Dietary protein supplies the body with nitrogen and amino acids, including the nine amino acids classified as dietary indispensable in humans (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine). Dietary proteins are found in animal products, plant products and single-cell organisms. Proteins are present in variable proportions in the different food sources and the different proteins also differ in their amino acid composition and dietary indispensable amino acid content. Amino acids are used through proteogenic pathways for the specific synthesis of body proteins and through non proteogenic pathways as precursors of other non-protein nitrogenous substances like peptide hormones, neurotransmitters, nucleic acids, glutathione, nitrogen monoxide or creatine. In addition, amino acids can be subjected to deamination and their carbon skeleton used in different energy metabolic pathways either directly as energy substrate or indirectly through gluconeogenesis. Protein nutritional quality is related to the capacity of these different protein food sources to achieve the different functions associated with the supply of nitrogen and amino acids in the body. In practice, different markers and approaches can be used to assess the nutritional quality of these different dietary proteins. A first group of approaches mainly related to nitrogen retention and nitrogen balance includes indispensable amino acid score, net protein utilisation and nitrogen efficiency for infant growth. Another group of approaches related to functional and metabolic responses to protein ingestion includes specific metabolism of target tissues (particularly muscle and bone) and/or specific responses of hormones (particularly insulin and related hormones). These markers take into account the different and specific metabolic function of nitrogen and amino acids related to both the proteogenic and the non proteogenic pathways whereas they do not consider the less specific role of amino acids in energy metabolic pathways.
Protein sources and dietary indispensable amino acid content: the protein-digestibility-corrected amino acid score (PDCAAS)
The nutritional value of dietary proteins can be related to their ability to meet nitrogen and dietary indispensable amino acid requirements in order to balance daily nitrogen losses for tissue maintenance and for the synthesis of non protein nitrogenous substances and to allow additional protein deposition in newly formed tissues for growth (in infants) and in specific physiological conditions such as pregnancy or lactation. Accordingly, the content and utilisation of dietary indispensable amino acids is considered as a valuable criterion for the evaluation of dietary protein as related to the capacity of a protein source provided at the mean level of protein requirement to satisfy the dietary indispensable amino acid requirement.
The protein requirement of adults has been defined as the lowest protein intake that will allow nitrogen equilibrium (zero nitrogen balance)(1). For an adult the mean protein requirement and the safe protein requirement is 0·66 g /kg/d and 0·83 g protein /kg/d, respectively and average requirements have also been defined for each of the 9 dietary indispensable amino acids(1, Reference Bos, Gaudichon and Tomé2). In addition, specific values for different selected age groups have been determined for protein and amino acid requirements using a factorial approach. The reference patterns of amino acids (mg/g protein) are calculated for the selected age groups by dividing the requirement for each amino acid (mg amino acid/kg bw/d) by the average requirement for protein (g/kg bw/d) (Table 1) (1). These indispensable amino acid reference patterns are used in the assessment of protein quality according to the chemical score and PDCAAS approach. Accordingly, in the amino acid score the dietary indispensable amino acid composition of the dietary protein is compared to the reference pattern which is assumed to meet requirements for dietary indispensable amino acids when the protein is provided at the mean level of protein requirement for the selected age groups. The ratio between the content in a protein and the content in the reference pattern is determined for each indispensable amino acid and the lowest value is used as the score. The PDCAAS corrects the amino acid score by the digestibility of the protein. A PDCAAS < 1 indicates that at least one amino acid is limiting whereas a score ≥ 1 indicates that there is no limiting amino acid in the food or diet. Scores above 100 % should by definition be truncated to 100 %, because any amino acid in excess of what is required is catabolized.
Table 1 Scoring pattern (dietary indispensable amino acid reference profiles) for infants, children, adolescents and adults(1)
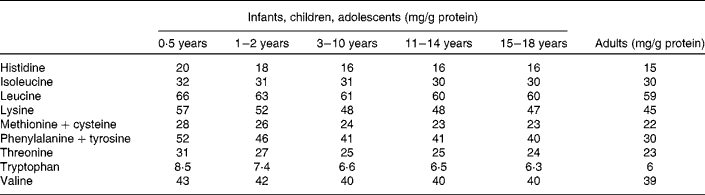
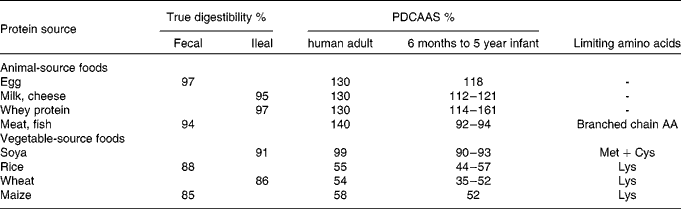
As reported in Table 2, dietary protein digestibility is relatively high in humans from 97 % to 86 % for egg and wheat, respectively. For adults, animal proteins like egg, milk product or meat have no limiting dietary indispensable amino acid whereas sulphur-containing amino acids (methionine and cysteine) are close to limiting for legume proteins such as soya protein, and lysine is the limiting amino acid for cereal proteins such as rice and wheat. In young infants, due to higher amino acid requirements than for adults, the PDCAAS values are usually lower than for adults with limiting amino acids being according to the protein sources: lysine, sulphur-containing amino acids (methionine and cysteine), threonine and tryptophan(Reference Michaelsen, Hoppe and Roos3). For young infants, cooked bean products not combined with cooked rice are for instance co-limiting in methionine plus cysteine and in tryptophan and bean products combined with rice are co-limiting in isoleucine and tryptophan and marginally limiting in lysine and valine whereas the methionine plus cysteine pattern is increased in diets containing rice(Reference Kannan, Nielsen and Mason4). Overall, with PDCAAS truncated to 100 % the value of proteins is in the order: animal protein (meat, milk, egg) ≥ legume (soya) protein >cereal protein. However, truncation can limit the information on the potency of a specific protein source to balance inferior proteins in mixed diets, and this may be particularly discussed in relation to the comparison of the value for animal protein (meat, milk, egg) and legume (soya) proteins. In this case the PDCAAS are equivalent when the score is truncated to 100 % but not when used as absolute values.
Table 2 True digestibility and PDCAAS values of dietary proteins in the human adult and 6–month-old to 5-year-old infants(1, Reference Michaelsen, Hoppe and Roos3, 42)
Protein source and efficiency of protein and nitrogen utilization
The nutritional efficiency of a protein can be determined from the extent to which dietary protein nitrogen is absorbed and retained by the organism and is able to balance daily nitrogen losses and to allow additional protein deposition in newly formed tissue for growth and in a specific physiological state such as pregnancy or lactation. The Net Protein Utilization (NPU) is the percentage of ingested nitrogen that is retained in the body and is determined by measuring digestive, metabolic (urinary) and miscellaneous nitrogen losses. NPU values are true or apparent depending on whether the loss of endogenous nitrogen is taken into account or not and this is critical to precisely determine the efficiency of dietary protein utilization and the quality of the different dietary protein sources. Endogenous intestinal (faecal) and metabolic (urinary) nitrogen losses can be obtained after feeding a protein-free diet or derived from the y-intercept of the regression line relating N intake to retention at different levels of protein intake, or can be directly determined from experiments using labeled amino acid or dietary proteins(Reference Fouillet, Bos and Gaudichon5, Reference Fuller and Tomé6).
As the post-prandial phase is critical for dietary protein utilization, the immediate retention of dietary nitrogen following meal ingestion represents a reliable approach for the assessment of protein nutritional efficiency. In the protein postprandial utilization approach the true dietary protein nitrogen retention has been directly measured in the post-prandial phase from experiments using 15N-labelled dietary proteins or 13C-labelled leucine infusion(Reference Fouillet, Bos and Gaudichon5, Reference Millward, Fereday and Gibson7). An average value of 70 % can be considered for NPU of dietary proteins(Reference Bos, Juillet and Fouillet8) when determined in such optimized controlled conditions in healthy adults but it can be modified by different factors including the diet and the physiological conditions. Comparison of the immediate post-prandial nitrogen utilization of different protein sources in adults adapted to an adequate level of protein intake shows values for post-prandial protein utilization ranging from 75–93 % to values of 63–66 % for high quality proteins such as milk protein and proteins with lower quality such as wheat, respectively (Table 3). Interestingly, it appears that these values are better related to PDCAAS when not truncated to 100 %, more particularly when comparing milk protein and legume protein. In addition, both the quality of protein and the previous adaptation of protein intake affect the distribution and metabolic use of dietary amino acids in splanchnic (intestine, liver, etc) and peripheral (muscle, skin, etc) tissues. Both the amino acid profile of the protein and the kinetics of amino acid delivery to the blood can modulate the postprandial splanchnic and peripheral uptake of amino acids in humans(Reference Fouillet, Mariotti and Gaudichon9, Reference Deglaire, Fromentin and Fouillet10). Increasing protein intake increases splanchnic catabolic use, and splanchnic catabolic use and peripheral anabolic use are inversely affected(Reference Fouillet, Juillet and Gaudichon11) (Fig. 1). Interestingly, this approach shows a nutritional value of proteins in the order: animal protein (milk) >legume (soya, pea) protein >cereal protein (wheat).
Table 3 Protein quality and Postprandial Protein (nitrogen) Utilization (NPPU(Reference Fouillet, Bos and Gaudichon5, Reference Bos, Juillet and Fouillet8), PPU)(Reference Millward, Fereday and Gibson7) in the healthy adult


Fig. 1 Post-prandial 8 h metabolic use of dietary nitrogen in adults after adaptation to and its modulation by both the protein source in the meal and the habitual protein intake, normal protein (NP 1 g/kg/d) and high protein (HP 2 g/kg/d) diets (Reference Fouillet, Juillet and Gaudichon11). Subjects in the milk, soya, and wheat groups twice ingested a mixed milk, soya, or wheat protein meal providing 4·85 mmol N/kg body weight, respectively, the first time after a normal protein diet adaptation (NP-milk, NP-soya, and NP-wheat groups, respectively) and the second time after high-protein diet adaptation (HP-milk, HP-soya, and HP-wheat groups, respectively). Open bars denote postprandial utilization of meal nitrogen for splanchnic catabolic, splanchnic anabolic, and peripheral anabolic purposes at 8 h. Hatched bars denote meal nitrogen enterohepatic recycling into the metabolic nitrogen pool through urea hydrolysis at 8 h.
An average value of 70 % was initially used for the protein efficiency of diets for both maintenance and additional protein deposition in newly formed tissue for growth and in specific physiological states such as pregnancy or lactation(12). However, from nitrogen balance studies, an average NPU value of 47 % was derived for healthy adults at maintenance and no differences were found between the results when the data were grouped by sex, diet, or climate(1, Reference Rand, Pellett and Young13). There are also different values used for efficiency of protein utilization for maintenance and for tissue deposition/growth in different populations and age groups including infants, pregnant or lactating women. From nitrogen balance studies, the protein quality of isolated soya protein appears to be comparable to that of animal protein sources such as milk and beef in adult humans(Reference Scrimshaw, Wayler and Murray14–Reference Young, Wayler and Garza16). In contrast, in preschool children soyabean protein isolate has a nutritive value of 82 % compared with skim milk as deduced from the amount of nitrogen needed to retain 24 mg nitrogen/kg/day based on regression of apparent nitrogen balance that was 0·61 g and 0·75 g for skim milk and soyabean protein isolate, respectively(Reference Torún, Cabrera-Santiago and Viteri17). In agreement with the indispensable amino acid scoring approach, the protein quality can be improved by mixing protein sources that can compensate for their individual deficiencies. Accordingly, in preschool children a corn–bean mixture (76 % corn, 24 % beans) or addition of cereal to the diet can satisfy all nitrogen balance requirements(Reference Viteri, Torun and Arroyave18, Reference Shulman, Gannon and Reeds19). From these results the nutritional quality can be classified as: animal protein (milk, meat) ≥ legume (soya) protein >cereal protein (wheat, corn).
Adequate dietary protein intake has been associated with adequate growth in infants. Milk protein is the main protein source for infant formulas and is usually used as a reference. Alternative protein sources include soya, other legumes and cereal proteins and different nutritional or pathological conditions are considered including normal healthy conditions, under-nutrition and allergy (mainly cow milk allergy) (Table 4). Soya protein formulas ensure normal growth and development in healthy term infants but have no nutritional advantages over cow's milk protein formulas and are considered if tolerance to soya protein is established with main indications including severe lactose intolerance, galactosaemia and the need to avoid foods of animal origin(Reference Turck20–Reference Lasekan, Ostrom and Jacobs22). Lentil-based high protein diets also appeared comparable to animal-based diets in respect to nitrogen absorption and nitrogen balance in malnourished children(Reference Hossain, Islam and Wahed24). Rice is among the first solid foods fed to infants in many cultures. Rice, as other cereals, is limiting in total protein as compared with dairy and other grain/legume protein sources, and is rich in methionine and cystine and is deficient in lysine and threonine. Fortification of rice protein with these two limiting amino acids improves its protein quality. Hypo-allergenicity efficacy, particularly for hydrolyzed rice protein-based formulas, has been reported, but limited data indicate that rice protein based infant formula may provide a potentially adequate alternative if standard milk- or soya protein-based formulas are not tolerated(Reference Koo and Lasekan29). In addition, Quality protein maize that consists of maize varieties bio-fortified with lysine and tryptophan could be used in preschool diets and could reduce or prevent growth faltering and may in some cases support catch-up growth in body weight(Reference Akalu, Taffesse and Gunaratna30). From these results the nutritional quality can be classified as: animal protein (milk, meat) ≥ legume (soya) protein >cereal protein (wheat, corn, rice).
Table 4 Protein source and determined efficiency of utilization for growth in preschool infants

Protein intake and response of target tissues (muscle, bone) and hormones (insulin, IGF1)
An increasing number of studies address the question of the role of protein in promoting optimal health at intakes beyond the recommended requirement, e.g. in relation to weight management and satiety, sarcopenia, bone health or insulin and glucose homeostasis. More particularly in older subjects it is questioned as to whether an increase in amino acid and protein availability could improve bone and muscle mass.
Increasing the intake of protein and the branched-chain amino acid leucine above the recommended requirement has been suspected to improve muscle mass. The major anabolic influences on muscle are feeding and contractile activity, and ingestion of sufficient dietary protein is required for muscle protein synthesis and maintenance. Amino acids are key components in promoting postprandial protein anabolism and skeletal muscle mass and contractility and the availability of amino acids is crucial for net anabolism in muscle. Dietary proteins, when ingested, result in systemic hyper amino acidaemia that supports muscle protein synthesis. In vivo, amino acids display an anabolic effect and have been shown to stimulate muscle protein synthesis(Reference Bohe, Low and Wolfe31, Reference Nair and Short32). In addition, the branched chain amino acids (BCAA) (leucine, valine, isoleucine) and particularly leucine have been demonstrated to act as a signal for muscle protein synthesis both in vitro and in vivo in animal models(Reference Balage and Dardevet33). Interestingly, compared to casein and cod protein, peanut protein intake alters body composition by reducing skeletal muscle mass and liver weight as well as muscle contractility and lipid metabolism in the rat, an effect that could originate from the relatively low leucine content of this protein(Reference Jacques, Leblanc and Papineau34).
However, there is no result showing that a dietary protein level above the recommended requirement of 0·8–1 g/kg/d can further modulate skeletal protein mass in non-exercising young healthy human subjects. There was no effect of a dietary protein intake above the average requirement on muscle mass and protein content and a high protein diet has not been demonstrated to modulate skeletal protein mass in non-exercising human subjects or animals(Reference Bolster, Pikosky and Gaine35, Reference Chevalier, Bos and Gryson36). Moreover, in adult humans there is no clear evidence that chronic leucine supplementation above the requirement as previously defined (i.e. 39 mg/kg bw/d in adult) is efficient in promoting muscle mass in non exercising subjects(Reference Koopman, Wagenmakers and Manders37). In older adults several studies concluded that the protein requirement may be greater than that for younger adults particularly in association with inactivity(Reference Gaffney-Stomberg and Insogna39–Reference Thalacker-Mercer, Fleet and Craig41). This was particularly deduced from an assumed lower efficiency of protein utilisation in the elderly(42). However, other studies did not show differences between young and elderly subjects after assessment of nitrogen balance(Reference Campbell, Johnson and McCabe43). In contrast, with the added stimulus of resistance exercise, muscle protein synthesis is stimulated and this anabolic effect is enhanced by increasing the availability of amino acids and protein which appear to be better than energy alone (as carbohydrate)(Reference Tang, Manolakos and Kujbida44). In some studies whey protein appears to be more efficient than casein and soya protein(Reference Phillips, Tang and Moore45, Reference Tang, Moore and Kujbida46) but the difference between whey protein and casein was not confirmed(Reference Reitelseder, Agergaard and Doessing47). Some authors also found that in older adults an increase in dietary protein alone does not change body composition or improve lean body mass unless accompanied by physical training programs(Reference Campbell and Leidy48, Reference Iglay, Apolzan and Gerrard49).
Protein and calcium are the main components of bone structure and it is widely accepted that protein deficiency increases the risk for bone fragility and fracture. In most epidemiological studies, bone mineral density is positively related to protein intake and this is also observed in infants, in premenopausal women and elderly people(Reference Geinoz, Rapin and Rizzoli50–Reference Alexy, Remer and Manz56). However, a positive relationship between protein intake above the safe level of protein intake based on nitrogen balance and the risk of bone fracture is not always significant and remains controversial(Reference Hegsted57–Reference Frassetto, Todd and Morris61). Whether increasing protein intake above the recommended intake further improves bone mineral density remains to be confirmed as well as the characteristics of protein quality in relation to bone health. In addition, although protein is essential for bone health, an increase in protein intake is often associated with an increase in urinary calcium excretion and it was first hypothesized that this could originate from an activation of bone resorption in order to provide calcium for the neutralization of the acid load produced by the oxidation of sulphur amino acids(Reference Barzel and Massey62). However, the association between protein intake and a decrease in bone mineral density was not confirmed and recent studies do not confirm that increasing protein intake above the recommended intake promotes skeletal bone mineral loss(Reference Fenton, Lyon and Eliasziw63–Reference Cao, Johnson and Hunt67).
Protein intake and protein quality influence insulin secretion and sensitivity in adults. High protein diets are accompanied by increased stimulation of glucagon and insulin within the endocrine pancreas; high glycogen turnover and stimulation of gluconeogenesis and correlation between daily protein intake and acute insulin response to glucose (as an estimate of insulin secretion capacities) indicates that protein intake potentiates insulin secretion(Reference Linn, Santosa and Grönemeyer68). The acute effect of protein meals on insulin and glucose in lean men could be different according to the protein source with an effect in the order: whey protein >fish protein >turkey protein >egg(Reference Pal and Ellis69). Contradictory results have been obtained for effects of an increase in protein intake on insulin sensitivity. Some studies suggested that exposure to high levels of branched chain amino acids could have a deleterious effect on insulin signalling leading to impaired insulin sensitivity and glucose tolerance(Reference Nair and Short32, Reference Patti, Brambilla and Luzi70–Reference Wang, Larson and Vasan72). However, prolonged leucine supplementation (7·5 g/d) in elderly type 2 diabetics habitually consuming an adequate amount of dietary protein did not modulate their glycaemic control(Reference Leenders, Verdijk and van der Hoeven73). In addition, an improvement of insulin sensitivity and glucose tolerance was reported with normal or reduced calorie high-protein diets regardless of weight loss(Reference Farnsworth, Luscombe and Noakes74–Reference Lacroix, Gaudichon and Martin77). In overweight or obese, post-menopausal women a casein and a whey daily supplement both improved insulin secretion, the homeostasis model assessment of insulin resistance (HOMA-IR) scores and triglycerides, and whey was more efficient than casein(Reference Pal and Ellis69, Reference Pal and Ellis78–Reference Pal, Ellis and Ho81).
Protein intake and protein quality influence IGF1 secretion in infants. Evidence increases for programming effects of early infant feeding choices on, among other factors, later obesity risk. In infants, high dietary protein intake has been associated with increased growth and this may be linked to increased concentrations of insulin-like growth factor I (IGF-I), which seem to be influenced by the diet, especially its protein component(Reference Larnkjaer, Hoppe and Mølgaard82). Interestingly, IGF-I and height were positively associated with intakes of animal protein, but not with the intake of vegetable protein and in addition high intakes of skimmed milk, but not meat, increased serum IGF-I and IGFBP-3 in eight-year-old boys(Reference Hoppe, Tina Udam and Lauritzen83–Reference Hoppe, Mølgaard and Michaelsen85). Moreover, whey protein significantly affects fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3 whereas this effect was not observed with casein(Reference Hoppe, Mølgaard and Dalum86).
Conclusion
Dietary proteins provide the body with nitrogen and amino acids as essential metabolic components with numerous specific metabolic functions related to both proteogenic and non proteogenic pathways. Different approaches can be used to assess dietary protein quality and properties including dietary indispensable amino acid content, nitrogen retention efficiency, nitrogen efficiency for growth, and the effect of protein intake on target tissues (muscle, bone, etc) and on insulin and related hormones.
The current approach to determining protein requirements and measures of protein quality associate body nitrogen balance and dietary indispensable amino acid requirements and although still debated, numerous results strongly suggest that this approach take into account the different and specific metabolic functions of nitrogen and amino acids related to both the proteogenic and the non proteogenic pathways. In contrast, it probably does not include the role of amino acids as energy substrate since the different energy substrates can be for a large part substituted one to one between carbohydrate, fat and protein. Combination of dietary amino acid composition and estimates of dietary indispensible amino acid requirements lead to PDCAAS which is a measure of protein quality. In the PDCAAS reference method, scores above 100 % are by definition truncated to 100 %, because any amino acid in excess of what is required should be catabolized. Overall, the method appears valuable to predict the nutritional efficiency of mixed diets to reach nitrogen balance. It is a relatively valuable index to predict protein quality related to the efficiency for growth in young infants.
Another current approach of protein quality evaluation relates to the efficiency of protein nitrogen utilization (NPU) determined either in acute studies (post-prandial) or more chronically (nitrogen balance). The acute measurement of post-prandial protein retention can determine the potential efficiency of the ingested protein. Interestingly, the post-prandial NPU values are better related to PDCAAS not truncated to 100 %, more particularly when comparing milk protein and legume protein. This observation suggests that in some conditions non truncated values of PDCAAS should be considered to evaluate the potential quality of a protein source. Moreover, if an average potential value of 70 % can be considered for the NPU of dietary proteins, this can be modified by different factors including the diet and the physiological conditions and this is reflected in the lower efficiency obtained more chronically by nitrogen balance studies with a lower potency to discriminate between the different protein sources.
Overall, protein quality measures derived from nitrogen balance and whole body nitrogen retention data represent the more ubiquitous evaluation of dietary protein quality whereas other criteria related to nitrogen retention in different parts of the body, the metabolic response of target tissues such as muscle and bone or the influence on hormones can be used additionally for more specific applications and specific situations (infants, exercise, older adults, etc). Some of these approaches could be of help in the future to more precisely define protein requirement and quality adapted to prevention of chronic and metabolic disease in specific sensitive populations, and particularly in infants and older adults.
Acknowledgements
The author has no conflict of interest. “This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.”







