High levels of sick leave cause concern in many developed countries, as this is a burden both for affected individuals and workplaces, and for the economy in general (Moncrieff & Pomerleau, Reference Moncrieff and Pomerleau2000; OECD, 2010). Sick leave benefits are granted for disease or injury that results in reduced work capacity (Soderberg & Alexanderson, Reference Soderberg and Alexanderson2003). Sick leave constitutes a complex phenomenon with many potential risk factors (Dekkers-Sanchez et al., Reference Dekkers-Sanchez, Hoving, Sluiter and Frings-Dresen2008). Definitions and processes of certification vary across countries (Henderson et al., Reference Henderson, Harvey, Overland, Mykletun and Hotopf2011) and this might partly explain the lack of an international research standard for defining sick leave, and for separating short- from long-term sick leave. The focus in this study is on long-term sick leave, hereafter referred to as LTSL. Research on LTSL is important, as individuals who have had one or more episodes of LTSL have increased risk for disability pensioning (Gjesdal et al., Reference Gjesdal, Haug and Ringdal2005; Hultin et al., Reference Hultin, Lindholm and Moller2012).
LTSL due to mental disorders has increased in western countries in the last two decades (Hensing et al., Reference Hensing, Andersson and Brage2006; Vaez et al., Reference Vaez, Rylander, Nygren, Asberg and Alexanderson2007). Anxiety and depression are important risk factors for LTSL (Knudsen et al., Reference Knudsen, Harvey, Mykletun and Overland2013). Mental disorders most often emerge in adolescence and early adulthood (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005), and may therefore be particularly detrimental to education and subsequent employment (Suvisaari et al., Reference Suvisaari, Aalto-Setala, Tuulio-Henriksson, Harkanen, Saarni, Perala and Lonnqvist2009). Despite the increased focus on mental disorders and sick leave, few studies have investigated effects of less common mental disorders, such as personality disorders (PDs) in young adults.
The DSM-IV Axis II system includes 10 PDs, ordered into three clusters (APA, 1994). PDs are characterized by persistent, maladaptive patterns of inner experience and behavior that leads to distress and impairment (APA, 1994). The worldwide prevalence of PDs has recently been estimated at 6.1% (Huang et al., Reference Huang, Kotov, de Girolamo, Preti, Angermeyer, Benjet and Kessler2009). There is extensive comorbidity among the different PDs (Coid et al., Reference Coid, Yang, Tyrer, Roberts and Ullrich2006; Marinangeli et al., Reference Marinangeli, Butti, Scinto, Cicco, Petruzzi, Daneluzzo and Rossi2000), indicating that multiple PD diagnoses are most common (Marinangeli et al., Reference Marinangeli, Butti, Scinto, Cicco, Petruzzi, Daneluzzo and Rossi2000). Although treated as categorical diagnoses in DSM-IV, strong empirical support exists to conceptualize PDs dimensionally (Livesley & Jang, Reference Livesley and Jang2000; Trull & Durrett, Reference Trull and Durrett2005; Widiger & Mullins-Sweatt, Reference Widiger and Mullins-Sweatt2009). Many have also argued that there are strong links between PDs and the general personality structure, where PDs may represent extremities of normal personality (McCrae et al., Reference McCrae, Lockenhoff and Costa2005; Widiger & Trull, Reference Widiger and Trull2007).
Much is known about the underlying genetic and environmental structure of PDs (Kendler et al., Reference Kendler, Aggen, Czajkowski, Roysamb, Tambs, Torgersen and Reichborn-Kjennerud2008, Reference Kendler, Aggen, Knudsen, Roysamb, Neale and Reichborn-Kjennerud2011; Roysamb et al., Reference Roysamb, Kendler, Tambs, Orstavik, Neale, Aggen and Reichborn-Kjennerud2011), but less about the consequences of PDs, particularly for work participation. Both PDs and extreme scores on normal personality traits decrease global functioning (McCrae et al., Reference McCrae, Lockenhoff and Costa2005; Skodol et al., Reference Skodol, Johnson, Cohen, Sneed and Crawford2007), and Global Assessment of Functioning (GAF) scores have been found to decrease with increasing number of PD criteria met (Nakao et al., Reference Nakao, Gunderson, Phillips, Tanaka, Yorifuji, Takaishi and Nishimura1992). Extreme scores on personality traits are also associated with impaired work functioning (Michon et al., Reference Michon, ten Have, Kroon, van Weeghel, de Graav and Schene2008) and short- and long-term sick leaves (Stormer & Fahr, Reference Stormer and Fahr2013; Vlasveld et al., Reference Vlasveld, van der Feltz-Cornelis, Anema, van Mechelen, Beekman, van Marwijk and Penninx2012). We are not aware of studies that have investigated the association between PDs and sick leave per se, but PDs have been found to be associated with problems maintaining job positions (Noren et al., Reference Noren, Lindgren, Haellstom, Thormaehlen, Vinnars, Wennberg and Barber2007). Further, borderline, dependent, schizoid, and schizotypal PDs are found to be significantly associated with disability pensioning (Knudsen et al., Reference Knudsen, Skogen, Harvey, Stewart, Hotopf and Moran2012; Østby et al., submitted).
Both PDs and LTSL are influenced by genetic as well as environmental factors. The heritability for dimensional representations of DSM-IV PDs varies between 0.21 and 0.38 (Kendler et al., Reference Kendler, Czajkowski, Tambs, Torgersen, Aggen, Neale and Reichborn-Kjennerud2006; Reichborn-Kjennerud et al., Reference Reichborn-Kjennerud, Czajkowski, Neale, Orstavik, Torgersen, Tambs and Kendler2007; Torgersen et al., Reference Torgersen, Czajkowski, Jacobson, Reichborn-Kjennerud, Roysamb, Neale and Kendler2008), and between 0.36 and 0.49 for LTSL defined as sick leave extending beyond 15–16 days (Gjerde et al., Reference Gjerde, Knudsen, Czajkowski, Gillespie, Aggen, Røysamb and Ørstavik2013; Svedberg et al., Reference Svedberg, Ropponen, Alexanderson, Lichtenstein and Narusyte2012). As no specific genes are expected for LTSL per se, it is important to investigate to what extent genetic contributions to mental disorders, such as PDs, can account for the heritability.
If an association exists between specific PDs and LTSL, it is necessary to establish the nature of this association. A natural next step after investigating heritability is to investigate whether these phenotypes share genetic and/or environmental contributions. This has, to our knowledge, not yet been investigated. By clarifying this, the question of causal pathways can also be illuminated. Although multivariate Cholesky models are not in themselves designed to establish causal pathways, some inferences can be made. For phenotypes that are influenced by both genetic and environmental factors, as is the case for both PDs and LTSL (Gjerde et al., Reference Gjerde, Knudsen, Czajkowski, Gillespie, Aggen, Røysamb and Ørstavik2013; Kendler et al., Reference Kendler, Czajkowski, Tambs, Torgersen, Aggen, Neale and Reichborn-Kjennerud2006; Reichborn-Kjennerud et al., Reference Reichborn-Kjennerud, Czajkowski, Neale, Orstavik, Torgersen, Tambs and Kendler2007; Svedberg et al., Reference Svedberg, Ropponen, Alexanderson, Lichtenstein and Narusyte2012; Torgersen et al., Reference Torgersen, Czajkowski, Jacobson, Reichborn-Kjennerud, Roysamb, Neale and Kendler2008), a pure genetic correlation would not indicate causality, but rather that the association is mediated through genetic factors shared between the phenotypes (De Moor et al., Reference De Moor, Boomsma, Stubbe, Willemsen and de Geus2008; Kendler et al., Reference Kendler, Neale, MacLean, Heath, Eaves and Kessler1993).
The aims of the present study were: (1) to investigate whether there is an association between DSM-IV PDs and LTSL defined as sick leave >16 days; (2) to identify which PDs are most important for the association; (3) to investigate to what extent the heritability of LTSL can be accounted for by genetic contributions to PDs; and (4) to explore whether the association between PDs and LTSL is causal or due to other factors.
Material and Methods
Sample
Data for the current analyses originate from the Norwegian Institute of Public Health Twin Panel (NIPHTP). The twins were identified through the national Medical Birth Registry, established on January 1, 1967. Our sample consisted of those who had participated in a psychiatric interview study conducted between June 1999 and May 2004 (90% within the end of 2002). Participants were recruited from 3,153 complete twin pairs who had given consent to be contacted again after a previous questionnaire study, and 68 twin pairs drawn directly from the NIPHTP. The response rate was 43.5% (2,801 out of 6,442), and 2,794 of the interview responses were valid. Non-participants consisted of 0.8% pairs not willing or able to participate, 16.8% pairs in which only one twin agreed to participate, and 38.9% pairs in which none responded after reminders. In 22 pairs where both twins initially agreed to be interviewed, the co-twin later declined. The high rate of attrition from the questionnaire studies to the interview study has been found not to affect twin analyses of mental health related variables (Tambs et al., Reference Tambs, Ronning, Prescott, Kendler, Reichborn-Kjennerud, Torgersen and Harris2009).
The interviews were mainly conducted by psychology students late in their training and psychiatric nurses who received a standardized training program by teachers certified by the World Health Organization (WHO). Members of a pair were assessed by different interviewers blind to the information obtained from the co-twin. The majority of interviews were conducted face to face, and 231 were interviewed over the phone.
By using the unique national identification numbers issued to all Norwegians at birth, data were in 2011 linked to the historical-event database (FD-Trygd). This database contains information regarding all social security benefits including, for example, sickness benefits, social assistance, rehabilitation allowance, disability pension, and unemployment benefits (Akselsen et al., Reference Akselsen, Lien and Siverstøl2007). As the register data at Statistics Norway are updated annually, we have obtained a detailed, longitudinal dataset on the twins, including annual information on the variables listed above from 1998 to 2008. The number of individuals with valid interview data after linkage was 2,771 (mean age in 1998: 25.6 years), as 23 declined to participate. For 4 of the 2,771 we lack information on zygosity. The sample for the twin modeling analyses was therefore 2,767, including 1,365 complete pairs, comprising 219 monozygotic (MZ) male pairs, 117 dizygotic (DZ) male pairs, 436 MZ female pairs, 257 DZ female pairs, 336 DZ opposite sex pairs, and 37 single responders.
Zygosity was initially determined using questionnaire items previously shown to classify correctly more than 97% of the twin pairs (Magnus et al., Reference Magnus, Berg and Nance1983), followed by DNA analyses on a subgroup of the sample. The discrepancy between classification based on the questionnaire and DNA markers implied an expected misclassification rate of <1% for the whole sample, which is unlikely to bias our results (Neale, Reference Neale2003).
For the interview study, approval was received from the Regional Ethical Committee and the Norwegian Data Inspectorate, and written informed consent was obtained from the participants after complete description of the study. The linkage of data from NIPHTP with registries at Statistics Norway was approved by the Regional Ethical Committee.
Measures
In Norway, the first 16 days of sick leave is paid for by the employers, and thereafter mandatorily covered by the Norwegian Insurance Scheme (NIS) for up to 52 weeks (Gjesdal & Bratberg, Reference Gjesdal and Bratberg2003). We defined LTSL as sickness absence of >16 days, the minimum sick leave period recorded in our dataset. After 52 weeks of sick leave, an individual who is still unable to work can be granted medical and/or vocational rehabilitation benefits in order to undergo treatment or training aimed at regaining work ability (NOU, 2000). We included periods of receiving rehabilitation benefits in the LTSL variable, as this reflects a continuation of LTSL. The total number of sick days, rehabilitation, and working days in the 11-year follow-up period were summed either up to the time of granted disability pension (N = 76), death (N = 12), or by the end of 2008. The LTSL variable was then constructed as a ratio (0–100%) between the cumulative number of sick days and rehabilitation days over potential working days (Gjerde et al., Reference Gjerde, Knudsen, Czajkowski, Gillespie, Aggen, Røysamb and Ørstavik2013). The LTSL proportion variable was positively skewed (skewness: 3.0, kurtosis: 9.0), and was thus further divided into four categories: 0 = no registered sick leave in the period (N = 2,646), 1 = up to 5% LTSL in the period (N = 2,249), 2 = 5–15% LTSL in the period (N = 1,411), and 3 = >15% LTSL in the period (N = 1,251). This variable had acceptable skewness and kurtosis values (0.4 and -1.1, respectively), and correlated 0.86 with the sum of the number of sick days in the observation period. A multiple threshold test confirmed that the LTSL categories reflect differences of severity on a normally distributed liability continuum. Only subjects eligible for sickness allowance during the period were included in the analyses; 153 twins were censored out, either due to no work in the period (N = 143) or for being granted disability pension before 2000 (N = 10).
PDs were assessed by a Norwegian version of the SIDP-IV (Pfohl & Zimmerman, Reference Pfohl, Blum and Zimmerman1995), a comprehensive semi-structured diagnostic interview for the assessment of all DSM-IV Axis II diagnoses. The instrument includes non-pejorative questions organized into topical sections to produce a natural flow in the interview. The questions address behaviors, cognitions, and feelings that have been predominant for most of the past 5 years, and thus are considered to be representative for the individual's long-term personality functioning. This 5-year assumption is supported by empirical evidence of high stability of normal personality traits during adulthood (McCrae & Costa, Reference McCrae and Costa1990). Each DSM-IV criterion is scored as 0 = absent, 1 = subthreshold, 2 = present or 3 = strongly present.
For the bivariate logistic regression analysis, we constructed a dichotomous variable defined as having at least one full categorical DSM-IV PD diagnosis (a score of ≥2 on at least three to five SIDP-IV criteria; APA, 1994).
In the rest of the analyses of the SIDP-IV data, we used a dimensional approach by constructing the PDs as ordinal variables. The number of criteria scored ≥1 was summed, assuming that the liability for each trait is continuous and normally distributed. Due to low prevalence of full PDs, the PD variables were truncated by collapsing the upper criteria counts into three to five categories to avoid empty cells in the twin analyses. This approach has been used in previous publications on the same sample (Gjerde et al., Reference Gjerde, Czajkowski, Roysamb, Orstavik, Knudsen, Østby and Reichborn-Kjennerud2012; Kendler et al., Reference Kendler, Czajkowski, Tambs, Torgersen, Aggen, Neale and Reichborn-Kjennerud2006, Reference Kendler, Myers, Torgersen, Neale and Reichborn-Kjennerud2007; Reichborn-Kjennerud et al., Reference Reichborn-Kjennerud, Czajkowski, Neale, Orstavik, Torgersen, Tambs and Kendler2007; Torgersen et al., Reference Torgersen, Czajkowski, Jacobson, Reichborn-Kjennerud, Roysamb, Neale and Kendler2008). The PD variables have been tested and approved with multiple threshold tests used to examine whether they can be regarded as differences of severity on a normally distributed continuum of liability (see, e.g., Kendler et al., Reference Kendler, Czajkowski, Tambs, Torgersen, Aggen, Neale and Reichborn-Kjennerud2006). Thus, for convenience we refer to PDs, but are in fact assessing dimensional representations of PDs.
Interrater reliability was assessed by two raters scoring 70 audio-taped interviews. Intraclass correlations for the number of endorsed PD criteria at the subthreshold level ranged from +0.81 to +0.96.
Statistical Analyses
Regression analyses
We first conducted a simple logistic regression analysis between the LTSL variable (dichotomized into above/below 15% sick leave days) and any categorical DSM-IV PD diagnosis, in order to demonstrate the crude association between PDs and LTSL.
To explore which dimensional representations of PDs were associated with LTSL, we conducted ordinal logistic regression analyses, first separately and then in a multivariate model, including the PDs that were significantly associated with LTSL. As the PDs consist of sum scores of criteria ≥1 truncated into three to five groups, the resulting odds ratios (OR) are not directly comparable between the PDs. We adjusted for sex in all the regression analyses, as prevalence rates for the PDs vary across sex. We corrected for dependency between twin pairs using generalized estimating equations (GEE; Dobson, Reference Dobson2002). The significance level was set to 0.05. All PDs have previously been found to correlate weakly to moderately (0.13–0.58; Roysamb et al., Reference Roysamb, Kendler, Tambs, Orstavik, Neale, Aggen and Reichborn-Kjennerud2011), indicating that multicollinearity was not an issue.
Twin model fitting
As our data are ordinal, we use a liability-threshold model (Falconer, Reference Falconer1965) to estimate the genetic and environmental contributions to twin resemblance on the variables. We assume that ordered categories are indicators of an unobserved, normally distributed liability that can be estimated as thresholds discriminating between the categories.
In the classical twin design (Jinks & Fulker, Reference Jinks and Fulker1970; Martin & Eaves, Reference Martin and Eaves1977), individual differences in liability are assumed to arise from additive genetic (A), shared environmental (C), and non-shared environmental (E) sources. As MZ twins share all, and DZ twins share on average half of their segregating genes, A would tend to make MZ twins correlate twice as high as DZ twins. C is defined as environmental factors contributing to similarity between twins, and is further assumed to have an equal effect on MZ and DZ twins. E is per definition not shared between twins in a pair, and hence does not contribute to twin similarity. E also contains measurement error. The influence of these factors on the variables can be estimated using structural equation modeling (SEM; Neale & Maes, Reference Neale and Maes2000). The liability-threshold models were fitted on raw data in OpenMx (Boker et al., Reference Boker, Neale, Maes, Wilde, Spiegel, Brick and Fox2011), which has the advantage of including single responders, and thus maximizing power. The difference in -2 times the log likelihood (Δ-2LL) is asymptotically χ2 distributed, allowing testing for significant deterioration in χ2 for nested submodels. If the difference in χ2 is non-significant, the simpler model is preferred over the more highly parameterized and complex model. In addition, as an index of parsimony, Akaike Information Criterion (AIC), calculated as χ2 - 2df (Akaike, Reference Akaike1987) was used to select the best fitting model. Preferred models are those with the lowest AIC value.
For the current study, multiple phenotypes were analyzed simultaneously. Multivariate analyses can be advantageous, compared to univariate analyses, as having multiple phenotypes makes it possible to use the additional information inherent in cross-twin cross-trait correlations (Martin & Eaves, Reference Martin and Eaves1977). A common multivariate method is the triangular Cholesky decomposition (Neale & Cardon, Reference Neale and Cardon1992), which is a convenient method for constraining maximum likelihood estimates of genetic and environmental covariance matrices to be positive definite. We first fitted a full ACE multivariate Cholesky model to the data, allowing for quantitative (scalar) sex differences. Quantitative sex differences involve the same genetic and environmental effects for males and females, but in different quantities for the sexes. We therefore constrained the A and C correlations to be equal for males and females based on the strategy suggested by Neale et al. (Reference Neale, Roysamb and Jacobson2006). We tested for quantitative sex differences (common sex limitation model [CSL]) by allowing the A, C, and E parameter effects to differ across male and female twins and then compared the fit of this model with a model constraining the parameters to be equal across sex (no sex limitation model [NSL]). We could not test for qualitative sex differences, which involve different genetic and/or environmental effects for males and females, as this is problematic in a multivariate Cholesky model (Neale et al., Reference Neale, Roysamb and Jacobson2006). After testing for sex differences, we ran submodels to test for significance of the A and C parameters by fixing selected parameters to be 0 in an AE-, CE-, and E model, consecutively.
Results
The prevalence of having had at least one episode of LTSL in the 11-year follow-up period was 63.9% (45.9% for males and 74.4% for females). The number of days of sick leave (from sick leave periods >16 days) and rehabilitation in the period ranged from 0 to 3,717 days (0–3,673 for males), although 95% of the sample was within a range of 0–1,300. Median days were 53 (0 for males and 117 for females). The mean number of sick leave periods >16 days was 1.9. By the end of the observation period (2008), 48.5% had achieved education at a tertiary level (undergraduate or postgraduate), which was slightly higher than the general population in the same age group (SSB, 2013), 65% had children, and 44.4% were married; 99.9% of the sample was registered as working at least one time during the observation period. After separating out those without work, work days ranged from 44 to 4,015 days (median: 2,841 days). The prevalence of any categorical PD diagnosis was 5.1%. The mean number of subthreshold PD-criteria varied between 0.4 for schizoid PD (SPD) and 1.9 for obsessive-compulsive PD (OCPD).
Regression Analyses
The OR for being in the highest LTSL group (>15% of working days) when fulfilling the criteria for at least one categorical DSM-IV PD diagnosis was 2.6 (1.8–3.8, 95% CI).
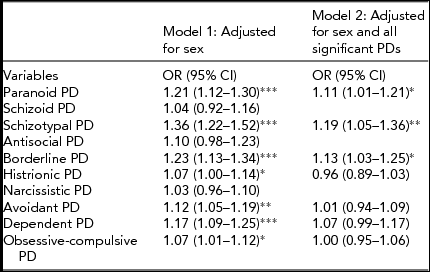
The results from the GEE ordinal logistic regression analyses are shown in Table 1.
TABLE 1 Results From Ordinal Logistic Regression Analyses: Odds Ratios for LTSL (Sick Leave >16 Days) by Dimensionally Measured Personality Disorder (PD) Traitsa

aPD traits are summed and truncated into three to five categories.
*Significant at p < .05; **Significant at p < .01; ***Significant at p < 0.001.
When testing each dimensional PD against LTSL and adjusting for sex, all PDs were positively and significantly associated with LTSL with the exception of: antisocial PD (APD), narcissistic PD (NPD), and schizoid PD (SPD). From the multiple GEE ordinal logistic regression analysis where we adjusted for sex and all of the significantly associated dimensional PDs, three PDs were uniquely and positively related to LTSL: schizotypal PD (STPD), borderline PD (BPD), and paranoid PD (PPD).
Twin Model Fitting
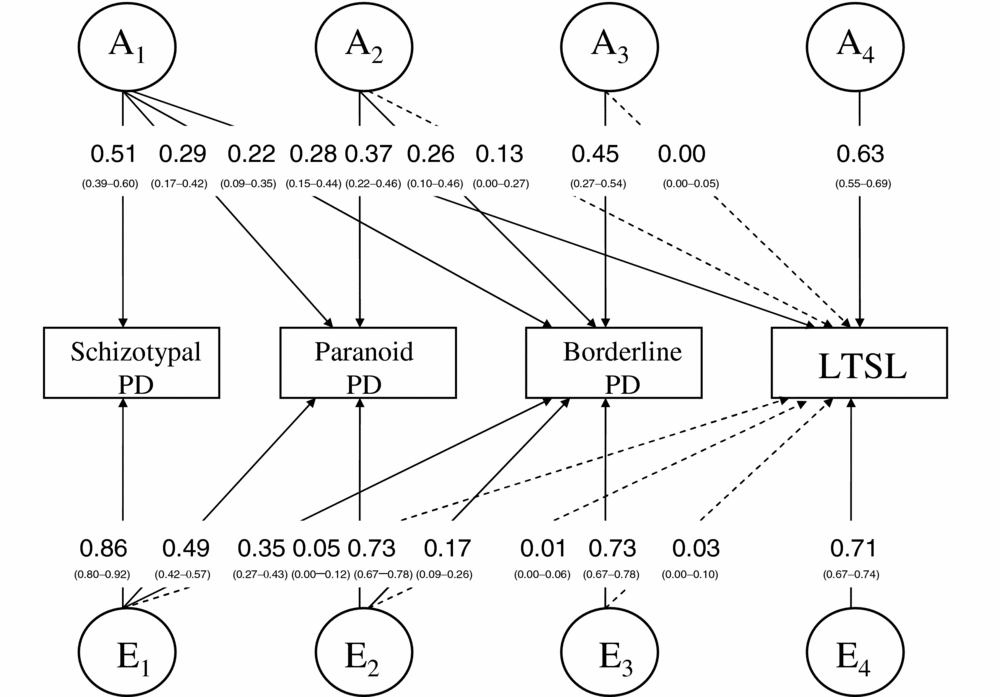
The three PDs significantly associated with LTSL were included in a tetravariate Cholesky model along with LTSL. STPD was placed first in the model, as this had the strongest association with LTSL in the regression analyses. PPD was placed second, as this is also a Cluster A PD, whereas BPD, from Cluster B, was placed third. The variables had different prevalence across sex. We therefore used separate thresholds for males and females. The results for the twin model fitting are shown in Table 2.
TABLE 2 Tetravariate Model Fitting Results for STPD, PPD, BPD, and LTSL

Best fitting model in bold type; STPD = schizotypal personality disorder; PPD = paranoid personality disorder; BPD = borderline personality disorder; LTSL = long-term sick leave; CSL = common sex limitation model; NSL = no sex limitation model.
Model 6, an AE-model with no sex differences, fitted significantly poorer than Model 1 to the data on a p = .05 level (Δχ2 = 38.78, Δdf = 24, p = .03, AIC = 2,333.72). However, as the AIC was lowest for Model 6, we selected this as best fitting. The parameter estimates for the best fitting model are shown in Figure 1. The genetic and environmental correlations between the variables are shown in Table 3. The phenotypic correlations between STPD, PPD, BPD, and LTSL were +0.19 (0.14–0.24, 95% CI), +0.17 (0.13–0.22, 95% CI), and +0.13 (0.08–0.18, 95% CI), respectively. The heritabilities for STPD, PPD, BPD, and LTSL were 0.26 (0.15–0.36, 95% CI), 0.22 (0.15–0.30, 95% CI), 0.32 (0.23–0.41, 95% CI), and 0.50 (0.46–0.55, 95% CI), respectively.
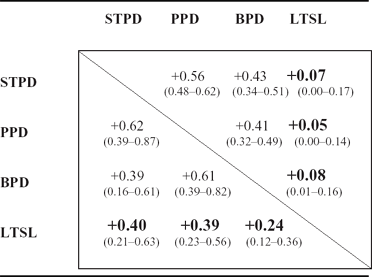
TABLE 3 Genetic and Environmental Correlations

The genetic correlations between the PDs and LTSL are shown in the lower triangle, and the environmental correlations are shown in the upper triangle. LTSL = long-term sick leave; STPD = schizotypal personality disorder; PPD = paranoid personality disorder; BPD = borderline personality disorder.

FIGURE 1 Best fitting tetravariate Cholesky model for schizotypal personality disorder, paranoid personality disorder, borderline personality disorder, and long-term sick leave (LTSL) with parameter estimates and confidence intervals. Dotted lines indicate non-significant effects.
Ninety percent of the phenotypic variance in LTSL was not related to the PDs. The remaining 10% was almost entirely due to the influence of shared genetic variance, as the unique environmental variance shared between the PDs and LTSL was less than 1%. Thus, 20% (0.282 + 0.132/0.50) of the heritability of LTSL was explained by the genetic variance shared with the PDs.
Discussion
We studied a population-based sample of 2,771 young adult Norwegian twins, born 1967–1979, for which we had an 11-year follow-up period (1998–2008) covering sick leave and rehabilitation days. Our first aim was to investigate whether there was a significant association between PDs and LTSL. We found that those in the highest LTSL category had an OR of 2.6 for having at least one categorical PD diagnosis when compared to those in the lowest LTSL category. This finding is in accordance with a previous study that found an OR of 2.76 between disability pensioning and any probable PD diagnosis (Knudsen et al., Reference Knudsen, Skogen, Harvey, Stewart, Hotopf and Moran2012). For our second aim, we found that most of the PDs were significantly associated with LTSL. When adjusting for the other significant PDs, only three PDs remained significantly associated with LTSL, namely STPD, PPD, and BPD, which may reflect the extensive comorbidity between PDs (Coid et al., Reference Coid, Yang, Tyrer, Roberts and Ullrich2006; Lenzenweger et al., Reference Lenzenweger, Lane, Loranger and Kessler2007; Marinangeli et al., Reference Marinangeli, Butti, Scinto, Cicco, Petruzzi, Daneluzzo and Rossi2000). Thus, the general PD tendency that increases risk for LTSL may be mediated through traits associated with these three disorders. A previous study on the same sample found that SPD, BPD, and dependent PD (DEPD) were significantly associated with disability pensioning after adjusting for other PDs, socio-economic status, and sex (Østby et al., submitted). These findings are similar to ours, as SPD is often associated with STPD (Lenzenweger et al., Reference Lenzenweger, Lane, Loranger and Kessler2007), but diverge with regard to DEPD and PPD. The different results may be explained by the different outcomes — sick leave versus disability pensioning, and the different designs.
Two of the PDs found to be significantly associated with LTSL, STPD, and PPD, are placed in the DSM-IV Axis II Cluster A group, characterized by odd and eccentric traits. BPD is placed in the Cluster B group, characterized by dramatic, emotional, and erratic traits (APA, 1994). STPD is associated with discomfort in close relationships and preference for spending time alone (APA, 1994). This could complicate workforce participation. Individuals with STPD may also be suspicious and have excessive social anxiety (APA, 1994), which makes it difficult to interact with colleagues and thus may increase the risk for LTSL. The most important characteristics of individuals with PPD are pervasive distrust and suspiciousness of other people (APA, 1994). These characteristics, along with a grudging attitude towards others and vigilance for possible attacks, will make it hard to function in a work environment. BPD is characterized by instability of interpersonal relationships, self-image, and affects (APA, 1994), and individuals suffering from BPD often experience high emotional distress along with varying degrees of mental and physical disability (Grant et al., Reference Grant, Chou, Goldstein, Huang, Stinson, Saha and Ruan2008; Holm & Severinsson, Reference Holm and Severinsson2008). Many of the criteria for BPD make it difficult to maintain and thrive in a job. Symptoms such as strong reactivity to interpersonal stresses, dysphoric mood, self-harm, and suicidal thoughts are all possible causes for frequent or long episodes of sick leave.
Our third aim was to estimate to what extent the genetic contributions to the selected PDs could account for the heritability of LTSL. We found that the genetic contributions to STPD, PPD, and BPD could account for a modest amount (20%) of the heritability of LTSL. The association was mainly due to one genetic factor shared in common for PDs and LTSL, as the second factor was not statistically significant. This finding is in accordance with a previous study that showed substantial genetic overlap between DSM-IV PDs (Kendler et al., Reference Kendler, Aggen, Czajkowski, Roysamb, Tambs, Torgersen and Reichborn-Kjennerud2008). The best fitting twin model according to AIC was an AE model with no sex differences, and hence we did not find evidence for shared environmental effects or genetic and environmental factors influencing PDs and LTSL to different degrees for males and females.
For the fourth aim, we explored the hypothesis of a causal pathway between PDs and LTSL. As PDs and LTSL were found to be influenced by both genetic and environmental factors (Figure 1), there should be both a genetic and an environmental correlation between at least one of the PDs and LTSL for us to argue that the association could be causal (De Moor et al., Reference De Moor, Boomsma, Stubbe, Willemsen and de Geus2008; Ligthart & Boomsma, Reference Ligthart and Boomsma2012). We found significant genetic correlations between all three PDs and LTSL, but small and mostly non-significant environmental correlations (Table 3). Thus, our results do not support a causal association. It could be that if most of the E effects were due to measurement error, and these could be separated out, we would expect higher and maybe significant E cross-loadings. However, there is no reason to assume that most of the E effects are measurement errors, as previous studies have shown that even after correcting for measurement error, there are substantial E effects for PDs (Gjerde et al., Reference Gjerde, Czajkowski, Roysamb, Orstavik, Knudsen, Østby and Reichborn-Kjennerud2012; Kendler et al., Reference Kendler, Myers, Torgersen, Neale and Reichborn-Kjennerud2007; Torgersen et al., Reference Torgersen, Myers, Reichborn-Kjennerud, Røysamb, Kubarych and Kendler2012). We do not know of any studies that have corrected for measurement error on LTSL, but as this is a population-based registry measure, we expect such errors to be of limited importance. To reach a more informed conclusion on the issue of causality a more formal model-testing approach should be used with a larger sample (Neale & Kendler, Reference Neale and Kendler1995).
Despite the absence of evidence for a causal association, early detection and treatment of STPD, PPD, and BPD is important, as these have previously been found to be the most impairing types of PDs (Nakao et al., Reference Nakao, Gunderson, Phillips, Tanaka, Yorifuji, Takaishi and Nishimura1992). The finding that PDs do not seem to be causally related to LTSL could be due to limited statistical power and should therefore be interpreted with caution.
Limitations
Due to the low prevalence of categorical PDs in the current sample, we used dimensional representations of PDs to be able to carry out the twin modeling analyses. There is, however, strong empirical support for conceptualizing PDs as dimensions (Widiger & Mullins-Sweatt, Reference Widiger and Mullins-Sweatt2009).
As PDs were measured between 1999 and 2004, although 90% within the end of 2002, whereas LTSL was measured between 1998 and 2008, it could be argued that we should have censored out LTSL before 2004. However, as PDs have an onset in adolescence or early adulthood (APA, 2000), and are measured as ‘during the last 5 years’, we feel confident that PDs preceded LTSL in the observation period.
We could not test for qualitative sex differences due to limitations posed by the multivariate Cholesky model (Neale et al., Reference Neale, Roysamb and Jacobson2006). Further, the finding of no quantitative sex differences or shared environmental effects could be due to limited statistical power. More studies with larger sample sizes are needed to conclude whether sex differences or shared environmental effects are important to explain individual differences in PDs and LTSL.
The sample used in the present study consisted of young adult Norwegian twins, and it is possible that the results are not representative for other age or ethnic groups.
Conclusion
The aim of the present study was to clarify the largely unexplored association between PDs and LTSL among young adults. STPD, PPD, and BPD were significantly associated with LTSL after adjusting for sex and comorbidity of other significant PD traits. Genetic contributions to these PDs accounted for 20% of the heritability of LTSL. The association between the PDs and LTSL was mainly due to one shared genetic factor, rendering a causal relationship between PDs and LTSL unlikely.
Acknowledgments
The work was supported by a grant from The Sickness Absence Research Programme at The Norwegian Research Council. The collection of twin data was also in part supported by previous grants from The Norwegian Research Council, The Norwegian Foundation for Health and Rehabilitation, and The Norwegian Council for Mental Health. We are very grateful to the twins for their participation.