reference Moreno, González-Sampériz, Morellón, Valero-Garcés and Fletcher2012; Lécuyer et al. Reference Lécuyer, Hillaire-Marcel, Burke, Julien and Hélie2021). With this expansion and considering the high altitude of the Cudó cave, snow covers were possibly present in the vicinity of Cudó cave during a major part of the year. This consequently increased the abundance of mid-European taxa with rocky habitat preferences, such as C. nivalis species, and demonstrate the importance of glacial deposit extension in high altitudes even in Mediterranean-affected environments that generally were experiencing milder conditions.
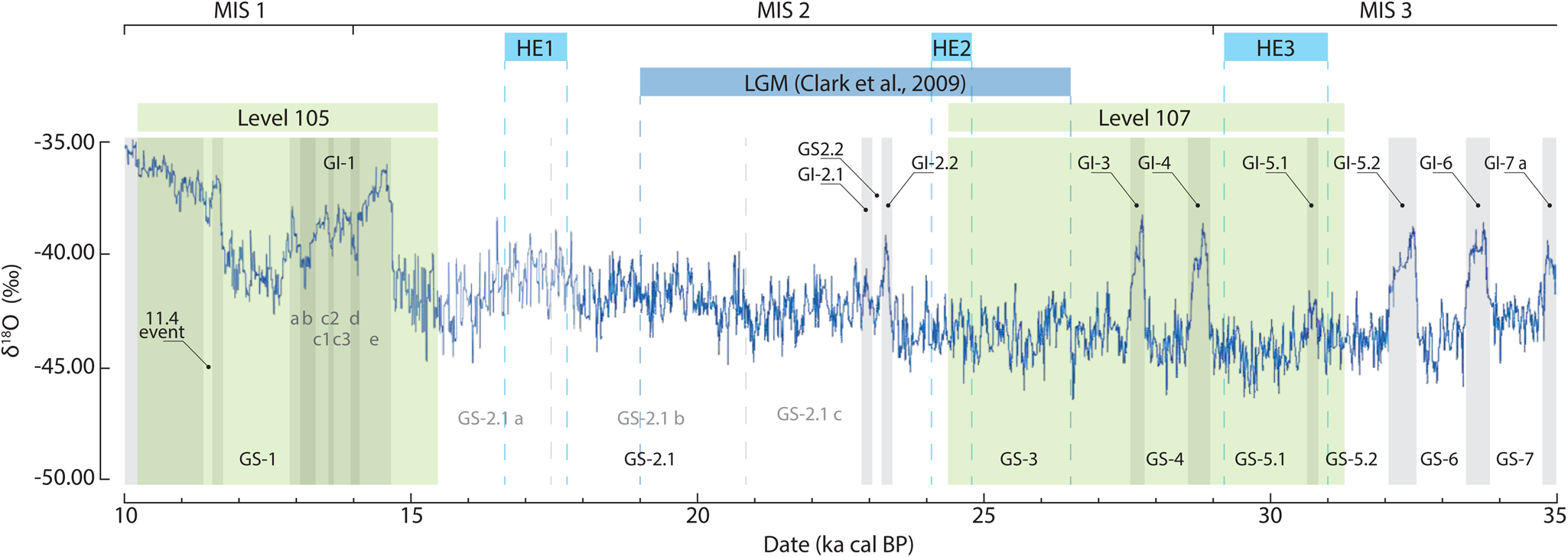
Figure 7 Chronological position of level 107 and 105 of Cudó cave among the global climatic events (Rasmussen et al. Reference Rasmussen, Bigler, Blockley, Blunier, Buchardt, Clausen, Cvijanovic, Dahl-Jensen, Johnsen, Fischer, Gkinis, Guillevic, Hoek, Lowe, Pedro, Popp, Seierstad, Steffensen, Svensson, Vallelonga, Vinther, Walker, Wheatley and Winstrup2014; Maier et al. Reference Maier, Mayr and Peresani2021).
The exceptionally cold and wet conditions of level 107 are probably related to HE3 or HE2 when the cold summer and humid winter conditions were experienced. It suggests that these events have different climatic conditions with the previous Heinrich Event 4 (HE4) event conditions. As Xaragalls cave C4 is recorded also in Terrasses de la Riera dels Canyars (López-García et al. Reference López-García, Blain, Bennàsar, Sanz and Daura2013), HE4 is distinctively warmer and drier than what is recorded in Cudó cave level 107. Furthermore, if Cudó cave level 107 is compared to level C from Arbreda cave (23.5–22.9 ka cal BP), the Cudó cave level 107 is still colder and significantly wetter (López-García et al. Reference López-García, Soler, Maroto, Soler, Alcalde, Galobart, Bennàsar and Burjachs2015). These discrepancies could be linked to the geographical condition where Cudó cave is situated. The mountainous relief with elevation reaching 1000 m.a.s.l. and the presence of cliffs could give this colder condition. Even so, the domination of mid-European taxa in level C of Arbreda cave could be evidence of the cold and humid climate after the HE2 and during the LGM.
From the taphonomic aspect, the same predator also has been identified from Galls Carboners assemblages (López-García et al. Reference López-García, Blain, Bennàsar, Alcover, Bañuls-Cardona, Fernández-García, Fontanals, Martín, Morales, Muñoz, Pedro and Vergés2014a). The quantification of digested marks on the incisors is comparable with Cudó cave level 107. The percentage of digested incisors from Galls Carboners, particularly from levels 106–108, ranges from 45.7% to 55.9%, while in Cudó cave level 107 it is 58.3%. These values fit to category 3 predator, including also B. africanus, B. bubo and A. noctua (Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016). Further studies point out that S. aluco was the main accumulator of small mammal remains in Galls Carboners (López-García et al. Reference López-García, Blain, Bennàsar, Alcover, Bañuls-Cardona, Fernández-García, Fontanals, Martín, Morales, Muñoz, Pedro and Vergés2014a). Thus, it can be demonstrated that the different analysis results between Cudó cave and Galls Carboners are not a result of hunting preferences of the predators.
Otherwise, the Cudó cave level 105 is in line with contemporaneous sites in north-eastern Iberia. It is roughly similar to level CE15 of Colomera cave (ca. 13 ka BP) (López-García et al. Reference López-García, Blain, Cuenca-Bescós, Ruiz-Zapata, Dorado-Valiño, Gil-García, Valdeolmillos, Ortega, Carretero, Arsuaga, de Castro and Carbonell2010), where the mid-European taxa become the most abundant group of the assemblage. The inferred habitat is also dominated with woodland (54%) and a quite high proportion of rocky habitat (24%). The climatic improvement also observed in Toll cave (Fernández-García & López-García Reference Fernández-García and López-García2013), from a roughly equal proportion of mid-European and Mediterranean taxa in level 3 (ca. 47.1–45.5 ka cal BP) become dominated by Mediterranean taxa in level 2. Unfortunately, there is no absolute date assigned to this level to build a better correlation.
Thus, the small mammal assemblage of level 105 clearly indicates a climatic improvement compared to level 107. This is particularly shown by the decreasing mid-European taxa and the increasing woodland habitat. However, this improvement is not exceptional if the older assemblages from the Prades Mountains are considered. The increasing Mediterranean taxa is not so much different from the Xaragalls cave C4, C3, and particularly the Galls Carboners assemblage. The woodland and open humid habitat composition even shows more similarities. Interestingly, the rocky habitat appears with a bigger proportion in Cudó cave level 105 than the Xaragalls cave C4 and C3 assemblages. This pattern could be related to several abrupt climatic events such as Older Dryas (GI-1d) and Inter-Allerød Cold Period (GI-1b) (Fig. 7).
5. Conclusions
Cudó cave that belongs to the Prades Mountains system yielded two archaeo-palaeontological stratigraphic levels, which are levels 107 and 105, dated between 31.2–24.4 ka cal BP and 15.5–10.2 ka cal BP, respectively. These two levels contain archaeological remains associated with the Upper Palaeolithic period, including lithic and faunal artefacts. Moreover, levels 107 and 105 of Cudó cave are rich in small mammal remains, with 1185 specimens being identified in this work corresponding to a minimum of 502 individuals. From this assemblage, 14 species have been identified.
Predatory activity has been identified as the main factor determining the accumulation of this assemblage, based on the high number of elements digested. Considering the digestion marks, S. aluco which belongs to the category 3 predators is the most possible accumulator of the assemblage. It is considered as an opportunistic hunter. It dwells on the cliffs, natural crevices, or woodland and preferentially hunts in an open landscape. However, from the inspection on the digestion pattern in relation to the predator's categories of both levels, it is deduced that the climatic factors indicate that the taphonomic explanation prevails. Apparently, the same type of predators was involved in the small mammal remains’ accumulation in both levels.
On the palaeoenvironmental aspect, it has been inferred that level 107 shows a moderate level of diversity comprising the predominance of C. nivalis, a mid-European species. The high abundance of this species also links to the predominance of rocky habitat as well as the estimated cool mean annual temperatures and high precipitation rates with respect to current conditions. Such an environmental pattern, together with the chronology proposed for this level, suggested this level could be correlated to the HE3 or HE2 as well as the beginning of LGM. This contrasts with level 105, where the environment was more diverse with a relatively low proportion of required mid-European taxa compared to Mediterranean taxa. The habitat also seems to be improved with a significant increase of woodland habitat and a decrease of rocky habitat. Furthermore, the mean annual temperatures and the precipitation rates also differ from the current condition. It could be an indicator of an environmental improvement in respect to level 107.
Despite of the climatic improvement, level 105 shows that the environmental conditions were not exceptionally different compared to the other sites such as Colomera cave level CE-15, the upper level of Toll cave, as well to the older levels of Xaragalls cave C4 and C3. It is indicated by the remarkable proportion of rocky habitat, the low temperature and the high precipitation rates that are still maintained in this level. This pattern might correspond to several abrupt climatic events during the period of 15.5–10.2 ka cal BP, such as Older Dryas (GI-1d) and Inter-Allerød Cold Period (GI-1b).
Finally, those patterns observed in the Cudó cave small mammal assemblages provide some global-to-regional climatic insights. First, consistent with previously published works, the HE3 and HE2 were cold and humid periods in north-eastern Iberia, with more severe climatic conditions than the HE4. Second, the environmental conditions in this area could have been more diverse despite the similar Mediterranean affected climate. Moreover, the environmental improvement after 15 ka as it seen in many studies does not appear so significant in all areas and within all proxies. Instead, the climatic changes were responded to differently depending on the proxies and the geographical area.
To sum up, this work advises to enlarge the taphonomic study in order to complete the taphonomic characterisation on the small mammal assemblages from this site and finally evaluate the ecological biases. With a similar goal, studies on other environmental proxies of Cudó cave, such as pollen, charcoal or phytoliths analysis, large vertebrate studies and the sedimentological analysis will be helpful to characterise the site. A better understanding of this site will be useful in a larger geographical scope than local studies, considering the scarcity of environmental data linked to the LGM context in north-eastern Iberia.
6. Acknowledgement
We are grateful to the fieldwork team of the Cudó cave site.
7. Financial support
This work is part of D.Q. Arjanto's master thesis (International Master on Quaternary Archaeology and Human Evolution from Universitat Rovira i Virgili, Tarragona under the Erasmus Mundus International Master of Quaternary Prehistory 2019 programe). This research was supported by the Catalonian Government project (CLT009/18/00053: ‘Evolució paleoambiental i poblament prehistoric a les conques dels rius Francoli, Gaià, Siurana i rieres del Camp de Tarragona’; P.I..: J.M.V.). J.M. López-García was supported by a Ramon y Cajal contract (RYC-2016-19386), with financial sponsorship of the Spanish Ministry of Science and Innovation. M. Fernández-García's research is funded by the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (grant agreement No. 818299-SUBSILIENCE project).
8. Conflicts of interest
None.
9. Authors’ contributions
Conceptualisation, investigation, data collection and formal analysis were mainly performed by D.Q. Arjanto. M. Fernández-García and J.M. López-García were actively involved in the conceptualisation, formal analysis, investigation, resources and writing and editing of the original draft. J.M. Vergès provided guidance in the conceptualisation, resources and reviewing–editing the manuscript. The first draft of the manuscript was written by D.Q. Arjanto and all authors commented on previous versions and approved the final manuscript.














