INTRODUCTION
Molecular epidemiological studies of Staphylococcus aureus have often focused on methicillin-resistant S. aureus (MRSA) in outbreak situations. Recent studies indicate that the prevalence of MRSA is low in general populations in European countries [Reference den Heijer1, Reference Sangvik2]. The clinical significance of methicillin-sensitive S. aureus (MSSA) is, however, also great [Reference Mostofsky, Lipsitch and Regev-Yochay3, Reference David4], and some spa types are indicated to be more common in clinical isolates compared to community isolates [Reference Ghasemzadeh-Moghaddam5]. Differences in the distribution of MSSA and MRSA strain types have been reported [Reference Miko6] and show a greater diversity of spa types in MSSA [Reference Sangvik2, Reference Ghasemzadeh-Moghaddam5–Reference Grundmann7], compared to the often observed clonal spread of MRSA strain lineages [Reference Grundmann7, Reference Goering8].
An association of specific MSSA spa types with age and gender in the general population has been suggested [Reference Sangvik2]. Moreover, nursing homes have been implicated as reservoirs for antimicrobial-resistant organisms, including S. aureus [Reference Bonomo9]. Only a few comprehensive S. aureus screening studies have been performed within these settings, and most focused primarily on MRSA [Reference Pfingsten-Wurzburg10, Reference Denis11].
The overall prevalence of nasal S. aureus colonization varies in different studies, and rates up to 55% have been reported [Reference Kluytmans, van Belkum and Verbrugh12]. Carriers are generally classified as persistent (∼20%), intermittent (∼30%) and non-carriers (∼50%) [Reference Nilsson and Ripa13, Reference Wertheim14]. The classification of an individual as a persistent carrier is of importance, since they appear to have an increased risk of endogenous infection compared to non-carriers [Reference Wertheim15, Reference von Eiff16], whereas intermittent carriers have an equally low risk as non-carriers [Reference van Belkum17]. However, it should be noted that classification of a persistent carriage state remains contentious [Reference Verhoeven18].
Recent studies have shown an equal or higher colonization rate of S. aureus in the throat compared to nares, and there are a number of individuals exclusively colonized in the throat [Reference Nilsson and Ripa13, Reference Mertz19, Reference Smith20]. A possible role of throat carriage for S. aureus transmission has also been recently indicated [Reference Mernelius21], and inclusion of throat culture in screening protocols is widely considered to be necessary for the optimal recovery of S. aureus [Reference Smith20, Reference Andrews22, Reference Olofsson23].
Knowledge of molecular epidemiology of S. aureus in nursing-home residents is important as this group represents a population with high comorbidity and frailty [Reference Ernsth24]. Furthermore, they have an increased frequency of hospital care with a risk of exchange of bacterial strains [Reference Bonomo9], which might have an influence on transmission patterns. The aim of this study was to determine the prevalence of S. aureus in Swedish nursing-home residents and investigate the molecular epidemiology of isolates in terms of the spatial and temporal spa gene type distribution and antimicrobial resistance.
METHODS
Study population
The study population (n = 290; 70% women, 30% men) was recruited from individuals, between the ages of 60 and 101 (median 85) years, included in the SHADES programme [Reference Ernsth24], a longitudinal, open cohort, multi-purpose study conducted in Swedish nursing homes for permanent residents, between 2008 and 2011. The nursing homes (n = 9) were located in three cities in the south of Sweden, Eslöv (n = 2) with 47% of the participants, Jönköping (n = 4) with 41% of the participants and Linköping (n = 3) with 11% of the participants. The colonization rates of S. aureus and antibiotic susceptibility results from the first culture have in part been reported previously [Reference Olofsson23]. Information about age, gender and length of stay were registered. The study was approved by the Regional Ethical Review Board at Linköping University (M150-07). Informed consent was obtained from all included individuals (or from persons close to them as appropriate).
Sample collection and S. aureus isolation and cultivation
Sampling was performed using a rayon-tipped swab (Copan Diagnostics Inc., Italy), from 1 January 2009 to 31 March 2011 and included samples (n = 1860) from the anterior nares, the throat, the groin and active skin lesions. If possible, samples were collected every 6 months during the study period.
The swabs were incubated for 16–20 h in a broth selective for S. aureus [Reference Nilsson and Ripa13] and then 10 μl broth was cultured on blood agar plates (n = 996), and from 1 February 2010 on BBL™ CHROMagar™ Staph aureus medium (Becton Dickinson, USA) (n = 864). Plates were incubated for 20–24 h, and S. aureus was confirmed by DNase activity or detection of the nuc gene [Reference Nilsson, Alexandersson and Ripa25]. Isolates were tested for antibiotic susceptibility [Reference Olofsson23] and stored at –80°C in skimmed milk.
spa typing
All S. aureus isolates (n = 466) were spa typed according to Kahl et al. [Reference Kahl26]. PCR products were sequenced at GATC Biotech (GATC Biotech AG, Germany). Ridom StaphType software (Ridom GmbH, Germany) was used to determine the spa types [Reference Harmsen27] and BURP clustering was performed [Reference Mellmann28].
Statistical analyses
Statistical analysis (χ 2 test and t test) was performed using the IBM SPSS Statistics version 20 statistical package (IBM Corporation, USA) and included the prevalence of colonization with S. aureus at the four body sites, the proportion of antibiotic-resistant isolates and the occurrence of spa types specified by individual characteristics and geographical region.
RESULTS
Prevalence
On the first sampling occasion 179/290 individuals were sampled from nares, throat and groin. From the remaining 111 subjects only samples from one or two of the body sites were available. There were no differences in age, gender and length of stay in the nursing home between individuals sampled on three body sites compared to those sampled from one or two body sites. Overall, 185 isolates of S. aureus from 747 samples were obtained. Table 1 shows that the highest prevalence was found in throat samples (34%) followed by anterior nares (31%), and by combining nares and throat samples the prevalence increased to 48%. There was no significant difference in gender (P = 0·52) or mean age (P = 0·14) between individuals colonized and not colonized.
Table 1. Detection of S. aureus and spa-type distribution, in different body sites, at the first sampling occasion
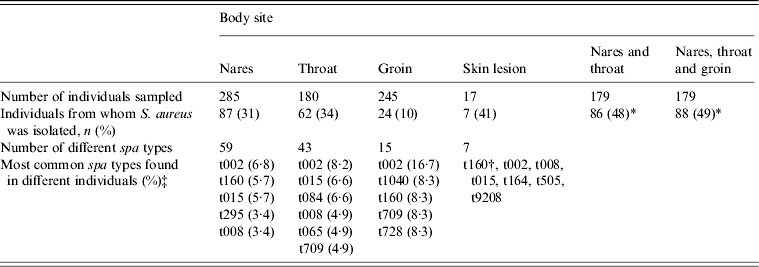
* S. aureus detected in one or more of the body sites.
† spa type t160 was found in two individuals, the rest in one each.
‡ The proportion of individuals carrying the spa type at the given body site.
In the longitudinal study the 290 individuals were sampled on various occasions (n = 1–5) for up to 2 years, due to the open cohort design; 28% once, 21% twice, 18% three times, 28% four times and 5% five times. A total of 1860 samples was obtained; nares (n = 739), throat (n = 355), groin (n = 665) and skin lesions (n = 101) and S. aureus was isolated from 466 of these cultures: nares (n = 240), throat (n = 110), groin (n = 63) and skin lesions (n = 53). The accumulated proportion of individuals from whom S. aureus could be isolated, increased from 31% to 60% and from 34% to 62% in nares and throat, respectively, by increasing the number of sampling occasions (n = 4), and by combining results from nares and throat the proportion increased to 67% (Fig. 1).
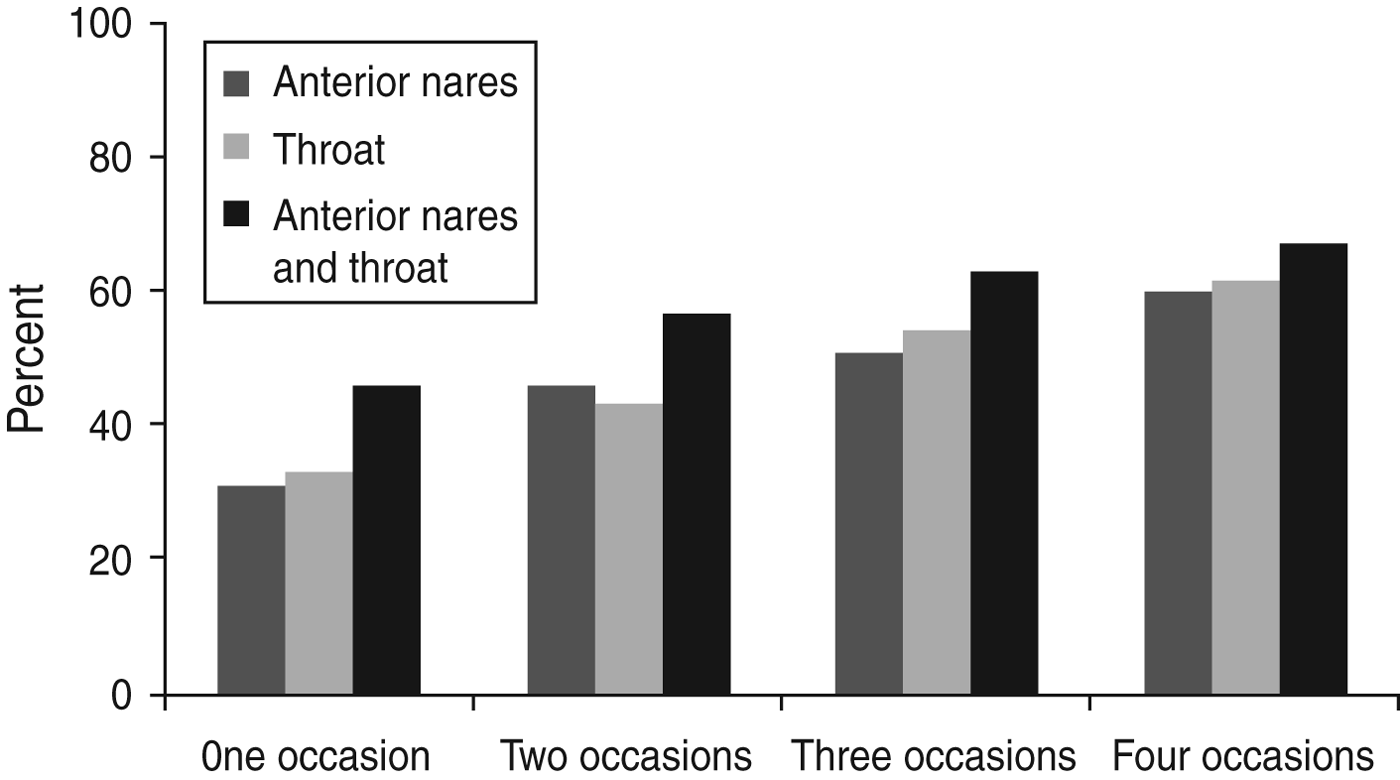
Fig. 1. The prevalence of S. aureus colonization rates obtained by the accumulation of results from increasing sampling occasions.
spa type distribution
Of the 185 isolates from the first sampling occasion 73 different spa types were identified; 47 (64%) were only found in single individuals, 14 (19%) in two, and 13 (18%) in three or more individuals. One isolate was non-typable. The most common spa types were t002 (8·3%), t160 (5·8%), t015 (5·0%), t084 (5·0%) and t705 (3·3%). No difference in distribution of common spa types was seen between the nares and throat samples (Table 1). All isolates from the groin and skin lesions belonged to spa types also isolated from other body sites. Carriage of two different spa types in one individual, was found in 10/43 (23%) individuals from whom S. aureus was isolated from more than one body site on the same occasion.
In the longitudinal study, no temporal differences in the distribution of major spa types were seen (data not shown). Fifty different spa types distributed over 82 individuals were detected in Eslöv, and in Jönköping 56 different spa types in 71 individuals were found. No spa type was carried by more than two individuals in Linköping. S. aureus of spa type t160 was found in nine individuals in one region, but was not found in the other geographical regions whereas other common spa types were equally distributed (Fig. 2). Seven of the individuals with spa type t160 were living at the same nursing home.

Fig. 2. Geographical distribution of spa types isolated from more than two individuals in the longitudinal study in (a) Eslöv and (b) Jönköping.
BURP cluster analysis of all spa types (n = 98) found in the longitudinal study revealed 10 clusters and 14 singletons (seven spa types shorter than five repeats were excluded from the analysis). The largest cluster was spa-CC 015 including 17% of all spa types followed by spa-CC 012 (15% of all spa types), spa-CC 084 (9% of all spa types) and spa-CC 005 (4% of all spa types). Of the 35 individuals that carried different spa types at any time during the study period, 21 carried the different strains at the same occasion and 18 changed spa type over time. Three individuals carried spa types closely related and in two of these individuals both spa types were present on the same sampling occasion.
Carriage of S. aureus over time
Carriage over time was evaluated in individuals sampled on three occasions (every 6 months) for 1 year. S. aureus was isolated from nares in 20% of the individuals, and 17% carried strains of the same spa types over the sampling period (Table 2). Similarly, 7% of the individuals exhibited throat carriage and all carried the same spa type. The proportion of individuals from whom S. aureus was never isolated was 49% for nares and 46% for throat samples. There was no significant difference in gender (P = 0·40), mean age (P = 0·40), or length of stay (P = 0·69) between those that always carried S. aureus and others. Carriage rates based on two sampling occasions within 6 months from nares (n = 216) showed S. aureus was present in 22% of the individuals, and 19% carried strains of the same spa type. S. aureus was not detected in 55% of the individuals sampled twice from the nares.
Table 2. Repeated detection of S. aureus in individuals sampled on three occasions during one year
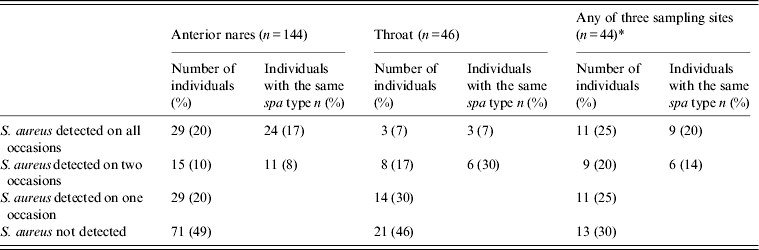
* Individuals sampled from nares, throat and groin on three occasions.
Antibiotic susceptibility testing
From the 290 individuals in the longitudinal study 338 isolates were available for antibiotic susceptibility testing. Of these 44 isolates from 26 (14 male, 12 female) individuals all but one were resistant to a single antibiotic and the other to two antibiotics (ciprofloxacin and fusidic acid). Overall, 18% of the colonized individuals carried an antibiotic-resistant isolate. None of the isolates was resistant to vancomycin, gentamicin, erythromycin, linezolid or rifampicin and no MRSA was detected. No specific spa type was associated with resistance. The proportion of individuals with resistant isolates was higher in the individuals colonized with two different strains compared to carriers with a single clone (P = 0·04). Five individuals who carried two different strains were simultaneously colonized with resistant and non-resistant strains.
DISCUSSION
In this study we investigated the molecular epidemiology of S. aureus in a population of the elderly living in nine nursing homes in the south of Sweden. We found a diverse distribution of spa types as well as a local spread of one spa type. As much as 23% of individuals were colonized simultaneously with isolates of two different spa types and in this group antimicrobial resistance was twice as common as for those colonized with a single strain, and five individuals simultaneously carried resistant and susceptible strains. Furthermore, we show that inclusion of throat sampling with nasal swabs as well as repeated sampling increases the sensitivity of S. aureus detection in screening programmes.
The high diversity and the most frequently identified spa types in our study are in agreement with previous data from general populations as well as from hospital settings [Reference den Heijer1, Reference Sangvik2, Reference Ghasemzadeh-Moghaddam5–Reference Grundmann7, Reference Mernelius29]. The four most common spa types were also the most prevalent in the Swedish HITS study, which was conducted in the regions of Jönköping and Linköping, but the fifth most common spa type was restricted to the region of Eslöv. This indicates a comparable distribution of spa types between regions and populations and suggests that some strains are more successful colonizers than others, as exemplified by the finding of spa type t160 in a single nursing home. There are conflicting results in the literature on spa-type distribution regarding age and gender [Reference Sangvik2, Reference Nilsson and Ripa13] but we found no differences in these parameters and body site sampled in our study population.
BURP clustering analysis revealed that only 3/35 individuals carrying different spa types at any time were carriers of closely related strains and that almost half of all spa types fell into the four largest clusters. The high rate (23%) of individuals harbouring simultaneously two strain types in two or more body sites on a single sampling occasion is noteworthy as only one isolate from each site was subjected to spa typing. A previous study calculated the rate of individuals carrying two different strains of S. aureus to be below 7%, based on typing of three colonies from the anterior nares [Reference Cespedes30]. More recently a study in children reported a similar rate (26%) to that found here when 4–15 colonies were typed [Reference Mongkolrattanothai31]. The issue of simultaneous carriage of more than one strain is of clinical importance as a more resistant S. aureus strain may remain undetected impairing antibiotic treatment, a situation underlined by the recovery of resistant and susceptible phenotypes of different spa types from the same individual, albeit from different body sites. Further studies are required to elucidate the clinical significance of these findings. Furthermore, in epidemiological and transmission studies undetected carriage of multiple strains could lead to misinterpretation of data. However, analyses of dual colonization with different strains based on selecting several colonies from agar plates would be laborious and simplified methods are needed [Reference Matussek32].
The highest prevalence of S. aureus carriage was found in the throat (34%), which is in accordance with a previous Swedish study [Reference Nilsson and Ripa13]; the nasal colonization rate of 31% is also comparably high, but in agreement with previous results from a Swedish population [Reference den Heijer1]. Screening protocols for S. aureus carriage based on nares sampling only may underestimate colonization rates. We were able to increase the recovery rate from 31% to 48% by combining the culture results obtained from the nares and the throat on one sampling occasion which has also been shown by other studies [Reference Smith20–Reference Andrews22]. In addition, repeated sampling occasions further increased the prevalence figures. Thus the use of a sensitive strategy of multiple body-site sampling on different occasions maximized the estimates of the prevalence of S. aureus colonization in this elderly population.
The issue of persistent carriage of S. aureus is important, since persistent nasal carriers appear to have an increased risk for endogenous infection [Reference Wertheim15–Reference van Belkum17]. There is no general consensus on the definition of persistent carriage [Reference Wertheim14, Reference Verhoeven18], and reported frequencies from nasal samples vary between 12% and 30% [Reference Kluytmans, van Belkum and Verbrugh12, Reference Nilsson and Ripa13, Reference van Belkum17, Reference Eriksen33–Reference Nouwen35]. We found 20% of carriers were repeatedly colonized in the nares on three sampling occasions over 1 year and this rose to 22% if the result was based on two sampling occasions. The importance of persistent throat carriage, as a risk for endogenous infections and in transmission to others has not been fully evaluated. Recently, a persistent throat carrier rate of 46% was reported using enrichment broth prior to plating [Reference Nilsson and Ripa13]. These are higher figures than the rates of 24% and 7% repeatedly colonized in the throat on two and three occasions, respectively, found in our study. However, we only succeeded in obtaining three throat samples from 46 individuals due to sampling difficulties.
When persistent carriage is to be defined, the type identity of the strain of S. aureus should be considered [Reference Nilsson and Ripa13]. It is not known if the risk inherent in being a persistent carrier is dependent on continuously carrying the same strain. We found that 17% of the elderly were colonized in the nares with S. aureus of the same spa type on all three occasions compared to 20% for all S. aureus strain types and the corresponding figures for two sampling occasions were 19% and 22%, respectively.
S. aureus was never detected in almost half of nasal (49%) and throat (47%) swabs of participants and they could thus be regarded as non-carriers, which is in agreement with other findings [Reference Kluytmans, van Belkum and Verbrugh12, Reference Wertheim14, Reference van Belkum17, Reference Eriksen33–Reference Nouwen35]. The basis for the resistance of individuals to colonization is not known, but some spa types seem to be more successful colonizers of humans [Reference Melles36], and little is known about bacterial factors of importance for specific strains to become virulent [Reference Stark37].
In conclusion, we found a high prevalence of S. aureus in this elderly population and a local accumulation of one strain was noted in one nursing home. Antibiotic resistance was rare and no MRSA was found, but in individuals colonized with two different strains resistance was higher and simultaneous detection of resistant and non-resistant strains was registered.
ACKNOWLEDGEMENTS
Financial support was provided by Futurum – the academy of Healthcare, County Council of Jönköping, Sweden, the Medical Research Council of South-East Sweden (FORSS) and the County Council of Östergötland, Sweden.
We acknowledge the clinical microbiology laboratory in Halmstad for providing S. aureus isolates from part of the study.
DECLARATION OF INTEREST
None.






