INTRODUCTION
Measles remains a leading vaccine-preventable cause of child mortality worldwide. In 2008, there were 164 000 measles deaths globally – nearly 450 deaths every day or 18 deaths every hour [1]. However, this disease can be eliminated, considering its exclusively human reservoir, lack of a long-term carrier state for the virus, and the availability of an effective and safe vaccine [Reference Godoy2]. A successful strategy for measles control includes high vaccine coverage, effective laboratory-supported disease surveillance, and appropriate clinical management [Reference Moss3, 4]. Although once considered a common illness in children, measles is now a sporadic infectious disease in Taiwan. Measles has been listed as a reportable disease since 1985 in Taiwan. From 1985 to 1992, there were four major measles outbreaks in Taiwan; 1985 (2219 cases), 1988 (1386 cases), 1989 (1060 cases) and 1992 (303 cases) [Reference Lee5, Reference Lee6]. The incidence of measles has decreased substantially in areas where measles vaccination was introduced. Live attenuated measles vaccine was first introduced to Taiwan in 1968 and a nationwide immunization programme was launched in 1978. In May 1990, the measles immunization programme in Taiwan was switched from a single-dose Schwarz vaccine at 12 months to a two-dose regimen at 9 and 15 months. Since mid-1991, Taiwan has had a project for the eradication of measles, congenital rubella syndrome, poliomyelitis and neonatal tetanus. Since 1992 there has been a universal vaccination of one-dose measles vaccine at age 9 months plus one dose of measles, mumps and rubella (MMR) vaccine at age 15 months [Reference Lee5–Reference Liu, Lei and Chiang7]. Two catch-up campaigns occurred in response to outbreaks for junior high-school students from 1992 to 1994 and elementary school students from 2001 to 2004 [Reference Cheng8]. A universal second booster MMR vaccination also started in 2001. In January, 2006, the stand-alone measles vaccine was discontinued and replaced by MMR. The vaccination schedule for the first dose of MMR was changed to 12 months in April 2009 after a measles cluster [Reference Lin9].
In June 2007, a 26-year-old medical student in a university hospital in southern Taiwan began to have a high fever and arthralgia after his tour to Japan in late May. Pustule-like skin rashes developed thereafter. Serological confirmation diagnosed him as having modified measles, despite the fact that he had had two doses of measles vaccination in childhood [Reference Lee10]. Meanwhile, a large measles outbreak occurred in Japan, causing a number of universities and other institutions to close in an attempt to contain the disease [Reference Nagano11]. This case highlights the waning immunity of subjects who have received vaccinations in a country like Taiwan but who frequently travel to neighbouring endemic places in Asia. In hospitals and outpatient facilities, healthcare workers (HCWs) are at risk of acquiring infectious diseases from patients, and in some instances HCWs were implicated in the transmission of diseases [Reference Mendelson12].
Nosocomial measles transmission is an important threat in industrialized countries and in urban settings in developing countries, where measles has not been endemic for many years [Reference Biellik and Clemens13]. Previous studies in the USA have shown that more than one fifth of measles transmission cases in medical settings occur in HCWs [Reference Atkinson14, Reference Davis15]. In a recent measles outbreak in southern Taiwan, unvaccinated HCWs became infected in hospitals [Reference Lin9, Reference Lee16]. Seroepidemiological data of the prevalence of susceptibility to measles in HCWs is crucial for disease control and prevention. The measles serology test has been included in routine preplacement health screening for all newly hired employees at our hospital since 2004. This study aimed to determine the current measles seroprevalence in HCWs at a major hospital in southern Taiwan.
METHODS
Study population and database
A cross-sectional, hospital-based study of measles seroprevalence was conducted at National Cheng Kung University Hospital from August 2004 to August 2009. The hospital is a 1200-bed public tertiary referring centre in southern Taiwan serving more than 4000 visiting patients a day. It has more than 2500 employees including physicians, nursing staff, and housekeeping personnel. The demographic data and results of measles serology tests were recorded in the computer database of the infection control centre of the hospital. All the data were analysed according to age, gender and profession.
Determination of IgG antibodies for measles virus
Blood samples were obtained in the preplacement health screening by standard laboratory techniques. All subjects were generally healthy and without underlying diseases or acute illness at the time of blood sampling. Subjects gave their informed consent. Sera were collected by centrifugation of the blood at 1500 rpm for 5 min and then stored at −20°C until tested. Enzyme-linked immunosorbent assays (ELISAs) using the Enzygnost® test kit (Siemens Healthcare Diagnostics, Germany) measured the specific immunoglobulin G antibodies against measles virus, following the standard procedures recommended by the manufacturer as described previously [Reference Weigle, Murphy and Brunell17]. Spectrophotometric measurement of the optical density (OD) at 450 nm of the wells and linear regression analysis were performed with calibrator control wells. The test results were grouped as negative for OD less than 0·1, equivocal for 0·1–0·2 OD and positive for above 0·2 OD. The equivocal samples were retested and then grouped if an equivocal result was found again.
Statistical analysis
The proportional differences of each group were compared with a χ2 test with SPSS version 11·5 software (SPSS Inc., USA). P values <0·05 were considered significant.
RESULTS
The study included 1584 newly hired HCWs who had undergone preplacement screening. All subjects were born on or before 1989. Two hundred and fifty (15·8%) of these HCWs were born before the launch of the measles mass vaccination programme of 1978. In other words, most of the HCWs had received measles vaccination. The majority of the participants were female (1177, 74·3%), with an average age of 27·7 years (median 25, range 20–57 years). Younger HCWs (aged 20–29 years) had a higher rate of negative or equivocal anti-measles antibody results than those aged 30–39 years (4·8% vs. 1·3%, P=0·0157 and 17·1% vs. 4·8%, P<0·0001) or those aged ⩾40 years (17·1% vs. 4·4%, P=0·0054) (Table 1). Further analysis showed that the younger the HCWs, the lower the anti-measles antibody positivity (71·8% in the 20–24 years group and 86·3% in the 25–29 years group, P<0·0001). Gender difference is not a factor in terms of anti-measles titres (Fig. 1).
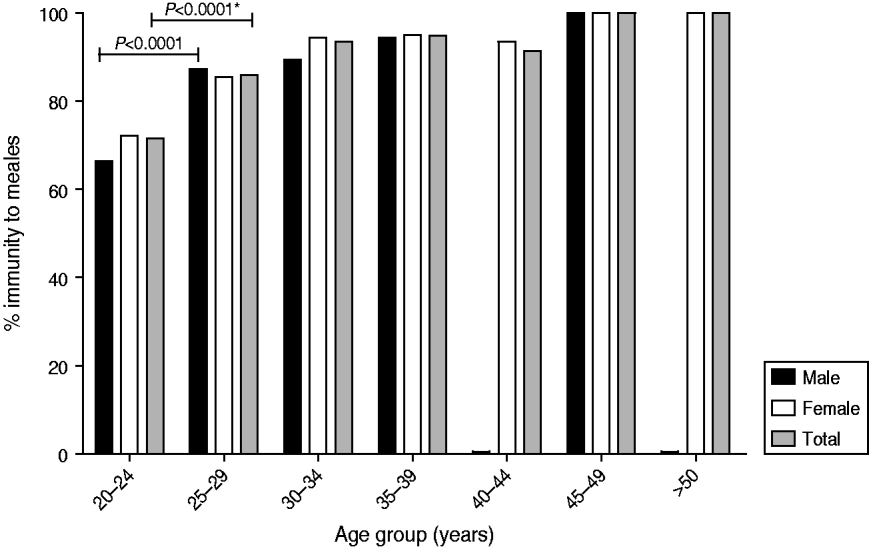
Fig. 1. Measles immunity in healthcare workers by gender and age group in southern Taiwan, 2004–2009. * χ2 tests, P<0·05 is considered significant.
Table 1. Immunoglobulin G antibodies against measles virus in healthcare workers (HCWs) during 2004–2009 in Taiwan, by age
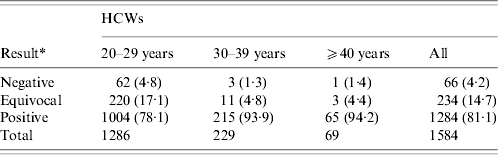
Data are presented as number (percentage) of HCWs in different groups.
* Results reported as negative for optical density (OD) less than 0·100, equivocal for 0·100–0·200 OD and positive for above 0·200 OD.
Eighteen percent of the newly hired HCWs were physicians (90% residents, 10% attending physicians), 50·2% were nursing staff, and 23·9% were housekeeping personnel including pharmacists, technicians, research assistants, and others. The positivity for physicians (91%) is comparable with nursing staff (88·1%) (P=0·2170) but higher than for medical students (81·8%, P=0·0130) and housekeeping personnel (58·5%, P<0·001) (Table 2). Housekeeping personnel had the highest rate of equivocal anti-measles antibody (36·1%). However, the highest rate of negative anti-measles antibody was in medical students (8·3%).
Table 2. Immunoglobulin G antibodies against measles virus in healthcare workers (HCWs) during 2004–2009 in Taiwan, by profession
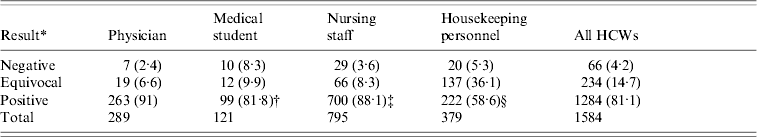
Data presented as number (percentage) of HCWs in different groups.
* Results reported as negative for optical density (OD) less than 0·1, equivocal for 0·1–0·2 OD and positive for above 0·2 OD.
† χ2 test, physician vs. medical student, P<0·001,
‡ χ2 test, physician vs. nursing staff, P=0·2170,
§ χ2 test, physician vs. housekeeping personnel, P=0·0130.
DISCUSSION
Measles is a highly contagious disease. The basic reproductive number (R 0) is the average number of secondary cases from a single case introduced into a very susceptible population. R 0 estimates for measles in Taiwan before the introduction of vaccine in 1978 ranged from 4·78 to 8·00 [Reference Chen18].
People who have a measles-specific antibody that is detectable by any serological test are considered immune. However, people with an equivocal test result should be considered susceptible unless they have other evidence of measles immunity or subsequent testing indicates they are immune [Reference Watson19]. Despite high measles vaccine coverage in Taiwan, we found waning immunity in HCWs aged <25 years in our survey. In a previous community-based measles seroepidemiological study in Taiwan conducted between 1995 and 1997, the seropositivity in people aged 19–25 years was 100% [Reference Lee20]. One possible explanation for the difference between the studies is different sources of measles immunity, natural infection or vaccination. Since mass measles immunization was started in 1978, individuals aged between 19 and 25 years, during the 1995–1997 period, got their immunity for measles mainly from natural infection. In contrast, most of the HCWs aged <25 years in this study acquired immunity to measles via vaccination.
Housekeeping personnel in this study had the highest susceptibility to measles. Although this group of HCWs is generally not involved in direct patient care, they could be a key minority factor in intra-hospital infections. The 2003 SARS outbreak in Taiwan also highlighted the role of housekeeping personnel, e.g. laundry attendants, in disease transmission [Reference McDonald21]. Medical students are also more susceptible to measles than other HCWs in this study. Medical students might be exposed to various infectious agents during their clinical rotations. Other previous surveys indicated a close relationship between measles outbreaks and relatively low immunity to measles in this group [Reference Yalcin, Kanra and Pehlivan22, Reference Wicker23]. Age difference might play an important role in determining measles antibody titres. Medical students in this study are younger compared to other professional groups.
A recent study in the UK also recommends assessing the measles immunity of not only newly hired workers but also all HCWs due to the low level of documented MMR vaccination [Reference Williams24]. Re-vaccination has been shown effective in lowering the number of susceptible people and the percentage of vaccines with low antibody titres [Reference Tischer and Gerike25]. Our results provide useful information for medical authorities in making vaccination plans for susceptible populations. All the new healthcare employees with negative or equivocal measles antibody titres in our hospital were required to receive one catch-up MMR vaccine. Recently, the Immunization Practices Advisory Committee (ACIP) of Taiwan CDC also issued new recommendations that all healthcare personnel without documented evidence of measles immunity receive two doses of MMR vaccine at least 1 month apart [Reference Wang26].
The prevention of measles through vaccination is still the safest and most efficient way to contain the disease. The duration of immunity following measles vaccination is more variable and shorter than that following a natural infection with measles, but it persists for decades even in countries where measles is no longer endemic and immunological boosting from wild-type measles infection does not occur [Reference Amanna, Carlson and Slifka27, Reference Dine28]. Although antibody levels induced by vaccination may decline over time, immunological memory persists and most people vaccinated produce a measles-specific immune response without clinical symptoms following exposure to wild-type measles virus [Reference Moss3]. A recent cohort study in Finland also showed that 95% of MMR vaccines remain seropositive for measles from ages 22 to 26 years [Reference Davidkin29].
Of the HCWs examined by haemagglutination inhibition assay and ELISA, 98·5% were immune to measles in Japan [Reference Hatakeyama30]. In Australia, the rate of seropositivity in HCWs for measles was reported to be 98·3% [Reference Fedeli, Zanetti and Saia31]. Among all the newly hired HCWs, 13% had negative IgG antibody test results for measles virus in Saudi Arabia [Reference Almuneef32]. In New Zealand, 86% were measles IgG seropositive, 9% were seronegative, and 5% had equivocal test results of the high-risk HCWs [Reference Williamson33]. There is concern that a considerable proportion (18·9%) of HCWs in Taiwan lack immunity to measles.
In summary, measles is still a serious infectious disease despite the existence of effective vaccines. In countries with suboptimal vaccination rates, the most cost-effective preventive measure is to increase the vaccination coverage. However, in highly vaccinated countries like Taiwan, the waning immunity in those already vaccinated is another challenge. This study demonstrates the importance of the waning measles immunity in HCWs and points out the benefits of regular surveillance and catch-up vaccinations both of which should be included in a hospital's infection control strategy.
DECLARATION OF INTEREST
None.





