First-degree relatives of individuals with type 2 diabetes (T2D) are a good human model to test for mechanisms involved in the early development of atherosclerosis, as long before developing overt diabetes, they show insulin resistance, dyslipidaemia, increased abdominal adiposity and muscle TAG content, and reduced first-phase insulin secretion in response to glucose(Reference Nyholm, Nielsen and Kristensen1, Reference Cnop, Vidal and Hull2).
Postprandial hyperlipaemia may promote coronary and carotid artery disease; moreover, the magnitude of postprandial triacylglycerolaemia is related to the intima-media thickness of the carotid arteries, a surrogate marker for early atherosclerosis(Reference Eberly, Stamler and Neaton3, Reference Boquist, Ruotolo and Tang4). Chronic consumption of fat-rich diets leads to atherosclerosis, inducing endothelial dysfunction and other changes in the vascular system(Reference Damjanovic and Barton5, Reference Keogh, Grieger and Noakes6); these alterations are accompanied by an increased production of some inflammatory molecules. Among these, IL-1β promotes migration and development of smooth muscle cells within the atherosclerotic lesion and stimulates vascular endothelial growth factor synthesis, thus contributing to the development of diabetic microangiopathy(Reference Aronson7); IL-6 is implicated in endothelial dysfunction and vascular inflammation, and promotes insulin resistance(Reference Fantuzzi8); IL-8 mediates leucocyte adhesion to endothelial cells and infiltration into the arterial wall(Reference Henrichot, Juge-Aubry and Pernin9); CD40 promotes restenosis after vascular injury, and enhanced expression of the CD40 ligand in platelets and elevated plasma levels of the soluble CD40 ligand (sCD40L) have been reported in subjects at high risk of developing CHD(Reference Li, Sanders and Bevard10–Reference Chakrabarti, Blair and Freedman13).
Circulating levels of intracellular adhesion molecule-1 (ICAM-1) and vascular cellular adhesion molecule-1 (VCAM-1) increase in diabetic patients(Reference Buolbou, Koukoulis and Makri14) and predict the development of the disease(Reference Meigs, Hu and Rifai15). Evidence suggests that postprandial hyperlipaemia may stimulate the release of these molecules, with oxidative stress as the putative mediator of such effect(Reference Nappo, Esposito and Cioffi16) and nitrotyrosine as a good marker of peroxynitrite and nitrosative stress generation. However, there is no information on the acute effects of a fat-rich meal on the appearance of these markers of early endothelial damage in subjects at risk of developing T2D due to a positive family history of the disease. We examined whether a lipid-rich meal modulates the synthesis of cytokines and stimulates the release of adhesion factors and parameters of oxidative stress in young healthy offspring of diabetic patients.
Subjects and methods
The participants in the study were sixteen non-smoking, healthy, young volunteers with one or both parents with T2D (FHD+) who were consecutively enrolled on a voluntry basis among post-doctoral students matching the inclusion criteria and compared with other sixteen subjects of similar age and BMI but without a family history of T2D (FHD − ). Inclusion criteria were negative history of chronic illness, normal glucose tolerance (assessed by an oral glucose tolerance test) and absence of clinical or biochemical signs of ongoing inflammation (highly sensitive C-reactive protein < 0·5 mg/l).
All subjects were asked to follow the same written diet for the 2 weeks preceding the study, a diet of 5842 kJ (1400 kcal) with 16 % protein, 58 % carbohydrate and 24 % fat. Alcohol consumption was moderate in fourteen subjects ( < 60 g/week) and occasional in six, and four were teetotallers. None of the participants practised strenuous physical exercise or was taking any medication. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board of the University of Pisa (Pisa, Italy). Written informed consent was obtained from all subjects.
Participants were admitted early in the morning after a 12 h fast. Blood pressure was measured four times in the sitting position after a 10 min rest; the mean of the last two measurements was used for statistical analysis. A blood sample was taken before the test meal; then, they consumed (within 15 min) a fat-enriched breakfast (butter 25 g, bread 100 g and 10 % fat ham 50 g). The energy content of the meal was 3051 kJ (730 kcal), with 13 % of energy from protein, 35 % from carbohydrate and 52 % from fat. At 2 and 6 h after the meal, blood samples were drawn for the isolation of monocytes and the determination of biochemical parameters and inflammatory molecules. Some measurements were also repeated after 12 h.
Biochemical parameters
Plasma cholesterol and TAG concentrations were measured by standard enzymatic assays. HDL-cholesterol was measured after precipitation of apoB-containing lipoproteins, and LDL-cholesterol was calculated by the Friedewald formula. Plasma NEFA assay was performed by a spectrophotometric method (Wako Chemicals, Neuss, Germany), plasma insulin by RIA (Linco Research, Inc., St Charles, MO, USA), with an intra-assay CV of 3·2 % and an inter-assay CV of 4·0 %.
Monocyte isolation
Blood was collected in vacutainers containing sodium heparinate, diluted (1:1) with sterile Dulbecco's PBS, slowly laid on a warm Ficoll-Hypaque solution (Histopaque 1077; Sigma-Aldrich, Milan, Italy) and centrifuged at 950 g for 20 min. To separate monocytes from lymphocytes, 1 ml of the cell suspension was added to a hyperosmotic Percoll solution (Sigma-Aldrich) in 3 ml final volume and centrifuged at 580 g for 15 min. Collected cells were added with Dulbecco's PBS up to 15 ml, centrifuged at 350 g for 7 min, thus obtaining a monocyte-enriched suspension. To discard platelets and dead cells, in each centrifuge tube, 3 ml of the iso-osmotic Percoll solution was overlaid with 1 ml of the monocyte-enriched suspension and centrifuged at 350 g for 15 min; newly resuspended in Dulbecco's-PBS and finally centrifuged at 350 g for 7 min, storing the pellet at − 80°C.
Cytokine gene expression
IL-1β, IL-6 and IL-8 mRNA levels were evaluated by RT-PCR from total RNA extracts obtained by the RNeasy mini kit (Qiagen, Milano, Italy). Total RNA was reverse-transcribed using the ImProm II™ RT-PCR System (Promega, Madison, WI, USA). Amplification primers for IL-1β, IL-6 and IL-8 and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase were as follows: IL-1β sense 5′-GGATATGGAGCAACAAGTGG-3′, antisense 5′-ATGTACCAGTTGGGGAACTG-3′; IL-6 sense 5′-GTCTCCTCATTGAATCCAGATTGG-3′, antisense 5′-AGCTCAGCTATGAACTCCTTCTC-3′; IL-8 sense 5′-TCTTGCACAAATATTTGATGC-3′, antisense 5′-CCACTGTGCCTTGGTTTC-3′; glyceraldehyde 3-phosphate dehydrogenase sense 5′-GTGAGGAGGGGAGATTCA-3′, antisense 5′-GCATCCTGGGCTACACTG-3′. Oligonucleotides were synthesised by M-Medical Genenco-Life Science (Firenze, Italy). Amplification was carried out with a Platinum PCR SuperMix High Fidelity kit (Invitrogen, Milano, Italy). Products were separated on 1 % agarose, stained with ethidium bromide and quantified by scanning densitometry.
To more precisely compare gene expression between the two groups, real-time PCR analysis was also performed. For first-strand complementary DNA synthesis, 1 μg RNA was reverse-transcribed in a 20 μl volume using random hexamers as primers, according to the manufacturer's instructions (First Strand complementary DNA Synthesis Kit for RT-PCR; AMV, Roche, Indianapolis, IN, USA). The primers and probe sequences were obtained from PE Applied Biosystems (Pre-Developed TaqMan Assay Reagents Control Kits; Foster City, CA, USA). The following assay ID were used: IL-1β, Hs00174097m1; IL-6, Hs00174131m1; IL-8, Hs00174103m1; glyceraldehyde 3-phosphate dehydrogenase, Hs99999905m1. PCR amplifications were performed in a total volume of 20 μl containing 20 ng of the complementary DNA sample, 200 nmol/l of each primer, 100 nmol/l of the corresponding probe, 12·5 μl of the TaqMan Universal PCR Master Mix. For each reaction, the polymerase was activated by preincubation at 95°C for 10 min. Amplification was then performed by forty cycles of switching between 95°C for 15 s and 60°C for 60 s. The quantity of each complementary DNA sample was normalised to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase.
Release of markers of inflammation and oxidative stress
IL-6, sCD40L, soluble VCAM-1, soluble ICAM-1 and plasma nitrotyrosine concentrations were analysed using commercially available ELISA (R&D Systems, Abingdon, UK and Cell Sciences, Canton, MA, USA).
Statistical analysis
Given the exploratory nature of the study, no prespecified outcome variable was selected, and a formal power analysis was not carried out. Plasma insulin and TAG levels were non-normally distributed and were therefore log-transformed for use in statistical analyses. Comparisons of baseline data were performed by the unpaired Student's t test. Differential changes between the two groups over time were tested by two-way ANOVA for repeated measures; results are given as significance of the time factor and the group × time interaction term. Simple and multiple regression analyses were performed by standard methods. Data were analysed using the StatView software package (SAS Institute Inc., Cary, NC, USA).
Results
FHD+ subjects were slightly older and heavier than FHD − subjects and showed a trend towards higher blood pressure and cholesterol levels; however, a slightly higher plasma TAG concentration was the only significantly different variable between the two groups (data not shown).
Following the test meal, TAG, NEFA and insulin all rose significantly, while total cholesterol and plasma glucose concentrations remained stable (Table 1). These changes were similar in the two groups.
Table 1 Biochemical parameters before and after a fat-enriched breakfast in subjects with (FHD+) or without (FHD−) a family history of type 2 diabetes
(Mean values and standard deviations)
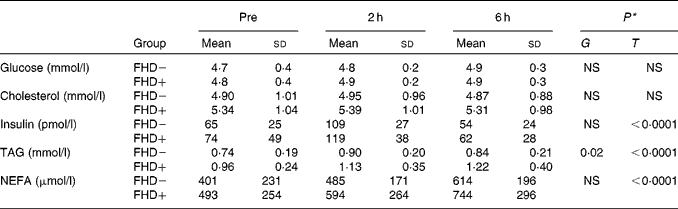
T, time; G, group × time interaction.
* P value for the effect of T and G.
No significant difference in the monocyte expression of the three cytokines was found between the FHD+ and FHD − subjects, either basally or at 6 h post-meal, nor did the meal alter the baseline expression pattern. This observation was confirmed by the values obtained by real-time PCR: no difference in the expressions of IL-6, IL-8 and IL-1β before and after the meal was observed either in FHD+ or FHD − subjects (Fig. 1). Moreover, median plasma IL-6 levels, similar in the two groups in the fasting state (0·43 (interquartile range 0·27) pg/ml in FHD − subjects and 0·57 (interquartile range 0·47) pg/ml in FHD+ subjects), did not change significantly at 2, 6 or 12 h (0·56 (interquartile range 0·33) v. 0·53 (interquartile range 0·49); 0·70 (interquartile range 0·55) v. 0·63 (interquartile range 0·55); 0·54 (interquartile range 0·45) v. 0·58 (interquartile range 0·58) pg/ml, all P = NS for either a group or group × time interaction). Median sCD40L levels followed the same trend, without any differences between the two groups either in the fasting state (2·48 (interquartile range 1·77) ng/ml in FHD − subjects and 1·69 (interquartile range 4·28) ng/ml in FHD+ subjects) or in the post-meal state (2·06 (interquartile range 1·71) v. 1·68 (interquartile range 3·92) and 2·07 (interquartile range 1·83) v. 1·27 (interquartile range 3·12) at 2 and 6 h, respectively, both P = NS). After 12 h, median sCD40L concentration was 2·15 (interquartile range 1·21) ng/ml in FHD − subjects and 1·13 (interquartile range 2·93)) ng/ml in FHD+ subjects, P = NS for either a group or group × time interaction.

Fig. 1 Representative RT-PCR for the monocyte mRNA expressions of IL-6, IL-8 and IL-1β in two subjects without a family history of type 2 diabetes (FHD − ; no. 1 and 2, representative of sixteen) and two subjects with a family history of type 2 diabetes (FHD+; no. 3 and 4, representative of sixteen) in the fasting state (pre) and 6 h after a fat-enriched breakfast (post), and the quantitative evaluation of IL-6, IL-8 and IL-1β expressions in sixteen FHD − and sixteen FHD+ subjects measured by real-time PCR in the fasting state (pre) and 6 h after a fat-enriched breakfast (post). Values are means, with standard errors represented by vertical bars. ■, FHD − ; ![]() , FHD+; M, marker.
, FHD+; M, marker.
With regard to the serum levels of the subjects' adhesion molecules, neither VCAM-1 nor ICAM-1 changed in FHD − subjects, while both increased in FHD+ subjects significantly, though still within the normal range (P ≤ 0·01 for the group × time interaction; Fig. 2). For both markers, the between-group difference persisted after adjusting (by multivariate regression) for age, insulin, total cholesterol or TAG. At 12 h, VCAM-1 and ICAM-1 tended to decline but were still significantly higher in FHD+ subjects. Nitrotyrosine levels were superimposable between the FHD − and FHD+ subjects at baseline but rose significantly higher in FHD+ subjects than in FHD − subjects at 2 and 6 h post-meal (Fig. 2); after 12 h, this difference was cancelled. In FHD+ subjects, nitrotyrosine was directly related to NEFA (r 0·53, P < 0·0001), TAG (r 0·32, P = 0·03) and insulin (r 0·36, P = 0·01) levels.

Fig. 2 Circulating levels of vascular cellular adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1) and nitrotyrosine at baseline and 2, 6 and 12 h after a fat-enriched breakfast in subjects with (FHD+) and without (FHD − ) a family history of type 2 diabetes. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different for the group × time interaction along the whole test (P ≤ 0·01). ●, FHD − ; ![]() , FHD+.
, FHD+.
In the whole study group, there was a positive correlation between the meal-induced ΔVCAM-1 and the baseline (r 0·450, P = 0·0098) and post-meal (r 0·514, P = 0·0031) TAG levels, while meal-induced ΔICAM-1 was significantly related to meal-induced nitrotyrosine (r 0·537, P = 0·0015).
Discussion
In the present study, we show that young healthy subjects with a parental history of T2D have a normal response to an oral fat load in terms of inflammatory cytokines expression and release; in contrast, the response of the circulating adhesion molecules is modestly but consistently increased in parallel with a meal-induced rise in nitrotyrosine, an index of oxidative stress. These data do not confirm those by Nappo et al. (Reference Nappo, Esposito and Cioffi16), who found an enhanced IL-6 and TNF-α release after either a glucose or a fat load also in normal subjects. One explanation for this discrepancy is that our subjects were carefully selected for being free from confounding factors such as glucose intolerance, obesity or hypertension. The test meal that we used did not induce changes in either IL-1β, a cytokine mainly released by neutrophils that might play a role in inducing β-cell apoptosis(Reference Donath, Schumann and Faulenbach17), or IL-6, a marker of insulin resistance whose levels have been shown to predict the risk of incident diabetes(Reference Hu, Meigs and Li18, Reference Pradhan, Manson and Rifai19), unmodified by a fatty meal in normal individuals as reported by Poppitt et al. (Reference Poppitt, Keogh and Lithander20). This suggests that family history of diabetes per se, in the absence of overt metabolic abnormalities, does not confer susceptibility to an inflammatory response to an acute stimulus represented by a lipid oral load. Similarly, the pro-inflammatory mediator sCD40L, which is expressed in CD4+T cells and activated platelets and participates in several inflammatory and pro-atherothrombotic functions(Reference Schonbeck and Libby21), was unaffected by an oral fat load in both groups, implying that familial predisposition to diabetes does not add prothrombotic risk.
At variance with the observations made by Ceriello et al. (Reference Ceriello, Quagliaro and Piconi22), VCAM-1 and ICAM-1 did not increase post-meal in FHD − subjects, but did so in FHD+ subjects, even remaining within the normal range, suggesting an early defect in vascular reactivity and endothelial function in these subjects, possibly outlined by the oral fat load. In this respect, the direct relationship between TAG and VCAM-1 appears of particular interest, supporting the role of circulating lipids in endothelial function(Reference Rubin, Claas and Pfeuffer23). We actually show that not only post-meal TAG but even fasting TAG might play a role in determining VCAM-1 variation in the post-absorptive phase, reinforcing the role of lipid metabolism as a strong determinant of endothelial function. The rise in nitrotyrosine levels (a useful marker of both peroxynitrite and nitric oxide activity) in the FHD+ subjects and their relation to TAG and FFA levels resonate with the concept that NEFA increase oxidative stress generation in humans(Reference Steinberg, Tarshoby and Monestel24). Further support to such a hypothesis might come from the relationship between the variation of ICAM-1 and nitrotyrosine levels, while such a correlation with VCAM-1 is lacking. This may be referred to the differences between these two adhesion molecules, given that ICAM-1 is mostly expressed at the level of endothelial cells, and its expression is enhanced by a variety of pro-inflammatory stimuli(Reference Wallen, Held and Rehnqvist25).
In conclusion, in young healthy subjects, parental diabetes appears to be associated with the evidence of abnormal endothelial reactivity and increased oxidative stress in response to an acute stimulus. The present study has several limitations, mainly its exploratory, hypothesis-generating nature and the small sample size. These observations await to be confirmed in larger cohorts of subjects.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. S. M. did the majority of laboratory determinations. V. C. recruited the volunteers and performed the tests. E. S. gave an important contribution to the experimental procedures. E. F. is the head of the section; he revised the manuscript and gave important suggestions to improve the quality of the manuscript. A. S. designed the experimental protocol, analysed the data and wrote the manuscript. The authors have no conflict of interest to disclose.





