It is well documented that oxidative stress, defined by Blomhoff(Reference Blomhoff1) as a “condition that is characterised by the accumulation of non-enzymatic oxidative damage to molecules that threaten the normal functions of a cell or the organism” is involved in the aetiology of a large number of human diseases. Typical examples are various forms of cancer (breast, colon and liver), neuropathological disorders such as Parkinson's and Altzheimer's disease, inflammations including hepatitis and inflammatory bowel diseases, different types of dermatitis as well as bacterial and viral infections (HBV, sepsis), diabetes, and rheumatoid arthritis (for review see(Reference Elahi and Matata2)). Also coronary heart diseases which are the major cause of death in industrialised countries(Reference Elahi and Matata2, Reference Lefer and Granger3) and idiopathic infertility(Reference Agarwal, Sharma, Nallella, Thomas, Alvarez and Sikka4, Reference Pasqualotto, Sharma, Kobayashi, Nelson, Thomas and Agarwal5), which has increased over the last decades in Western countries seem to be causally related to reactive oxygen species (ROS) meditated damage and it has been stressed that oxidative damage is also involved in several diseases of ageing, e.g. Werner's syndrome(Reference Von Kobbe, May, Grandori and Bohr6), progeria(Reference Yan, Li, Jiang and Oberley7), amyotrophic lateral sclerosis, cataract formation and decreased immune functions(Reference Kohen and Nyska8–Reference Calabrese, Guagliano, Sapienza, Mancuso, Butterfield and Stella10).
Already half a century ago it was found that the acute toxic, DNA-damaging and carcinogenic effects of ionising radiation which are predominantly caused by the formation of ROS can be reduced by antioxidant vitamins such as C, E, and A(Reference Zimmermann and Kimmig11–Reference Gebhart13). In the following decades, it became apparent that plant derived foods as well as beverages contain a large number of compounds which protect against oxidative damage and its consequences. Typical examples for such antioxidants which have been defined as “redox-active compounds that reduce pro-oxidative stress by reacting non-enzymatically with a reactive oxidant”(Reference Blomhoff1) are flavonoids and phenolic acids contained in fruits and vegetables(Reference Li, Tsao, Yang, Liu, Zhu and Young14–Reference Erdman, Balentine, Arab, Beecher, Dwyer, Folts, Harnly, Hollman, Keen, Mazza, Messina, Scalbert, Vita, Williamson and Burrowes17), allyslulfides in Allium species(Reference Kim, Chang, Kim, Chang and Chun18), hydroxycinnamic acids in coffee(Reference Iwai, Kishimoto, Kakino, Mochida and Fujita19, Reference Fujioka and Shibamoto20), phenolic compounds in wines(Reference Lopez-Velez, Martinez-Martinez and Del Valle-Ribes21) and vegetable oils(Reference Hidalgo, Leon and Zamora22), catechins in teas(Reference Luximon-Ramma, Neergheen, Bahorun, Crozier, Zbarsky, Datla, Dexter and Aruoma23), specific ingredients of common spices such as capsaicin in chillies(Reference Antonious, Kochhar, Jarret and Snyder24), gingerol(Reference Masuda, Kikuzaki, Hisamoto and Nakatani25) and curcumin(Reference Somparn, Phisalaphong, Nakornchai, Unchern and Morales26), chlorophylls(Reference Hsu, Yang, Chen, Chao and Hu27), anthocyanins in berries(Reference Xiong, Melton, Easteal and Siew28) as well as carotenoids(Reference Semba, Lauretani and Ferrucci29) to name only a few.
The increasing evidence of the strong impact of the redox status on human health has stimulated intense research activities in this field. It has been estimated that around 10 papers dealing with oxidative stress and/or antioxidants are published daily(Reference Blomhoff1) and many of them concern the identification of dietary compounds in the diet and investigations concerning their mode of action. The results of these efforts have a strong impact on the development of nutritional recommendations and led to the development of supplements which contain high levels of food derived antioxidants(Reference Aruoma, Sun, Fujii, Neergheen, Bahorun, Kang and Sung30) and to the production of functional foods. A broad variety of different methods are currently used to study antioxidants in human foods and to identify and characterise their active principles. The models include chemical–analytical and physical measurements, experiments with subcellular fractions and intact cells, animal studies as well as human intervention trails. In the last decade, new biomarkers have been developed and validated which can be used in human studies and the rapid development of -omics techniques (in particular the use of microarrays and two dimensional gel electrophoresis) offers the possibility to explore the effects of antioxidants on gene expression and protein levels and to study alterations of disease related patterns(Reference Narayanan31–Reference Scandalios33).
The aim of the present article is it to give a critical overview on the advantages and limitations of the different approaches which are currently used with particular emphasis on the newly developed methods. We anticipate that it will help in the interpretation of existing data and lead to the development of improved strategies concerning the detection of antioxidants.
The formation of ROS as well as their physical and chemical properties, their reactions with organic molecules and their inactivation by antioxidants have been extensively described in the scientific literature(Reference Choe and Min34–Reference Karihtala and Soini36). Therefore, these topics are confined in the present article to short descriptions which are essential to understand the subsequent chapters.
Formation of reactive oxygen species (ROS)
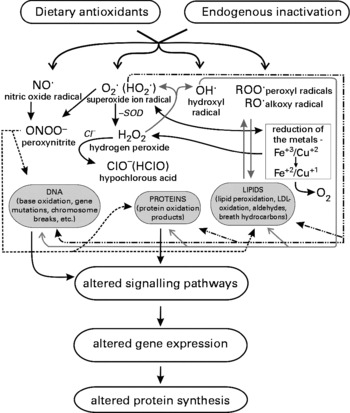
Pro-oxidants (often termed as reactive oxygen species) can be classified in two groups, namely radicals and non-radicals. Radicals (O2, ![]() , OH∙, ROO∙, RO∙, and NO∙) contain unpaired electrons in the shells around the nucleus which causes the high reactivity of these species (except O2), due to their ability to donate or receive other atoms to obtain stability. Important non-radicals comprise hyperchlorous acid (HOCl), hydrogen peroxide (H2O2), organic peroxides, aldehydes and ozone (O3). The most relevant ROS as well as some of their main reactions are shown in Fig. 1.
, OH∙, ROO∙, RO∙, and NO∙) contain unpaired electrons in the shells around the nucleus which causes the high reactivity of these species (except O2), due to their ability to donate or receive other atoms to obtain stability. Important non-radicals comprise hyperchlorous acid (HOCl), hydrogen peroxide (H2O2), organic peroxides, aldehydes and ozone (O3). The most relevant ROS as well as some of their main reactions are shown in Fig. 1.
Fig. 1 Different form of reactive oxygen species and their interaction with organic molecules.
Superoxide ![]() , which is formed for example during respiration in mitochondria (as a consequence of reduction of oxygen required for APT production) forms at low pH hydroperoxyl (HO2 which penetrates the cell membranes more easily than the charged form(Reference Fridovich37, Reference Halliwell and Gutteridge38). Enzymatic as well as non-enzymatic dismutation leads to formation of hydrogen peroxide (H2O2) which can be detoxified enzymatically (catalase, glutathion peroxidase). H2O2 molecules can damage cells at low concentrations and degrade haem proteins and oxidise DNA, enzymes, –SH groups and keto acids and are also the source of more deleterious species such as HOCl and OH∙. The latter radical is short lived and reacts at a high rate with most organic molecules (DNA, amino acids, sugars, proteins, lipids). Transition metals (first row of the D block of the periodic table) contain unpaired electrons (except Zn) and can therefore be considered as radicals. In particular copper and iron are contained at relatively high concentrations in many organisms. At physiological pH, most of the metals are present in oxidised forms (Fe3+∙, Fe2+), but after reduction (e.g. by ascorbic acid or via the Haber-Weiss reaction
, which is formed for example during respiration in mitochondria (as a consequence of reduction of oxygen required for APT production) forms at low pH hydroperoxyl (HO2 which penetrates the cell membranes more easily than the charged form(Reference Fridovich37, Reference Halliwell and Gutteridge38). Enzymatic as well as non-enzymatic dismutation leads to formation of hydrogen peroxide (H2O2) which can be detoxified enzymatically (catalase, glutathion peroxidase). H2O2 molecules can damage cells at low concentrations and degrade haem proteins and oxidise DNA, enzymes, –SH groups and keto acids and are also the source of more deleterious species such as HOCl and OH∙. The latter radical is short lived and reacts at a high rate with most organic molecules (DNA, amino acids, sugars, proteins, lipids). Transition metals (first row of the D block of the periodic table) contain unpaired electrons (except Zn) and can therefore be considered as radicals. In particular copper and iron are contained at relatively high concentrations in many organisms. At physiological pH, most of the metals are present in oxidised forms (Fe3+∙, Fe2+), but after reduction (e.g. by ascorbic acid or via the Haber-Weiss reaction ![]() ), they can undergo “Fenton type” reactions (e.g. Fe2+ + H2O2 → Fe3+ + OH + OH−). These two processes explain the formation of OH∙in vivo. However, it is notable that in organisms, metals are always bound to proteins and membranes and it has been shown that they can undergo in this state the aforementioned reactions and produce OH∙ at a single site and convert non-reactive radicals to highly reactive species.
), they can undergo “Fenton type” reactions (e.g. Fe2+ + H2O2 → Fe3+ + OH + OH−). These two processes explain the formation of OH∙in vivo. However, it is notable that in organisms, metals are always bound to proteins and membranes and it has been shown that they can undergo in this state the aforementioned reactions and produce OH∙ at a single site and convert non-reactive radicals to highly reactive species.
Nitric oxide (NO∙) is produced by oxydation of the terminal guanidine-nitrogen atoms of arginine(Reference Halliwell and Gutteridge38–Reference Czapski and Goldstein40). This reaction is catalysed by nitric oxide synthetases (NOS, i.e. neuronal NOS, endothelial NOS and inducible NOS). NO∙ can react with different radicals, the most important reaction under physiological conditions is the formation of peroxynitirite (ONOO− ) in which ![]() is involved(Reference Halliwell and Gutteridge38, Reference Czapski and Goldstein40). ONOO− can cause damage similar to that induced by OH∙(Reference Beckman and Koppenol39).
is involved(Reference Halliwell and Gutteridge38, Reference Czapski and Goldstein40). ONOO− can cause damage similar to that induced by OH∙(Reference Beckman and Koppenol39).
The biological significance of the different ROS species has been discussed quite controversially. Some authors suggested that ![]() and NO∙ are the most relevant ones, while other stressed that peroxyl radicals may be even more important(Reference Kohen and Nyska8).
and NO∙ are the most relevant ones, while other stressed that peroxyl radicals may be even more important(Reference Kohen and Nyska8).
Several exogenous factors contribute to oxidative stress. Ionising radiation causes toxic effects in organisms primarily via ionisation of intracellular water(Reference Shadyro, Yurkova and Kisel41). Also non-ionising radiation (UV light) can indirectly produce a variety of ROS species including O3 (Reference Pentland42). Other major sources of exposure are air pollutants such as cigarette smoke(Reference Koren43) and car exhausts(Reference Victorin44), drugs (bleomycin, doxorubicine)(Reference Yu and Anderson45) as well as pesticides and herbicides(Reference Wiseman, Ridgway, Goldfarb and Woods46) and industrial chemicals(Reference Parke and Sapota47). Also pathogenic microorganisms may produce oxygen species, but the most relevant external source is nutrition as most of the foods we consume are oxidised and contain oxidants such as peroxides, aldehydes, fatty acids and transition metals(Reference Ames48, Reference Kanner and Lapidot49).
The most important endogenous processes of ROS production are respiration processes in the mitochondria and the massive continuous production of radicals is even increased in ageing cells(Reference Richter, Gogvadze, Laffranchi, Schlapbach, Schweizer, Suter, Walter and Yaffee50, Reference Fleury, Mignotte and Vayssiere51). Another source are white blood cells involved in immune responses which can undergo a respiratory bust that is characterised by an up to 20-fold increase of oxygen production(Reference Dhalla, Temsah and Netticadan52, Reference Forman and Torres53). During this reaction, NAPDH serves as a donor of electrons which results in the production of ![]() from oxygen. The enzyme myeloperoxidase catalyses the production of HOCl by interaction between H2O2 peroxides and chlorides(Reference Hawkins, Brown and Davies54, Reference Rodrigues, Rodriguez, Henrique Catalani, Russo and Campa55).
from oxygen. The enzyme myeloperoxidase catalyses the production of HOCl by interaction between H2O2 peroxides and chlorides(Reference Hawkins, Brown and Davies54, Reference Rodrigues, Rodriguez, Henrique Catalani, Russo and Campa55).
Targets of oxidative damage
The continuous exposure to ROS from exogenous and endogenous sources results in oxidative damage of many cell components and alterations of cellular functions; some of these changes can be used as markers of oxidative stress and to investigate putative protective effects of phytochemicals.
Proteins
Radicals react in particular with nucleophilic amino acids for example with tryptophane, histidine and cysteine(Reference Davies56, Reference Grune, Reinheckel and Davies57). Apart from direct oxidation of SH-groups by H2O2 and ![]() , organic radicals may bind covalently to cellular proteins which are part of cell membranes or have enzymatic functions. One of the major adducts which can be easily detected is 3-nitrotyrosine which is produced by interactions between ONOO– and other nitrogen reactive radicals with the amino acid tyrosine(Reference Levine and Stadtman58). Also relatively resistant amino acids such as lysine and proline can be hydroxylated non-enzymatically by OH∙(Reference Stadtman59).
, organic radicals may bind covalently to cellular proteins which are part of cell membranes or have enzymatic functions. One of the major adducts which can be easily detected is 3-nitrotyrosine which is produced by interactions between ONOO– and other nitrogen reactive radicals with the amino acid tyrosine(Reference Levine and Stadtman58). Also relatively resistant amino acids such as lysine and proline can be hydroxylated non-enzymatically by OH∙(Reference Stadtman59).
It is also known that ROS can destroy peptide bonds and cause drastic alterations of their structures resulting in changes of their cellular functions (for review see(Reference Karihtala and Soini36)). NO∙ reacts in particular with Fe–S centres of proteins which transport electrons and this affects the functions of mitochondria(Reference Kushnareva, Murphy and Andreyev60). Another important feature is their reaction with thiol groups of proteins; a typical example is the S-nitrosylation of caspases which are part of cell signalling processes(Reference Squadrito and Pryor61).
Lipids
All cellular membranes are vulnerable to oxidation due to their high concentrations of unsaturated fatty acids. Damage of lipids by ROS (lipid peroxidation, LP) occurs in three stages. In the first (“initiation phase”), double bonds of fatty acids are attacked by radicals which leads to formation of fatty acids radicals. During the “propagation”, a chain reaction takes place which leads to continuous formation of these radicals. The last stage (chain determination) occurs following interactions ROO∙ or with other radical types and/or antioxidants (for a detailed description of LP see(Reference Halliwell and Gutteridge38, Reference Younes and Siegers62, Reference Horton and Fairhurst63)). Important marker molecules formed during LP are aldeydes and ketones, for example malonedialdehyde (MDA) and 4-hydroxynonenal (4-HNE) as well as the family of isoprostanes which are excreted in the urine. Oxidation of fatty acids can be measured with the relative change in the fatty acid pattern and the formation of conjugated dienes.
Blood cholesterols can be oxidised by ROS to form oxidised low density lipoproteins (LDL). The oxidative modification hypothesis of atherosclerosis assumes that circulating LDL particles are modified by oxidation and that these particles are then taken up by macrophages inside the arterial wall and form the start of atherosclerotic plaques(Reference Zock and Katan64, Reference Forrester and Shah65).
DNA damage
ROS can damage DNA either directly or indirectly (via LP). The major part of damage is attributable to OH∙ radicals which interact either with the sugar phosphate chain or oxidise bases and form reaction products such as thymine glycol, 5-hydroxy-uracil and 8-hydoxy-desoxyguanosine (8-OH-dG)(Reference Moller and Wallin66, Reference Barzilai and Yamamoto67). Also NO∙ can damage DNA indirectly via formation of peroxynitrite, another pathway of damage is the formation of covalent bonds between radicals and nucleobases(Reference Halliwell and Gutteridge38, Reference Niles, Wishnok and Tannenbaum68, Reference Tsutsui, Ide and Kinugawa69).
The most important indirect form of DNA damage is caused by LP (for review see(Reference Vaca, Wilhelm and Harms-Ringdahl70)). Lipid radicals formed during the chain reaction cause adduct formation, strand breaks and DNA-protein crosslinks. The former lesions are also induced by specific end products of the reaction such as alkenes and alkanes(Reference Ehrenberg, Osterman-Golkar, Segerbck, Svensson and Calleman71, Reference Segerback72) and numerous studies have been published which concern the genotoxic effects of reaction products such a MDA and 4-HNE(Reference Vaca, Wilhelm and Harms-Ringdahl70). Nuclelotide- as well as base excision repair mechanisms (BER and NER) prevent the persistence of oxidative lesions. Oxidised guanosine is removed by the action of glycosylases such as 8-oxoguanine DNA glycosylase 1 and 2 (OGG1 and OGG2); mispaired adenines by MYH and MTH1. NEIL glycosylases containing β/δ-elimination activities excise a broad range of oxidatively damaged bases, including 5-hydroxyuracil (5-OHU), thymine glycol (Tg), uracil, 8-oxoguanine (8-oxoG) and ring-fragmented purines(Reference Doublie, Bandaru, Bond and Wallace73). Also pyrimidine derived lesions are a substrate of this latter enzyme, important uracil specific enzymes are for example UNG and SMUG1. A detailed description of repair of oxidised DNA can be found in the articles of Sanderson et al. (Reference Sanderson, Bennett, Sung and Mosbaugh74), Krokan et al. (Reference Krokan, Drablos and Slupphaug75) and Cooke et al. (Reference Cooke, Evans, Dizdaroglu and Lunec76).
Oxidative stress, cell signalling and activation of transcription factors
The exposure of organisms to ROS causes dramatic changes in gene regulation patterns and protein synthesis. In lower eucaryotes, e.g. in yeast the expression of up to 1/3 of the genes is affected by oxidative stress(Reference Kim, Chang, Chung, Park and Seo77), mammalian cells are somewhat less flexible but still hundreds of genes have been identified which react towards oxidative damage. During the last decades, complex signalling pathways have been discovered which activate transcription factors involved in gene regulation. The networks involved in ROS mediated cellular responses have been described in a number of reviews(Reference Valko, Rhodes, Moncol, Izakovic and Mazur78–Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser84). In the present article we will give a short overview on the most important processes since the mode of action of dietary antioxidants may involve changes of signalling pathways which cause activation of cellular defence systems(Reference Ma, Battelli and Hubbs85).
Alterations of cell signalling pathways
Oxidants can impinge signalling either by influencing redox dependent protein–protein interactions or via altering enzyme activities, i.e. oxidations leading to inhibition of phosophor/serine/threonine-, phosphothyrosine- and phospholipid-phosphatases(Reference Mathers, Fraser, McMahon, Saunders, Hayes and McLellan82). The key reactions which have been identified are interactions with sulphydryl groups on their cysteine residues(Reference Poli, Leonarduzzi, Biasi and Chiarpotto86).
The intracellular responses can be grouped in two categories namely receptor-mediated ones and non-receptor effects. Fig. 2 gives a schematic overview on the different processes.

Fig. 2 Impact of ROS on cell signalling and activation of transcription factors.
Growth factors and cytokines (e.g. TNF-α, IL-1) cause ROS production in non-pathogenic cells and activate intracellular receptor mediated signalling which affect mitogen activated protein kinases (MAPKs). Growth factor receptors are tyrosine kinases (RTLs), apart from these, also non-receptor protein kinases have been identified which are also activated by ROS and belong for example to the Src family(Reference Abe and Berk87, Reference Esposito, Chirico, Montesano Gesualdi, Posadas, Ammendola, Russo, Cirino and Cimino88). Activated Src binds to membranes and initiates MAPKs, NfκB and PI3K signalling pathways(Reference Valko, Rhodes, Moncol, Izakovic and Mazur78). Other important targets involved in signalling are Ras (membrane bound G proteins involved in the regulation of cell growth), protein tyrosine phosophatases (PTP) and seronine/threonine kinases. The most important representative of the latter group is protein kinase C (PKC), its catalytic site is a zinc finger domain containing several cysteine rich regions which can be modified by various oxidants(Reference Gopalakrishna and Jaken89).
MAPKs (for a detailed description see(Reference Kyriakis and Avruch90)) relay signals generated by exogenous or endogenous stimuli to intracellular space via phosphorylation of proteins. During this process, the kinases interact also with downstream mediators including transcription factors(Reference Lopez-Ilasaca, Crespo, Pellici, Gutkind and Wetzker91). Studies on the upregulation of MAPKs have shown that these processes are type and stimuli specific. For example, it was found that endogenous H2O2 production by respiratory burst induces ERK but not p38 kinase(Reference Iles and Forman92) while exogenous peroxide treatment activates the latter enzyme(Reference Torres and Forman93).
Activation of transcription factors by ROS
The most significant effects of ROS on MAPKs concern the activation of transcription factors which control the expression of protective genes, arrest division of damaged cells and induce apoptosis (programmed cell death).
AP-1 is a collection of dimeric basic region-leucine zipper proteins which are for example induced by metals and H2O2 (Reference Rao, Luo and Hogan94, Reference Pinkus, Weiner and Daniel95) and regulate cell growth, differentiation and apoptosis.
NFκB is a DNA binding protein which is sequestered in the cytoplasm because of an interaction with a member of the inhibitory IκB family. Activation via ROS causes dissociation and allows NFκB to enter the nucleus and activate genes involved in inflammatory responses, transformation and angiogenesis(Reference Amiri and Richmond96). A number of investigations showed that that activation by different stimuli can be blocked by antioxidants including N-acetlylcysteine, cysteine, vitamin E, thiols and green tea polyphenolics(Reference Valko, Rhodes, Moncol, Izakovic and Mazur78).
Another important factor which plays a key role in protecting cells from malignant transformation is p53, also termed a “tumour suppressor” since it arrests cell cycle and induces apoptosis(Reference Hofseth, Hussain and Harris97). p53 is directly activated by oxidants and its overexpression leads to increase of intracellular ROS levels. One of the important functions of p53 is the up regulation of proteins that play a role in ROS mediated apoptosis namely ferrodoxin reductase (FDXR) and a novel stress-response gene Redd1/HIF-1 originally isolated as an HIF-1-response gene(Reference Jin, An, Lee, Woo, Seo, Choe, Yoo, Lee, Um, Lee, Park, Kim, Hong, Rhee and Park98). It was shown in a number of in vitro studies that antioxidants reduce apoptosis rates due to interaction with p53(Reference Li, Jang and Surh99, Reference Sablina, Budanov, Ilyinskaya, Agapova, Kravchenko and Chumakov100). In a recent human intervention study we observed a drastic reduction of the apoptosis frequencies in lymphocytes after consumption of wheat sprouts which is probably due to antioxidant effects(Reference Ehrlich, Hoelzl, Nersesyan, Winter, Koller, Ferk, Fenech, Dusinska, Knasmüller, Ďuračková, Slámenova and Micksche101).
Two other transcription factors affected by ROS are nuclear factor of activated T cells (NFAT) and HIF. The former family regulates muscle growth and differentiation as well as cytokine formation and angiogenesis(Reference Rao, Luo and Hogan94, Reference Jauliac, Lopez-Rodriguez, Shaw, Brown, Rao and Toker102); while the latter is a heterodimer controlling genes encoding for vascular endothelial growth factor (VEGF), aldolase, enolase and lactate dehydrogenase(Reference Semenza103).
Probably the most important contribution to cell defence against oxidative stress is mediated through transcriptional activation of genes via a cis-acting enhancer known as antioxidant responsive element (ARE) which was discovered by Pickett and co-workers(Reference Huang, Nguyen and Pickett104–Reference Favreau and Pickett106) and identified in the 5′flanking regions of many genes. A number of studies showed that the transcription factor Nrf2 which belongs to the CNC (cap‘N'collar) basic leucine zipper family and is represented in many tissues is the key mediator of ARE dependent activation(Reference Gupta, Dobashi, Greene, Orak and Singh107, Reference Klaunig and Kamendulis108). Comparative investigations with genetically altered rodents (Nrf2+/+ and Nrf2 − / − ) showed that numerous genes are regulated by the element including those which encode for protection against ROS such as glutamate cystein ligase (GCL) which catalyses the rate limiting step of glutathione synthesis, NADPH quinone oxidoreductase (NQO), glutathione S-transferase (GST), aldehyde dehydrogenase (ADH), glutathione peroxidase, glutathione reductase, peroxiredoxin I (PrxI), superoxide dismutase (SOD), catalase, and thioredoxin(Reference Michiels, Raes, Toussaint and Remacle109, Reference Fridovich110). Also enzymes which are involved in the supply of reducing equivalents (e.g. glucose-6-phosphate dedydrogenase) as well as xenobiotic drug metabolising enzymes (e.g. CYPs), chaperones, and stress response proteins are regulated by Nrf2(Reference Kwak, Wakabayashi, Itoh, Motohashi, Yamamoto and Kensler111, Reference Thimmulappa, Mai, Srisuma, Kensler, Yamamoto and Biswal112).
Recent investigations showed that the actin binding protein Kelch-like Ech-associated protein (Keap1) regulates transcription factor Nrf2 by controlling its stability and subcellular localisation(Reference McMahon, Itoh, Yamamoto and Hayes113–Reference Kwak and Kensler115). The disruption of the Keap-Nrf2 complex by oxidative stress leads to Nrf2 accumulation in the nucleus where it is associated with small MAF transcription factors and mediates ARE dependent gene expression (see Fig. 2).
It is well documented that chemicals which release ROS such as metals(Reference He, Lin, Chen, Zhang and Ma116, Reference He, Chen, Lin and Ma117) and vascular diseases (arteriosclerosis, diabetes, chronic renal failure, preeclampsia) both cause induction of the different transcription factors described above(Reference Mann, Niehueser-Saran, Watson, Gao, Ishii, de Winter and Siow118).
The interaction of phytochemicals with these processes has been reviewed in several articles(Reference Mann, Niehueser-Saran, Watson, Gao, Ishii, de Winter and Siow118–Reference Surh, Kundu, Na and Lee121). It was shown that phenolics such as EGCG and resveratrol and spice ingredients (e.g. capsaicin, curcumin) inhibit the transcription factors NFkB, AP-1 and β-catenin-TcF signalling via interaction with upstream signalling pathways (IKK phosphorylation, MAPK phosphorylation and PI3K/Akt phosphorylation), in parallel proinflammatory mediators (TNF-α, IL, PGE2 and NO) and the activities of proinflammatory enzymes (COX 2, iNOS) were reduced(Reference Kundu and Surh119). In the case of COX 2, it is known that inhibition by synthetic compounds such as non-steroidal anti-inflammatory drugs (NSAIDS) is paralleled by decreased rates of colon and colorectal cancers in humans(Reference Stange122, Reference Chia, Newcomb, Bigler, Morimoto, Thibodeau and Potter123). On the contrary, the induction of detoxifying enzymes (including those which inactivate ROS) is due to activation of the transcription factor Nrf2. Typical example for dietary antioxidants which cause an induction of this transcription factor are synthetic and tea specific phenolics, isothicoyanates and sulforaphane(Reference Kong, Owuor, Yu, Hebbar, Chen, Hu and Mandlekar124), curcumin(Reference Nishinaka, Ichijo, Ito, Kimura, Katsuyama, Iwata, Miura, Terada and Yabe-Nishimura125) and flavonoids(Reference Tanigawa, Fujii and Hou126, Reference Gonzalez-Gallego, Sanchez-Campos and Tunon127).
The molecular mechanisms by which phytochemicals interact with signal transmission cascades are not precisely known. It is supposed that the downregulation of transcription factors may be due to the direct scavenging of ROS. In the case of Nrf2 it was shown that the activation by sulforaphane and synthetic alkylating compounds is due to modifications of cysteine residues of Keap1, a sensor protein which regulates Nrf2(Reference Hong, Freeman and Liebler128–Reference Eggler, Liu, Pezzuto, van Breemen and Mesecar131). However it cannot be excluded that Nrf2 activation seen with certain phytochemicals may be due to release of ROS; it is known that antioxidants (in particular phenolics) can act under certain conditions as pro-oxidants.
Conventional and new methods for the detection of dietary antioxidants
A large number of different techniques have been developed to monitor oxidative damage and its consequences; these approaches can be also used to identify dietary antioxidants and their mode of action. These methods are often applied in experimental systems in which oxidative stress is induced by specific treatments or diseases. The most frequently employed models are described in the next chapter; the following sections describe physics–based and biochemical methods, techniques for the detection of oxidative DNA-damage, approaches used to investigate alterations of signalling pathways as well as the advantages and disadvantages of -omics techniques (Fig. 3).

Fig. 3 Overview of different methods used for the detection of dietary antioxidants.
Induction of oxidative stress in biological systems
In experiments with subcellular fractions and in in vitro experiments with cells, ROS are in most cases generated by chemical reactions, for example with the xanthine/xanthine oxidase system by hydroquinone oxidation(Reference Abdelwahed, Bouhlel, Skandrani, Valenti, Kadri, Guiraud, Steiman, Mariotte, Ghedira, Laporte, Dijoux-Franca and Chekir-Ghedira32, Reference Le, Hailer, Buhrow, Wang, Flatten, Pediaditakis, Bible, Lewis, Sausville, Pang, Ames, Lemasters, Holmuhamedov and Kaufmann132, Reference Wiseman133) which generate ![]() . Transition metals such as copper and iron play a major role in Fenton type reactions thereby forming mainly OH∙ radicals. Another frequently used approach is the use of chemicals such as H2O2, t-butyl-hydrogenperoxide or bleomycin which release
. Transition metals such as copper and iron play a major role in Fenton type reactions thereby forming mainly OH∙ radicals. Another frequently used approach is the use of chemicals such as H2O2, t-butyl-hydrogenperoxide or bleomycin which release ![]() and OH∙ or of compounds such as menadione(Reference Criddle, Gillies, Baumgartner-Wilson, Jaffar, Chinje, Passmore, Chvanov, Barrow, Gerasimenko, Tepikin, Sutton and Petersen134), paraquat(Reference McCarthy, Somayajulu, Sikorska, Borowy-Borowski and Pandey135), and plumbagin(Reference Srinivas, Gopinath, Banerji, Dinakar and Srinivas136) which form O2.
and OH∙ or of compounds such as menadione(Reference Criddle, Gillies, Baumgartner-Wilson, Jaffar, Chinje, Passmore, Chvanov, Barrow, Gerasimenko, Tepikin, Sutton and Petersen134), paraquat(Reference McCarthy, Somayajulu, Sikorska, Borowy-Borowski and Pandey135), and plumbagin(Reference Srinivas, Gopinath, Banerji, Dinakar and Srinivas136) which form O2.
Activated phagocytic cells produce oxygen radicals as part of their defence system and a burst of ROS can be induced by exposing such cells to bacteria, particles or certain chemicals; one of the most powerful responses can be evoked with the tumour promotor phorbol myristate acetate(Reference Phillips, Anderson and Gangolli137).
Chemicals which generate ROS are rarely used in animal studies due to their high reactivity. A more convenient way to cause oxidative damage which has been also used in numerous in vitro experiments is ionising radiation. Indirect approaches are feeding of vitamin E deficient diets(Reference Minamiyama, Takemura, Hai, Suehiro and Okada138), iron overload(Reference Isomura, Fujie, Shibata, Inoue, Iizuka, Takebe, Takahashi, Nishihira, Izumi and Sakamoto139) or inhalation of oxygen(Reference Gregorevic, Lynch and Williams140).
Organ specific inflammations can be induced with certain chemicals; for example liver cirrhosis with CCl4(Reference He, Luo, Wang, Wang, Fu, Xu, Zhao and Liu141) or thioacetamide(Reference Bruck, Ashkenazi, Weiss, Goldiner, Shapiro, Aeed, Genina, Helpern and Pines142). 2,4,6-Trinitrobenzene sulfonic acid, oxazolone and dextran sodium sulfate are used in models for inflammatory bowel diseases(Reference Wirtz, Neufert, Weigmann and Neurath143); diabetes can be caused by the antibiotic streptozotocin.
In the last years, a variety of genetically altered mice and rat strains have been developed as models for ROS-related diseases for example animals which are deficient in specific SOD forms(Reference Fujimoto, Arakawa, Shibaya, Miida, Ando, Yasumo, Hara, Uchiyama, Iwabuchi, Takasaki, Manabe and Yamoto144) and GST isozymes(Reference Fujimoto, Arakawa, Shibaya, Miida, Ando, Yasumo, Hara, Uchiyama, Iwabuchi, Takasaki, Manabe and Yamoto144).
Disease related models include those of ataxia-telangiectasia(Reference Erker, Schubert, Elchuri, Huang, Tarin, Mueller, Zielen, Epstein and Wynshaw-Boris145), Alzheimer's(Reference Ashe146) and Parkinson's(Reference Hamaue, Ogata, Terado, Ohno, Kikuchi, Sasaki, Tashiro, Hirafuji and Minami147) disease, ageing models(Reference Zha, Le, Higami, Shimokawa, Taguchi and Razzaque148). In addition, also rodent species have been developed which are deficient in specific genes encoding for repair of oxidative DNA damage such as Ogg1 (Reference Larsen, Kwon, Coin, Egly and Klungland149) and Myh (Reference Xie, Yang, Cunanan, Okamoto, Shibata, Pan, Barnes, Lindahl, McIlhatton, Fishel and Miller150).
Most human studies on dietary antioxidants are carried out with healthy volunteers. In this context it is notable that a number of parameters such as age, sex, body mass index, and seasonal variations were found to affect the redox status and should be taken into consideration in dietary studies(Reference Glasziou and Sanders151). In the last years, a number of antioxidant studies has been conducted in which oxidative stress was induced by physical exercise(Reference Hartmann, Niess, Grunert-Fuchs, Poch and Speit152, Reference Sacheck, Milbury, Cannon, Roubenoff and Blumberg153) or hyperbaric treatment(Reference Dennog, Radermacher, Barnett and Speit154) and also with patients with ROS related diseases such as diabetes(Reference Astley, Langrish-Smith, Southon and Sampson155–Reference Sampson, Astley, Richardson, Willis, Davies, Hughes and Southon157), HIV(Reference Jaruga, Jaruga, Gackowski, Olczak, Halota, Pawlowska and Olinski158), atherosclerosis and coronary artery diseases(Reference Soccio, Toniato, Evangelista, Carluccio and De Caterina159), cancer(Reference Hercberg, Czernichow and Galan160, Reference Flora161), uremia(Reference Kan, Undeger, Bali and Basaran162) or systemic lupus erythematosus(Reference Evans, Cooke, Akil, Samanta and Lunec163).
Conventional methods used for the detection of oxidative stress or for the identification of antioxidative dietary components
This part of the review will focus on conventional methods used to describe oxidative stress, and will be divided into five parts namely (1) physics based approaches, (2) methods used for the determination of the antioxidant compounds; (3) biochemical methods used to monitor the oxidation of macromolecules and their oxidation products, (4) approaches for the detection of ROS induced DNA-damage and (5) methods used to measure antioxidant enzymes and transcriptional factors.
Trapping of reactive species
The only technique that can measure free radicals directly and specify them is electron spin resonance (ESR), because it detects the presence of unpaired electrons. However, ESR can only be used to monitor only fairly unreactive radicals, since reactive ones do not accumulate at high-enough levels. One solution to this problem is to add ‘traps’ or ‘probes’, i.e. agents that intercept reactive radicals reacting with them to form a stable radical that can be detected by ESR. Whole-body ESR techniques are being used with rodents(Reference Berliner, Khramtsov, Fujii and Clanton164) but are currently not applicable to humans due to the lack of human safety data on the probes. A wide range of traps is available for use in animals and cell culture systems, not only N-tertbutyl-p-phenylnitrone (PBN) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO)(Reference Khan, Wilmot, Rosen, Demidenko, Sun, Joseph, O'Hara, Kalyanaraman and Swartz165) which were frequently used, but also “newcomers” as 1,1,3-trimethyl-isoindole N-oxide (TMINO)(Reference Bottle, Hanson and Micallef166), 5,5-diethylcarbonyl-1-pyrroline N-oxide (DECPO)(Reference Karoui, Clément, Rockenbauer, Siri and Tordo167), N-2-(2-ethoxycarbonyl-propyl)-a-phenyl-nitrone (EPPN)(Reference Stolze, Udilova, Rosenau, Hofinger and Nohl168), 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO)(Reference Khan, Wilmot, Rosen, Demidenko, Sun, Joseph, O'Hara, Kalyanaraman and Swartz165) and 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO)(Reference Zhao, Joseph, Zhang, Karoui and Kalyanaraman169). A generally underestimated problem is, that the reaction products giving an ESR signal, can be rapidly removed in vivo and in cultured cells, both by enzymic metabolism and by direct reduction by agents such as ascorbate(Reference Halliwell and Whiteman170).
Approaches to determine the total antioxidant capacity
Two main approaches have been developed to evaluate the antioxidant capacity in foods and human material (in particular plasma and LDL). The first measures the ability of a substance to transfer one electron to reduce compounds like radicals, carbonyls or metals. The most popular tests which belong to this category are the ferric iron reducing antioxidant parameter (FRAP), the Trolox equivalent antioxidant capacity (TEAC), and the diphenyl-1-picrylhydrazyl test (DPPH). Methods which fall into the second category are based on their ability to quench free radicals by hydrogen donation. Some scientists believe that these reactions are similar to the reaction mechanisms of antioxidants(Reference Aruoma171). The most popular methods are the oxygen radical absorbance capacity test (ORAC), the total radical trapping antioxidant parameter (TRAP), the total oxidant scavenging capacity (TOSC) method (all measuring effects in the hydrophilic compartment of the plasma) and the inhibition of linoleic acid and LDL oxidation.
Free radical quenching methods
The ORAC assay, which is based on the work of Ghiselli et al. (Reference Ghiselli, Serafini, Maiani, Azzini and Ferro-Luzzi172), Glazer(Reference Glazer173) and Cao et al. (Reference Cao, Alessio and Cutler174) measures the antioxidant inhibition of ROO∙ induced oxidations. Therefore, it reflects the classical H donating ability of antioxidants in the hydrophilic compartment. The peroxyl-radical reacts with a fluorescent probe thereby forming a non-fluorescent product which can be quantitated by following the fluorescence over time. In earlier studies, β-phycoerythrin was used as the fluorescent agent emitting in the visible region (Exc 495 nm, Em 595 nm), but due to shortcomings and inconsistencies of the results, fluorescein or dichlorofluorescein are currently used, since they are less reactive and more stable(Reference Prior, Wu and Schaich175). The antioxidative activity can be expressed as the lag time or the net integrated area under the fluorescence curve (AUC). ORAC values are reported as Trolox equivalents. Originally, the ORAC assay was limited to the measurement of hydrophilic chain breaking antioxidant capacity. A newer protocol, in which lipophilic and hydrophilic compounds are selectively separated by extraction, allows now also the quantification of lipophilic antioxidants using a mixture of acetone and water(Reference Huang, Ou, Hampsch-Woodill, Flanagan and Deemer176). The advantage of the ORAC assay is that it can be automated. Convincing results have been obtained with 48 or 96 well plates coupled with a microplate reader(Reference Huang, Ou, Hampsch-Woodill, Flanagan and Prior177). One important parameter is the temperature control (37°C), as small temperature differences decrease the reproducibility of the test(Reference Lussignoli, Fraccaroli, Andrioli, Brocco and Bellavite178). A principal drawback of the test is, that the effect of oxidation of the photoreceptor of the protein used does not necessarily reflect protection against oxidative damage of the protein itself(Reference Frankel and Meyer179).
The TRAP assay, proposed by Wayner et al. (Reference Wayner, Burton, Ingold and Locke180) is based on the use of 2,2′-azobis(2,4-amidinopropane)dihydrochloride (AAPH), a hydrophilic azo-compound which generates peroxyl-radicals. AAPH decomposes at 37°C spontaneously with a known rate. Various substances like 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), R-phycoerythrin or 2′-7′-dichlorofluorescin (DCFH)(Reference DeLange and Glazer181–Reference Rota, Chignell and Mason183) have been used as oxidizable agents. A comprehensive review on the different modifications has been published by Ghiselli et al. (Reference Ghiselli, Serafini, Natella and Scaccini184). The basic reactions of the procedure are similar to those of the ORAC assay. The probe reacts with ROO∙ radicals at low concentrations with a significant spectroscopic change in between the native and the oxidized sample and no radical chain reaction beyond sample oxidation should occur. The antioxidant capacity is determined as the time required to consume all antioxidants by extension of the lag time for appearance of the oxidized probe when antioxidants are present. TRAP values are usually expressed as the lag time of the sample compared to the corresponding times for Trolox. The test is relatively complex to perform, requires experience and is time-consuming. The use of the lag phase is based on the assumption that all antioxidants show a lag phase and that the lag phase corresponds to the antioxidative capacity.
One drawback of TRAP and ORAC is the interference of proteins which contribute by ⩾80 % to the total antioxidant capacity(Reference Prior, Wu and Schaich175, Reference Ghiselli, Serafini, Natella and Scaccini184). Therefore, Trolox can be used as an internal standard or the samples must be deproteinized prior to the measurements.
The TOSC assay was initially used for environment related studies on marine organisms(Reference Winston, Regoli, Dugas, Fong and Blanchard185, Reference Regoli and Winston186). It is based on the inhibition of the radical-dependent formation of ethylene from ketomethiolbutyric acid by antioxidants. This procedure permits testing against three different ROS species (i.e. peroxyl-, hydroxyl-radicals as well as peroxynitrite) with physiological relevance and different reactivities. It can be conducted at physiological temperature and pH; non-linear concentration-dependent activity variations can be examined easily and different types of antioxidant reactions (retardant or fast-acting) can be distinguished. However, high throughput analyses are not possible and multiple injections of each sample are required in order to observe ethylene formation. Further limitations are the multiple endpoints of calculated 20, 50 and 80 % TOSC and the DT50 (first derivative of TOSC of 50 %) since it was shown that there is no linear relationship between the different multiple endpoints(Reference Lichtenthaler and Marx187).
The chemiluminescence assay (CL) is a modification of the TRAP assay. Radical formation is followed by CL or photo-CL (PCL). CL is characterized by low emission intensity and by the fact that reactions with oxidants emit CL. The most widely used marker is luminol(Reference Lundqvist, Kricka, Stott, Thorpe and Dahlgren188, Reference Whitehead, Thorpe and Maxwell189), but also biuminescent proteins like pholasin are becoming popular(Reference Witko-Sarsat, Nguyen, Knight and Descamps-Latscha190–Reference Glebska and Koppenol192). The antioxidant capacity is the time of depressed light emission, which is measured at 10 % recovery of light output.
Recently Popov et al. (Reference Popov, Völker and Lewin193) have described the PCL, a commercial test system termed PHOTOCHEM for the determination of the integral antioxidative capacity towards O2(Reference Popov, Völker and Lewin193). In a strict sense, the method measures antiradical capacity. In contrast to many other assays used to determine AOC, this procedure requires no standardisation of the pH and of the temperature. However, to date, the system is only marketed by one company (Analytic Jena, Germany) and reagents for the hydrophilic and lipophilic assays are only available from the manufacturer; furthermore, a high throughput is not possible. Ascorbic acid is normally used for the determination of hydrophilic and Trolox for the lipophilic antioxidative capacity.
Low-density lipoprotein (LDL) oxidation is based on the autoxidation of linoleic acid or LDL which is induced in vitro mainly by Cu2+ or some other azo-initiators(Reference Esterbauer, Jurgens, Quehenberger and Koller194–Reference Rice-Evans, Leake, Bruckdorfer and Diplock196). LDL-oxidation is of higher physiological relevance when tested under in vivo conditions and not ex vivo. The oxidation is monitored at 234 nm for conjugated dienes or by peroxide values for lipid hydroperoxides. LDL has to be freshly isolated from blood which is a time- and material-consuming procedure which requires ultracentrifugation. During the preparation, low temperature and light protection are essential(Reference Wagner, Tomasch and Elmadfa197). Further, conjugated dienes can be formed in presence of polyunsaturated fatty acids.
Recently, fluorescence and UV based ELISA assays with plasma became available, for which no complicated and time consuming LDL isolation is needed. This methods can also be used for larger human trials. Also the procedure developed by Holvoet et al. (Reference Holvoet, Donck, Landeloos, Brouwers, Luijtens, Arnout, Lesaffre, Vanrenterghem and Collen198) who measured oxidized LDL levels by a competitive ELISA utilizing a specific murine monoclonal antibody (mAb-4E6) based on UV is employed quite often at present(Reference Holvoet, Donck, Landeloos, Brouwers, Luijtens, Arnout, Lesaffre, Vanrenterghem and Collen198). The AOC is determined in all these experiments either as AUC or as the lag time until the antioxidants are consumed. An important modification was developed by Frankel et al. (Reference Frankel, German and Davis199) who determined the secondary oxidation product hexanal from LDL. Hexanal was chosen, since it is the major oxidation product of n-6 fatty acids and is monitored with head space gas chromatography, the percentage inhibition of hexanal formation is used as a parameter for AOC. In many ex vivo studies LDL was isolated, subsequently the substances were added and tested on their ability to delay oxidation. This scenario does not reflect in vivo conditions. Furthermore, not all oxidation inducers which are used ex vivo can be used for in vivo testing(Reference Frankel and Meyer179).
The Crocin bleaching assay monitors the protection of AAPH-induced crocin bleaching, by antioxidants(Reference Bors, Michel and Saran200). Crocin is a mix of natural pigments and absorbs, similar to carotenoids, at 450 nm. Therefore, the interpretation of the results can be complicated in experiments with food samples. Initially, the test was used for the analysis of plasma samples(Reference Tubaro, Ghiselli, Rapuzzi, Maiorino and Ursini201). One of its limitations is that crocin is not commercially available, but high sample throughput with microplates is possible.
Single electron transfer methods
In these assays, the sample itself is an oxidant that abstracts an electron from the antioxidant, thereby causing colour changes which are proportional to the AOC. When the change of absorbance is plotted against the antioxidant concentration, the slope of the curve reflects the total reducing capacity. In contrast to the methods described in the last chapter, no oxygen radicals are present in the system; therefore, it can be assumed that the reducing capacity is equal to the antioxidant capacity.
The TEAC-assay is a spectrophotometric test which was developed by Miller et al. (Reference Miller, Rice-Evans, Davies, Gopinathan and Milner202). 2,2-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) is oxidized by ROO∙ to a green-blue radical cation. The ability of antioxidants to delay colour formation is expressed relative to Trolox. Originally, the test used metmyoglobin and H2O2, and the ABTS radical was measured at 734 nm. Meanwhile, various modifications have been developed(Reference Re, Pellegrini, Proteggente, Pannala, Yang and Rice-Evans203, Reference Van Den Berg, Haenen, Van Den Berg and Bast204). After generation of the ABTS radical, the sample to be tested is added, subsequently other chemicals like manganese dioxide, ABAP, potassium persulfate or enzymes are used to generate the ABTS radical(Reference Prior, Wu and Schaich175, Reference Miller, Rice-Evans, Davies, Gopinathan and Milner202, Reference Re, Pellegrini, Proteggente, Pannala, Yang and Rice-Evans203, Reference Miller, Sampson, Candeias, Bramley and Rice-Evans205). Temperatures higher than 37°C, which are not physiological and different absorption maxima (415, 645 734 or 815 nm) are frequently used. Depending on the protocol, the decrease or increase in ABTS radical absorbance in presence of the test sample or Trolox at a fixed time point is measured and the antioxidant capacity is calculated as Trolox equivalents. ABTS is not a physiological substance. It reacts fast with aqueous and organic solvents and substances with a low redox potential show a good response. Therefore, phenolic compounds or ascorbic acid react quite well with ABTS whereas lipophilic compounds respond more weakly. The test can be adapted to microplates and is not restricted to a narrow pH range, but high haemoglobin concentration in the plasma may interfere with the measurements.
The FRAP assay determines the reduction of 2,4,6-tripyridyl-s-triazine (TPTZ) in plasma to a coloured product(Reference Benzie and Strain206) and has also been adapted for food samples(Reference Benzie and Szeto207, Reference Ou, Huang, Hampsch-Woodill, Flanagan and Deemer208). Similar to the TEAC assay, compounds with a redox potential < 0·7 V are detected. FRAP not enable to monitor compounds that quench radicals like proteins or thiol compounds such as glutathione (similar to all the “reducing assays”) and can therefore underestimate the AOC. In order to maintain the solubility of Fe, the assay is conducted at acidic conditions (pH of 3·6). Most redox reactions take place within a few minutes, therefore both tests consider most of the substance effects (like polyphenols) but not all, since some substances have longer reaction times(Reference Prior, Wu and Schaich175). This was recently shown for polyphenols like caffeic-, tannic-, ferulic- or ascorbic acids, where the absorption steadily increased for hours(Reference Pulido, Bravo and Saura-Calixto209). Since the test reflects only the reducing potential and does not consider H transfer, it should only be considered in combination with other methods to give a more complete picture. Similar to the TEAC, it is a relatively easy test procedure, can be done manually or fully automated and requires no expensive equipment.
The copper reducing assay (CUPRAC, AOP-90) is a modification of the FRAP in which iron is replaced by Cu; Cu2+ is reduced to Cu1+(Reference Apak, Guclu, Ozyurek and Karademir210). The assay is conducted at 490 nm with bathocuprine, or at 450 nm with neocuproine. Results are expressed as uric acid equivalents. Since copper has a lower redox potential than iron, but enhances the redox cycling potential, its pro-oxidative potential can be more sensitive(Reference Prior, Wu and Schaich175). The limitations regarding the underestimation of slower reactive molecules is similar to the other assays but one of it advantages is that almost every antioxidant including thiols can be detected(Reference Prior, Wu and Schaich175).
The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical used in the DPPH-assay is stable, deep purple, commercially available and has not to be generated. The test was developed by Brand-Williams et al. (Reference Brand-Williams, Cuvelier and Berset211). The loss of DPPH colour at 515 nm after reaction with test compounds is measured either by decrease of absorbance or by electron spin resonance(Reference Bondet, Brand-Williams and Berset212). The concentration of a 50 % decrease of the DPPH radical is defined as EC50, the time to reach it as TEC50. Since the DPPH assay uses a wavelength of 515 nm, it can interfere with substances with a similar spectrum like carotenoids(Reference Nomura, Kikuchi, Kubodera and Kawakami213). The test considers both, electron transfer as well as hydrogen (H) transfer reactions with the focus on the prior. Again, smaller molecules have better access to the radical site and contribute to a higher extent to the AOC(Reference Prior, Wu and Schaich175). The test procedure itself is quite simple and fast and requires only a spectrophotometric device.
For all the electron transfer tests it can be assumed that they “overestimate” smaller molecules and hydrophilic substances like ascorbic acid, uric acid or polyphenols and reflects not always the situation in the organism(Reference Huang, Ou and Prior214).
From the evaluation of the different assays it becomes clear that no single test reflects the overall antioxidative capacity of antioxidants. Both hydrophilic and lipophilic activities must be considered, as well as H transfer and single electron transfer mechanisms and additional tests which reflects the inactivation of various reactive oxygen/nitrogen species are needed to fully estimate the AOC (Table 1).
Table 1 Comparison of methods used to determine the total antioxidant capacity (TAC)
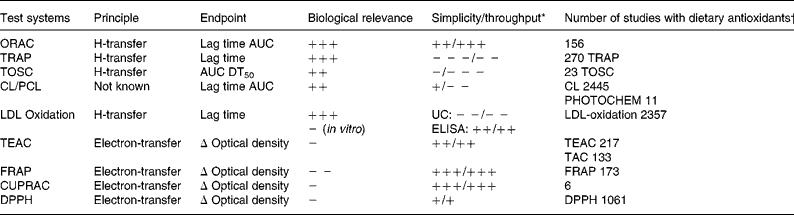
CL, chemiluminescence assay; CUPRAC, cupric reducing antioxidant capacity; DPPH, 2,2-diphenyl-2-picrylhydrazyl assay; ELISA, enzyme-linked immunosorbent assay; FRAP, ferric reducing ability of plasma; LDL, low density lipoprotein; ORAC, oxygen radical absorbance capacity test; PCL, photo-chemiluminescence assay; TEAC, trolox equivalent antioxidative capacity; TOSC, total oxidant scavenging capacity; TRAP, total radical trapping antioxidant parameter.
* +,++,+++, positive; − , − − , − − − , negative; UC, ultracentrifugation.
† Number of antioxidant studies conducted with the different methods identified by use of a computer aided search (Scopus database).
Oxidation of macromolecules
Biomarkers of lipid oxidation
Cell membranes are highly susceptible to LP due to their specific composition which is charactesized by high amounts of polyunsaturated fatty acids (PUFAs)(Reference Spiteller215). LP oxidation is a chain reaction (see above) and leads to structural and functional damage of membranes as well as to the formation of lipid hydroperoxides which are unstable and degrade to various secondary oxidation products.
The formation of malondialdehyde (MDA) is the most widely used parameter of PUFA peroxidation in the thiobarbituric acid-reacting substances (TBARS) assay. One of the oldest and still most widely used methods is based on the precipitation of protein nearly at boiling conditions(Reference Yagi216). The samples are heated for 1 h with TBA at low pH and the pink chromogen formed absorbs at 532 nm. The sample preparation has been criticized since it is far from physiological conditions and not the free MDA in the original sample is measured but the amount generated by decomposition of lipid peroxides during heating(Reference Gutteridge217). Furthermore, it is known that also other compounds like sugars, amino acids or bilirubin are able to react with TBA(Reference Meagher and Fitzgerald218). The sensitivity of the test can be increased by combining it with HPLC to separate such compounds before acidic heating(Reference Wong, Knight, Hopfer, Zaharia, Leach and Sunderman219).
In the last few years, several innovations have been introduced to improve the specificity of the test and to reduce known bias. In particular the temperature at the deprotenization step has been reduced to physiological conditions. In addition, several methods have been developed which do not require derivatization(Reference Karatas, Karatepe and Baysar220, Reference Wilson, Metz, Graver and Rao221) or new derivatization agents like 2,4-dinitrophenylhydrazine(Reference Sim, Salonikas, Naidoo and Wilcken222) or diaminonaphthalene(Reference Steghens, Van Kappel, Denis and Collombel223). Very recently, GC/MS based methods have been developed which possess high sensitivity showing an overestimation of MDA levels(Reference Cighetti, Allevi, Anastasia, Bortone and Paroni224, Reference Stalikas and Konidari225). Although it is questionable whether MDA measurements are a reliable method for LP, it is well documented by a large number of studies that increased levels are found in patients with ROS related diseases(Reference Del Rio, Stewart and Pellegrini226).
The first step of PUFA oxidation is the conjugation of double bonds leading to formation of conjugated dienes (CD) which absorb at around 234 nm. They can either be absorbed by lipids but also in plasma samples. The plasma preparation is more physiological and requires no heat treatment. In plasma samples, CD are usually analysed with HPLC–UV detection(Reference Ramel, Wagner and Elmadfa227). Determination of the diene levels cannot be used alone to describe oxidative stress, but when measured with HPLC–CD, the findings can support results obtained with other more reliable parameters of oxidative stress. Although MDA and CD are both primary oxidation products, they can develop differently in the same sample due to different mechanisms of formation(Reference Konig, Wagner, Elmadfa and Berg228).
Isoprostanes are stable oxidation products from arachidonic acid, initially formed from phospholipids and released into circulation before the hydrolyzed form is excreted in urine(Reference Morrow, Hill, Burk, Nammour, Badr and Roberts229). A large number of endproducts can theoretically be generated but interest has focused mainly on F2α-isoprostanes(Reference Milne, Musiek and Morrow230); the must promising marker for oxidative stress/injury being 8-prostaglandin F2α(Reference Roberts and Morrow231). At present it is regarded as one of the most reliable markers of oxidative stress, although the presence of detectable concentrations of isoprostanes in biological fluids requires continuous lipid peroxidation(Reference Young232). Several favourable characteristics make isoprostanes attractive as reliable markers for oxidative stress, i.e. they are specific oxidation products, stable, present in detectable quantities, increased strongly at in vivo oxidative stress, and their formation is modulated by antioxidants(Reference Roberts and Morrow231). Various approaches such as gas chromatography–mass spectrometry (GC–MS), GC–tandem MS, liquid chromatography–tandem MS and immunoassays are available for the detection of F2α-isoprostanes(Reference Schwedhelm and Boger233). The first results were produced by use of the MS technique, and various isoprostanes can be separated with this method(Reference Schwedhelm and Boger233). Recently, immunoassays have been developed which correlate apparently quite well with the results obtained with GC–MS measurements in urine but some discrepancies might occur with plasma samples when they were not tested on cross reactivity with other prostaglandin metabolites(Reference Young232). Nevertheless, their use might be appropriate in intervention studies with various blood samplings from the same subject, thereby focusing not on absolute levels but on relative changes.
The measurement of breath hydrocarbons is a non-invasive method which allows to determine LP through exhaled breath by measuring trace volatile hydrocarbons(Reference Frank, Hintze, Bimboes and Remmer234). Ethane formation results from n-3 oxidation, pentane formation is caused by n-6 oxidation. Although the data reported on their consistency to describe LP are quite convincing, the limiting factor is their detection. They are mainly employed with GC–FID, but one concern is the background level in the breath since bacteria were shown to produce significant amounts of theses hydrocarbons in vivo (Reference Romero, Bosch-Morell, Romero, Jareno, Romero, Marin and Roma235). Furthermore, the separation of different hydrocarbons is not easy due to similar boiling points(Reference Risby and Sehnert236).
Aldehydes represent stable products of PUFA oxidation. 4-Hydroxynonenal (4-HNE) and hexanal are mainly formed by n-6 fatty acid oxidation, while propanol and 4-hydroxyhexenal result from n-3 fatty acid oxidation(Reference Frankel, German and Davis199, Reference Esterbauer, Schaur and Zollner237, Reference Benedetti, Comporti and Esterbauer238). High concentrations of 4-HNE have been shown to trigger well-known toxic pathways such as induction of caspases, the laddering of genomic DNA, and release of cytochrome c from mitochondria, which may lead to cell death(Reference Cahuana, Tejedo, Jimenez, Ramirez, Sobrino and Bedoya239). The most frequently used methods for determination of the aldehydes are GC–MS, GC–head space or HPLC(Reference Holley, Walker, Cheeseman and Slater240). Also polyclonal or monoclonal antibodies directed against 4-HNE-protein conjugates are now frequently used for 4-HNE measurements(Reference Sharma, Brown, Awasthi, Yang, Sharma, Patrick, Saini, Singh, Zimniak, Singh and Awasthi241).
Biomarkers of protein oxidation
Markers of protein oxidation are less frequently used than lipid oxidation parameters (Table 2). They are mainly applied in combination with LP markers, although their formation has been associated with several diseases(Reference Agarwal242).
Table 2 Main biomarkers for lipid and protein oxidation

AOPP, advanced oxidation protein products; CD, conjugated dienes; GC/MS, gas chromatography–mass spectrometry; ELISA, enzyme-linked immunosorbent assay; 4-HNE, 4-hydroxynonenal; HPLC, high-performance liquid chromatography; MDA, malondialdehyde; TBARS, thiobarbituric acid reactive substances. Distribution of the total studies to in vitro (iv), animal (an) and human (hu) studies: iv%, percentage in vitro; an%, percentage animal studies; %hu, percentage human studies.
* The number of antioxidant studies conducted with the different methods was assessed by use of a computer aided search (Scopus database).
Formation of protein bound carbonyls is most abundant endpoint used to monitor protein oxidation(Reference Winterbourn and Buss243, Reference Levine, Wehr, Williams, Stadtman and Shacter244) by a conventional colorimetric assay using 2,4-dinitrophenylhdrazine(Reference Levine, Garland, Oliver, Amici, Climent, Lenz, Ahn, Shaltiel and Stadtman245). The test is easy to perform, but large quantities of solvents are required. Recently, an ELISA method has been developed and the results correlated well with the spectrophotometric method(Reference Alamdari, Kostidou, Paletas, Sarigianni, Konstas, Karapiperidou and Koliakos246).
Advanced oxidation protein products (AOPP) are predominantly albumin and its aggregates damaged by oxidative stress(Reference Witko-Sarsat, Friedlander, Capeillere-Blandin, Nguyen-Khoa, Nguyen, Zingraff, Jungers and Descamps-Latscha247). They contain abundantly dityrosines which cause crosslinking, disulfide bridges and carbonyl groups and are formed mainly by chlorinated oxidants such as hypochloric acid and chloramines resulting from myeloperoxidase activity(Reference Capeillere-Blandin, Gausson, Descamps-Latscha and Witko-Sarsat248). AOPP have several similar characteristics as advanced glycation endproducts-modified proteins. Induction of proinflammatory activities, adhesive molecules and cytokines is even more intensive than that caused by advanced glycation end products (AGEs). They are referred to as markers of oxidative stress as well as markers of neutrophil activation(Reference Witko-Sarsat, Friedlander, Khoa, Capeillére-Blandin, Nguyen, Canteloup, Dayer, Jungers, Drüeke and Descamps-Latscha249). Protein oxidation products mediated by chlorinated species (HOCl) generated by the enzyme myeloperoxidase were found in the extracellular matrix of human atherosclerotic plaques and increased levels of advanced oxidation protein products were postulated to be an independent risk factor for coronary artery disease(Reference Kaneda, Taguchi, Ogasawara, Aizawa and Ohno250, Reference Kalousova, Zima, Tesar, Dusilova-Sulkova and Skrha251). AOPPs are expressed as chloramine-T equivalents by measuring absorbance in acidic conditions at 340 nm in presence of potassium iodide. The test is easy to perform and can be carried out with microprobes.
Markers of oxidative DNA damage used in studies with dietary antioxidants
During the last fifty years, a broad variety of genotoxicity test procedures have been developed which are used for routine testing of chemicals, in environmental research and also in human studies concerning the impact of occupational exposure, lifestyle factors and nutrition on DNA-integrity. Due to the conservative structure of the genetic material, mutagenicity experiments can be carried out with a broad variety of indicator organisms including bacteria, yeasts, plants, invertebrates including Drosophila, laboratory rodents and also with cultured mammalian cells(Reference Kilbey, Legator, Nichols and Ramel252–Reference Fahrig254). The advantages and limitations of the different methods for the detection of DNA-protective dietary factors have been described by Knasmüller and coworkers(Reference Knasmüller, Steinkellner, Majer, Nobis, Scharf and Kassie255–Reference Hoelzl, Bichler, Ferk, Simic, Nersesyan, Elbling, Ehrlich, Chakraborty and Knasmuller257). One of the main problems (which is encountered also relevant for studies of the effects of dietary antioxidants) encountered in experiments with mammalian cells and lower organisms concerns the inadequate representation of the metabolism of the test compounds which may lead to results which cannot be extrapolated to humans. Nevertheless, all these methods have been used to study oxidative DNA-damage and to investigate putative protective effects of phytochemicals. At present, the most widely used endpoints are gene mutation assays with bacteria and mammalian cells (Salmonella typhimurium/microsome test, HPRT gene mutation assay), chromosome analyses in metaphase cells which can be conducted with stable cell lines (e.g. CHO, V79) and lymphocytes in vitro but can be also scored in in vivo and ex vivo experiments with blood cells. Another important endpoint are micronuclei which are formed as a consequence of chromosome breakage (clastogenicity) and aneuploidy and are less time consuming to evaluate as chromosomal aberrations(Reference Knasmüller, Majer, Buchmann, Remacle and Reusens256, Reference Fenech258). The most frequently used approaches to monitor antioxidant effects of dietary factors are described in the subsequent chapters.
The most widely used bacterial mutagenicity test procedure is the Salmonella/microsome assay, which has been developed by B. Ames in the 1970s(Reference Ames, Sutton and Harris259). The test is based on the detection of back mutations in specific genes which encode for histidine biosynthesis. One of the disadvantages of the initial set of tester strains was, that none of them was highly sensitive towards oxidative effects, therefore new polyplasmid strains (TA102 and TA104) were constructed which are in particular suitable for the detection of mutagenic effects caused by ROS(Reference Levin, Hollstein, Christman, Schwiers and Ames260). Since the target gene is located in these strains (in contrast to the classical tester strains) on plasmids, it can be easily lost and due to the high spontaneous reversion rates many groups encountered difficulties with these derivatives. Nevertheless, the Salmonella strains are at present widely used in antioxidant experiments; mutations are induced either by radiation or chemically and putative protective compounds or complex mixtures are plated on histidine free selective media plates together with the indicator bacteria. After incubation, the differences in the numbers of his+ revertants serves as an indication of protective effects. The test procedure has been standardised for routine testing of chemicals (see for example OECD guideline 471(261)) and the criteria which have been defined (sufficient number of plates, inclusion of positive and negative controls, testing of different doses) can also be applied for the detection of antioxidants. One of the problems which affects the reliability of the test results, concerns the fact that false positive effects may be obtained with compounds which cause bactericidal or bacteriostatic effects since these parameters are not monitored under standard conditions. A typical example are the protective effects seen with cinnamaldehyde(Reference Rutten and Gocke262). With complex dietary mixtures, difficulties may be encountered due to their histidine contents(Reference Wurgler, Friederich, Furer and Ganss263).
Apart from the Salmonella/microsome assay, also a number of other bacterial genotoxicity tests have been developed which are less frequently used, e.g. assays, based on the scoring of backward or forward mutations with E. coli strains, tests based on the induction of repair processes, such as umu and SOS chromotest or differential DNA-repair assays based on the comparison of the survival of strains differing in their DNA-repair capacity(Reference Knasmüller, Majer, Buchmann, Remacle and Reusens256).
Single cell gel electrophoresis (SCGE) assays are based on the determination of DNA migration in an electric field which leads to formation of COMET shaped images. In the initial version, the experiments were carried out under neutral conditions which allowed only the detection of double strand breaks(Reference Ostling and Johanson264). Subsequently, Tice et al. (Reference Tice, Agurell, Anderson, Burlinson, Hartmann, Kobayashi, Miyamae, Rojas, Ryu and Sasaki265) and Singh et al. (Reference Singh, McCoy, Tice and Schneider266) developed protocols for experiments in which the cells are lysed under alkaline conditions (pH>13), which enables the additional detection of single strand breaks and apurinic sites. One of the main advantages of this test procedure is, that it does not require cell divisions (which are a prerequisite for gene mutation and micronucleus experiments), therefore only short incubation periods are required so that not only stable cell lines can be used for in vitro studies but additionally also primary cells from different organs. Furthermore, it is also possible to carry out in vivo experiments with rodents and to study effects in a broad variety of tissues(Reference Sasaki, Sekihashi, Izumiyama, Nishidate, Saga, Ishida and Tsuda267). In most human studies, peripheral lymphocytes have been used as target cells, few experiments were conducted with exfoliated epithelial cells(Reference Dhillon, Thomas and Fenech268–Reference Szeto, Benzie, Collins, Choi, Cheng, Yow and Tse271) which are problematic due to their low viability. Very recently, also results from a human intervention trail with antioxidants were reported in which bioptic material from the colon was analysed(Reference DeMarini272).
Collins et al. developed in the 1990s(Reference Collins, Duthie and Dobson273) a protocol in which isolated nuclei are treated with lesion specific enzymes (endonculease III, formadidopyrimidine glycosylase, FPG). This approach has been used intensely to study the prevention of endogenous formation of oxidised purines and pyrimidines. However, it is crucial in these experiments to determine the optimal amounts of the enzymes due to their instability. More recently, Collins and coworkers published an additional modified version of the SCGE test which enables to monitor the impact of compounds on the repair capacity of the cells(Reference Collins and Horvathova274, Reference Collins275) and it was shown in a few model studies that antioxidants may alter DNA-repair processes(Reference Chakraborty, Roy and Bhattacharya276, Reference Collins, Horska, Hotten, Riddoch and Collins277). In order to obtain information concerning alterations of the sensitivity towards exogenous ROS mediated damage, it is possible to treat the cells with H2O2 other radical generating chemicals or radiation (ROS-challenge).
In the guidelines published by Tice et al. (Reference Tice, Agurell, Anderson, Burlinson, Hartmann, Kobayashi, Miyamae, Rojas, Ryu and Sasaki265) and Hartmann et al. (Reference Hartmann, Agurell, Beevers, Brendler-Schwaab, Burlinson, Clay, Collins, Smith, Speit, Thybaud and Tice278), a number of criteria are defined which are essential to obtain reliable results. They concern for example the number of parallel cultures and cells which are required in in vitro studies, the number of animals and treatment periods and also adequate statistical methods. It was generally agreed, that different parameters such as tail lengths of the comets, as well as percentage DNA in tail and tail moment are acceptable and that apart from automated image analysis systems also manual scoring methods are acceptable(Reference Burlinson, Tice, Speit, Agurell, Brendler-Schwaab, Collins, Escobar, Honma, Kumaravel, Nakajima, Sasaki, Thybaud, Uno, Vasquez and Hartmann279). An important point which is also relevant for antioxidant studies concerns the fact that multiple doses should be tested to substantiate effects and that it is necessary to monitor acute toxic effects. It is well documented that cell death leads to degradation of DNA, therefore it is essential to determine the viability of the cells after treatment with appropriate methods in in vitro experiments and to monitor toxic effects in inner organs by histopathology(Reference Burlinson, Tice, Speit, Agurell, Brendler-Schwaab, Collins, Escobar, Honma, Kumaravel, Nakajima, Sasaki, Thybaud, Uno, Vasquez and Hartmann279) and exclude conditions under which strong acute effects are observed in animal experiments. These criteria are fulfilled in most recent studies, but not in a number of older investigations.
The SGCE technique has been used in numerous in vitro investigations to study antioxidant effects of individual compounds and complex mixtures in stable cell lines and in human lymphocytes. Typical examples are investigations of flavonoids by Anderson and coworkers with blood and sperm cells(Reference Anderson, Yu, Phillips and Schmezer280, Reference Anderson, Basaran, Dobrzynska, Basaran and Yu281), experiments concerning antioxidant effects of coffee and its constituents(Reference Bichler, Cavin, Simic, Chakraborty, Ferk, Hoelzl, Schulte-Hermann, Kundi, Haidinger, Angelis and Knasmuller282), studies on the protective effects of tea catechins(Reference Elbling, Weiss, Teufelhofer, Uhl, Knasmueller, Schulte-Hermann, Berger and Micksche283, Reference Kundu, Bhattacharya, Siddiqi and Roy284) to name only a few. Also quite a substantial number of in vivo studies with laboratory animals have been published, e.g. with carotenoids(Reference Vanitha, Murthy, Kumar, Sakthivelu, Veigas, Saibaba and Ravishankar285), quercetin(Reference Kapiszewska, Cierniak, Papiez, Pietrzycka, Stepniewski and Lomnicki286), vitamins E and C(Reference Gabbianelli, Nasuti, Falcioni and Cantalamessa287) and garlic oil(Reference Liu and Xu288).
The results of human studies are described in the reviews of Moller & Loft(Reference Moller and Loft289–Reference Moller and Loft291). Most of them were conducted as intervention trails which have the advantage that inter-individual variations can be reduced. In total, 76 investigations have been published since the first trial was conducted by Pool-Zobel et al. in 1997(Reference Pool-Zobel, Bub, Muller, Wollowski and Rechkemmer292). Evidence for antioxidant effects was observed in approximately 64 % of the studies (25/39); the highest number of protective effects concerned the prevention of endogenous formation of oxidised pyrimidines, (39 % of the trials, 7/19), while in few studies evidence for reduced sensitivity towards ROS and protection against formation of oxidised purines was found. The lowest rate of protective effects was seen when the SCGE analyses were carried out under standard conditions (i.e. without ROS challenge and enzymes). Clear evidence for protective effects was observed for example in experiments with lycopene and tomatoes(Reference Riso, Visioli, Erba, Testolin and Porrini293), cruciferous and leguminous sprouts(Reference Gill, Haldar, Porter, Matthews, Sullivan, Coulter, McGlynn and Rowland294) and carotenoid supplementation(Reference Zhao, Aldini, Johnson, Rasmussen, Kraemer, Woolf, Musaeus, Krinsky, Russell and Yeum295). In our own experiments we found strong protective properties of sumach (a common spice) and its main active principle gallic acid(Reference Ferk, Knasmüller, Dusinska, Brantner, Uhl, Chakraborty, Bronzetti, Ferguson and De Flora296), wheat sprouts(Reference Ehrlich, Hoelzl, Nersesyan, Winter, Koller, Ferk, Fenech, Dusinska, Knasmüller, Ďuračková, Slámenova and Micksche101), after consumption of coffee(Reference Bichler, Cavin, Simic, Chakraborty, Ferk, Hoelzl, Schulte-Hermann, Kundi, Haidinger, Angelis and Knasmuller282) and in experiments with Brussels sprouts(Reference Hoelzl, Glatt, Meinl, Sontag, Heidinger, Kundi, Simic, Chakraborty, Bichler, Ferk, Angelis, Nersesyan and Knasmüller297). The latter observation is of particular interest; since no effects were seen in a large intervention trial in which the participants consumed 600 g of fruits and vegetables/d(Reference Moller, Vogel, Pedersen, Dragsted, Sandstrom and Loft298) our findings may be taken as an indication that coffee intake may contribute to a higher extent to ROS protection than plant derived foods.
Moller & Loft (2004) discuss in their papers also the possible shortcomings of intervention trails and emphasize the importance of controlled study designs which should include a sufficient number of participants and additionally also either placebo groups or washout periods(Reference Moller and Loft290). These latter criteria are not fulfilled in some of the older studies.
Micronucleus (MN) experiments have nowadays largely replaced conventional chromosomal aberration (CA) analyses (which have been used extensively in the past to study protective effects of antioxidants towards radiation and chemically induced DNA-damage) as they are less time consuming and laborious. Both endpoints can be used in in vitro experiments with cell lines and lymphocytes; one of the most promising newer developments is the use of human derived liver cell lines (HepG2, HepG3 etc). HepG2 cells have retained the activities of drug metabolising enzymes including those which metabolise plant specific antioxidants and thus may reflect the effects in humans more adequately than cell lines which are commonly used in genetic toxicology (for reviews see(Reference Knasmüller, Mersch-Sundermann, Kevekordes, Darroudi, Huber, Hoelzl, Bichler and Majer299–Reference Mersch-Sundermann, Knasmüller, Wu, Darroudi and Kassie301)).
The most widely procedure used in animal experiments is the bone marrow MN assay. Since it is problematic to induce MN with chemical oxidants in vivo, most experiments were conducted with radiation. Standardised guidelines have been published for this test system, e.g. by OECD(302), and one of the most important parameters relevant for AO studies is the treatment period. It is also notable, that one of the main shortcomings of this test is due to the fact that reactive molecules may not reach the target cells; this explains why negative results were obtained with a number of potent genotoxic carcinogens, also antioxidant effects may not be detected due to this limitation. Typical examples for AO studies in which this assay was used are experiments with epigallocatechin gallate, vitamin C, lipoic acid, ubidecarenone or coenzyme Q(10)(Reference Hosseinimehr, Azadbakht, Mousavi, Mahmoudzadeh and Akhlaghpoor303–Reference Prahalathan, Selvakumar, Varalakshmi, Kumarasamy and Saravanan308).
The development of the cytokinesis block micronucleus (CBMN) method by Fenech et al. (Reference Fenech and Morley309, Reference Fenech310) allows to monitor MN formation in peripheral blood cells in vitro and can be also used in dietary human studies with lymphocytes. It is based on the use of the mitogen phytohaemagglutinin (which stimulates nuclear division), in combination with cytochalasin B (which stops cellular division), so that MN can be scored in binucleated cells. Typical examples for the use of this approach are supplementation studies with vitamin C and intervention trials with red wine(Reference Fenech, Stockley and Aitken311–Reference Greenrod, Stockley, Burcham, Abbey and Fenech313), in vitro experiments with wine specific phenolic compounds(Reference Greenrod and Fenech314) as well as tests with the antioxidant drugs amifostine and melatonin(Reference Kopjar, Miocic, Ramic, Milic and Viculin315).
More than 100 different oxidative modifications of DNA have been described in the literature(Reference Dizdaroglu316) and it is notable that oxidative adducts occur at a frequency of 1 or more orders of magnitude higher than non-oxidative adducts(Reference Ames, Shigenaga and Hagen317). The dominant oxidative modification of DNA is 8-hydroxylation of guanine, which leads to formation of 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodG). In most studies, this oxidation product was measured in urine and in leukocytes, but it is also possible to monitor it in various inner organs, e.g. in liver, lung and kidneys(Reference Chater, Abdelmelek, Douki, Garrel, Favier, Sakly and Ben Rhouma318–Reference Kim, Suzuki, Laxmi, Umemoto, Matsuda and Shibutani321). The first investigations were conducted in the late 1980s(Reference Shigenaga, Gimeno and Ames322) and a number of different methods have been developed namely gas chromatography coupled with mass spectrometry(Reference Dizdaroglu323), liquid chromatography prepurification followed by gas chromatography coupled with mass spectrometry(Reference Gackowski, Rozalski, Roszkowski, Jawien, Foksinski and Olinski324), liquid chromatography coupled with tandem mass spectrometry(Reference Cooke, Olinski and Evans325), high-performance liquid chromatography with electrochemical detection(Reference Kasai326–Reference Shigenaga, Aboujaoude, Chen and Ames328), high-performance liquid chromatography with mass spectrometry(Reference Harman, Liang, Tsitouras, Gucciardo, Heward, Reaven, Ping, Ahmed and Cutler329) and enzyme-linked immunosorbent assay based methods(Reference Chiou, Chang, Chan, Wu, Tsao and Wu330).
It is well known that guanine is easily prone to oxidation if stringent precautions are not taken during preparation of samples for analysis(Reference Hofer and Moller331), therefore much of what is measured could be artefacts. In order to prepare compounds for analysis by gas chromatography coupled with mass spectrometry which enables the simultaneous analysis of a number of different adducts(Reference Wiseman, Kaur and Halliwell332), DNA is chemically hydrolysed and converted into a volatile form by derivatization with a suitable reagent. This reaction is normally carried out at high temperature. During the derivatization reaction, oxidation occurs to an extent that overwhelms the amount originally present(Reference Douki, Delatour, Bianchini and Cadet333–Reference Ravanat, Turesky, Gremaud, Trudel and Stadler335). Generally speaking, gas chromatography coupled with mass spectrometry estimates of DNA oxidation have been higher than high-performance liquid chromatography with electrochemical detection estimates by about a factor of 10(Reference Douki, Delatour, Bianchini and Cadet333). The cause of this difference (an overestimate due to artificial oxidation with gas chromatography coupled with mass spectrometry vs an underestimate due to inefficient enzymatic digestion with high-performance liquid chromatography with electrochemical detection) has now been settled(Reference Ravanat, Turesky, Gremaud, Trudel and Stadler335). In addition, GC/MS analyses are difficult to perform, labour-intensive and exhibit poor sensitivity or inadequate specificity when they are used to test urinary samples(Reference Hu, Wang, Chang and Chao336). After enzymatic hydrolysis of DNA to nucleosides it is possible to analyse the probes with chromatography by high-performance liquid chromatography(Reference Shigenaga, Aboujaoude, Chen and Ames328). 8-Oxo-dG and its corresponding base 8-oxo-Gua are electrochemically active, lending themselves to sensitive electrochemical detection. The relative simplicity and sensitivity of high-performance liquid chromatography with electrochemical detection of 8-oxo-dG have made it the most popular method for monitoring of DNA-oxidation(Reference Beckman and Ames337). The high-performance liquid chromatography with electrochemical detection method itself has been criticized for its high variability(Reference Adachi, Zeisig and Moller338, Reference Finnegan, Herbert, Evans, Griffiths and Lunec339). Estimates of the ratio of 8-oxodG to dG have ranged from approximately 0·25 × 10− 5 to ⩾10− 4, possibly due to artificial oxidation(Reference Beckman and Ames337, Reference Adachi, Zeisig and Moller338). Dreher & Junod(Reference Dreher and Junod340) described in detail how the formation of artefacts that occurs with high-performance liquid chromatography with electrochemical detection can be avoided. Also this method is prone to oxidation of samples during preparation for analysis but by use of antioxidants and anaerobic conditions during isolation and hydrolysis of DNA, these effects can be minimised.
Immunoassays have, by far, demonstrated the greatest versatility in terms of the matrices to which they can be applied (urine, serum, plasma, cell culture medium, DNA hydrolysates), and simplicity of use(Reference Chiou, Chang, Chan, Wu, Tsao and Wu330), but comparisons with urinary 8-oxodG levels by chromatographic techniques revealed strong discrepancies of the results(Reference Cooke, Evans, Herbert and Lunec341–Reference Yoshida, Ogawa and Kasai343). The precise reason for the higher enzyme-linked immunosorbent assay based methods values remains unknown although there is a correlation between the data obtained by this and HPLC based methods(Reference Cooke, Evans, Herbert and Lunec341–Reference Yoshida, Ogawa and Kasai343). Nevertheless, this method can be applied to studies comparing relative urinary 8-oxo-dG values among several groups if the determination of the exact concentratins of 8-oxo-dG in urine is not required(Reference Shimoi, Kasai, Yokota, Toyokuni and Kinae342, Reference Yoshida, Ogawa and Kasai343).
The European Standards Committee on Oxidative DNA Damage(261, 344–Reference Gedik and Collins347) studied systematically the reasons for the discrepancies of the results obtained with different methods. Comparative analyses of baseline 8-oxo-dG levels in mammalian cell DNA, by different methods in different laboratories showed that estimates of 8-oxo-dG in pig liver, using chromatographic techniques, ranged between 2.23 and 441 per 106 guanines, and in HeLa cells DNA between 1.84 and 214. In the case of chromatographic methods, it was argued that spurious oxidation during work-up has been a problem and the trustworthiest results are the lowest. Most laboratories employing HPLC–ECD were able to measure chemically induced damage with similar efficiency and dose response gradients for seven of the eight sets of results were almost identical. GC–MS and HPLC–MS/MS, employed in three laboratories, did not convincingly detect dose response effects. In another study, Gedik & Collins(Reference Gedik and Collins347) evaluated data obtained in various laboratories in studies on 8-oxodG in calf thymus DNA, pig liver, oligonucleotides, HeLa cells and lymphocytes. The authors conclude that HPLC–ECD is capable of measuring 8-oxo-dG induced experimentally in the different types of samples. On the contrary, GC–MS failed to detect a dose response of physically (photosensitizer Ro 19-8022 and visible light) induced 8-oxodG formation and was not regarded as a reliable method for measuring low levels of damage; also HPLC–MS/MS was not found capable of detecting low levels of oxidative DNA damage.
8-Oxo-dG studies were used in a number of animal studies with laboratory rodents for example with cyanidin-3-glycoside(Reference Duthie, Jenkinson, Crozier, Mullen, Pirie, Kyle, Yap, Christen and Duthie348), curcumin(Reference Tunstall, Sharma, Perkins, Sale, Singh, Farmer, Steward and Gescher349), grape juice(Reference Jung, Wallig and Singletary350), green tea extract, catechins(Reference Hong, Ryu, Kim, Lee, Lee, Kim, Yun, Ryu, Lee and Kim351, Reference Kaneko, Tahara and Takabayashi352) and coffee(Reference van Zeeland, de Groot, Hall and Donato353) to name only a few.
The formation of oxidised guanine was also monitored extensively in human studies. It was shown that certain diseases such as cancer, Parkinson's and Alzheimer's diseases, multiple sclerosis, HIV, cystic fibrosis of lung, muscular dystrophy, diabetes mellitus, rheumatoid arthritis(Reference Cooke, Olinski and Evans325) as well as occupational exposure to coal dust(Reference Schins, Schilderman and Borm354), polyaromatic hydrocarbons and aromatic amines(Reference Loft, Poulsen, Vistisen and Knudsen355, Reference Marczynski, Rihs, Rossbach, Holzer, Angerer, Scherenberg, Hoffmann, Bruning and Wilhelm356), asbestos(Reference Yoshida, Ogawa, Shioji, Yu, Shibata, Mori, Kubota, Kishida and Hisanaga357), arsenic(Reference Hu, Wang, Chang and Chao336) and smoking(Reference Hu, Wang, Chang and Chao336, Reference Loft, Astrup, Buemann and Poulsen358) lead to increased urinary excretion.
The results of dietary intervention trials are summarised in the reviews of Moller & Loft(Reference Moller and Loft289, Reference Moller and Loft290). At present results of 23 studies are available, and in 12, protective effects were found. Evidence for reduced formation was seen for example in intervention trials with individual vitamins (C and E) and with supplements containing different vitamins, red ginseng, green tea and red wine and in newer investigations with specific vegetables(Reference Thompson, Heimendinger, Diker, O'Neill, Haegele, Meinecke, Wolfe, Sedlacek, Zhu and Jiang359, Reference Thompson, Heimendinger, Gillette, Sedlacek, Haegele, O'Neill and Wolfe360). Machowetz et al. (Reference Machowetz, Poulsen, Gruendel, Weimann, Fito, Marrugat, de la Torre, Salonen, Nyyssonen, Mursu, Nascetti, Gaddi, Kiesewetter, Baumler, Selmi, Kaikkonen, Zunft, Covas and Koebnick361) published recently an interesting study in which they found a significant (13 %) reduction of the formation of oxidised bases after consumption of olive oil which could be not explained by the phenolic contents of the different oils. Examples for studies in which no effects were detected are intervention trials with β-carotene, low vitamin E levels in combination with polyunsaturated fatty acids and cranberry juice(Reference Duthie, Jenkinson, Crozier, Mullen, Pirie, Kyle, Yap, Christen and Duthie348, Reference Jenkinson, Collins, Duthie, Wahle and Duthie362).
Correlations between different markers of oxidative stress
The questions if and to which extent relationships exist between the sensitivity of commonly used biomarkers of oxidative stress and endpoints of oxidative DNA damage, is addressed in the review of Dotan et al. (Reference Dotan, Lichtenberg and Pinchuk363). The authors analysed the results of studies in which two or more methods were used under identical experimental conditions and conclude that good correlations exist between measurements of peroxidation products such as MDA, lipid hydroperoxides, F-2 isoprostanes, conjugated dienes, glutathione and protein carbonyls but not with other criteria of the oxidative status such as the concentrations of antioxidants (TAC) and DNA migration (SCGE assay).
The evaluation of recent studies with dietary antioxidants shows that in general good correlations are observed between different biomarkers in in vitro experiments while this is not the case for animal and human studies. Results obtained with curcurmin, lycopene and ellagic acid in X-radiation experiments in vitro showed that the antioxidants increased the levels of GSH and reduced lipid peroxidation (MDA formation), in parallel a decrease of MN formation was observed(Reference Srinivasan, Sudheer, Pillai, Kumar, Sudhakaran and Menon364–Reference Sudheer, Muthukumaran, Devipriya and Menon366). Also in a study with colon derived cell lines (HT29, CaCo2), and phenolic apple juice extracts, improvement of the intracellular redox status (measured with dichloroflurecein assay) was paralleled by decreased DNA-damage in SCGE experiments(Reference Schaefer, Baum, Eisenbrand and Janzowski367).
In a recent Indian study(Reference Gandhi and Nair368) with mice, a clear correlation was observed between protection against radiation induced DNA-migration in lymphocytes and TBARS formation by gallic acid, while no such relations could be seen between these parameters in experiments with vitamin E deficient rats. Although the TBARS levels were significantly increased, formation of 8-oxodG and comet formation were not affected(Reference Duthie, Gardner, Morrice, Wood, Pirie, Bestwick, Milne and Duthie369).
Also in human studies controversial results were obtained in multiple endpoint studies. Consumption of anthocyanin/polyphenolic rich fruit juices reduced in an intervention trial (n = 18) DNA-migration in lymphocytes and increased the levels of reduced GSH, while other parameters (MDA in plasma, excretion of isoprostanes in urine) were not affected(Reference Weisel, Baum, Eisenbrand, Dietrich, Will, Stockis, Kulling, Rufer, Johannes and Janzowski370). Also in an earlier study (n = 27) with two juices containing either cyanidin glycoside or EGCG, a significant decrease of ENDO III lesions was observed in COMET assays, while other markers of the redox status (FRAP, TBARS, and FOX2) were not altered(Reference Bub, Watzl, Blockhaus, Briviba, Liegibel, Muller, Pool-Zobel and Rechkemmer371). In another larger study (n = 36), significant decreases of plasma antioxidant capacity and reduced ratio of GSH:GGSG were found while the concentration of reduced GSH and 8-oxo-dG in lymphocytes were not altered(Reference Janse van Rensburg, Erasmus, Loots, Oosthuizen, Jerling, Kruger, Louw, Brits and van der Westhuizen372).
Also changes of markers of oxidative DNA-damage did not correlate in all studies. In workers exposed to coke ovens and of graphite electrode producing plants significant increases of both 8-oxo-dG concentrations and FPG lesions were observed in white blood cells(Reference Marczynski, Rihs, Rossbach, Holzer, Angerer, Scherenberg, Hoffmann, Bruning and Wilhelm356). Also Gedik et al. (Reference Gedik, Boyle, Wood, Vaughan and Collins373) found a good overall correlation between 8-oxo-dG excretion in urine and FPG lesions in lymphocytes in unexposed individuals (n = 8), while no associations were detected at the individual level. On the contrary, no correlations between these two endpoints were found in a much larger study (n = 99)(Reference Hofer, Karlsson and Moller374). Only few investigations have been conducted in which MN and comet formation were studied in parallel. In in vitro experiments with radiated lymphocytes both parameters were reduced by several dietary antioxidants(Reference Srinivasan, Sudheer, Pillai, Kumar, Sudhakaran and Menon364–Reference Sudheer, Muthukumaran, Devipriya and Menon366), but in a recent trial with wheat sprouts (n = 13), a significant decrease of FPG lesions was detected in lymphocytes whereas no differences in the comets were detected under standard conditions with Endo III and the frequencies of MN were also not altered in the same target cells(Reference Ehrlich, Hoelzl, Nersesyan, Winter, Koller, Ferk, Fenech, Dusinska, Knasmüller, Ďuračková, Slámenova and Micksche101). It is notable that the differences between formation of 8-oxo-dG and comet formation may be due to the fact that the latter parameter detects a broader spectrum of lesions. The discrepancies between results of MN assays and comet measurements may be due to the fact that DNA oxidation is efficiently repaired and does not necessarily lead to double strand breaks which cause formation of chromosomal aberrations and MN.
The discrepancies seen in comparative experiments raise the question which of the parameters is the most reliable. In terms of prevention of diseases the most reliable endpoints are probably those which are directly related to specific pathologic conditions. For example, oxidized LDL is known to be related to coronary heart disease(Reference Domanski375) while for chromosomal aberrations and micronuclei it is well known that they are predictive for increased cancer risks(Reference Bonassi, Znaor, Ceppi, Lando, Chang, Holland, Kirsch-Volders, Zeiger, Ban, Barale, Bigatti, Bolognesi, Cebulska-Wasilewska, Fabianova, Fucic, Hagmar, Joksic, Martelli, Migliore, Mirkova, Scarfi, Zijno, Norppa and Fenech376, Reference Norppa, Bonassi, Hansteen, Hagmar, Stromberg, Rossner, Boffetta, Lindholm, Gundy, Lazutka, Cebulska-Wasilewska, Fabianova, Sram, Knudsen, Barale and Fucic377). The association between comet formation in lymphocytes and human diseases is not fully understood at present but it is known that occupationally exposed individuals as well as individuals with diseases which are associated with elevated cancer risks show increased comet formation(Reference Hoelzl, Bichler, Ferk, Simic, Nersesyan, Elbling, Ehrlich, Chakraborty and Knasmuller257, Reference Knudsen and Hansen378, Reference Maluf and Erdtmann379).
Measurement of antioxidant enzymes and of proteins involved in cell signalling and transcription
In many AO studies, the activities of enzymes were measured, which inactivate ROS and are part of the endogenous defence system. Table 3 lists up examples of such studies, a detailed description of currently used methods for the measurement of SOD, GPx and CAT can be found in the paper of Vives-Bauza et al. (Reference Vives-Bauza, Starkov and Garcia-Arumi380).
Table 3 Overview on currently used methods for the detection of antioxidant enzymes and examples of their induction by food constituents

* SM, spectrophotometric measurement; t.o., target organ; GSH, glutathione; GSSG, oxidised form of glutathione; NADPH, nicotinamide adenine dinucleotide phosphate; n.i., not indicated; RT-PCR, real time polymerase chain reaction; WB, Western blot.
† ↑ , increase; ↓ , decrease; ↔ , unchanged; bw, body weight; h, hours; AP-1, activating protein-1; CAT, catalase; DAS, diallyl sulphide; DATS, diallyl trisulfide; EGCG, epigallocatechin-3-gallate; GPx, glutathione peroxidase; HO-1, heme oxigenase-1 protein; LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; NAD(P)H, nicotinamide adenine dinucleotide phosphate; QR, quinone reductase; SOD, superoxide dismutase; TGF-α, transforming growth factor-α.
In many investigations it was found, that dietary factors cause an induction of SOD. For example, significant effects were seen in human trials with coffee(Reference Bichler, Cavin, Simic, Chakraborty, Ferk, Hoelzl, Schulte-Hermann, Kundi, Haidinger, Angelis and Knasmuller282), Brussels sprouts(Reference Hoelzl, Glatt, Meinl, Sontag, Heidinger, Kundi, Simic, Chakraborty, Bichler, Ferk, Angelis, Nersesyan and Knasmüller297), gallic acid(Reference Ferk, Chakraborty, Simic, Kundi, Knasmüller, Gee and Fogliano381), plant extract supplements(Reference Nelson, Bose, Grunwald, Myhill and McCord382) and phenolics rich diets(Reference Kim, Kim and Sung383). However, in some studies e.g. in an animal study by Akturk and coworkers(Reference Akturk, Demirin, Sutcu, Yilmaz, Koylu and Altuntas384), a decrease of its activity was found with antioxidants. Also in the case of heme oxygenase-1 (HO-1) induction effects were observed in most investigations (see Table 3), while glutathione peroxidase (GPx) was not altered in the majority of human and animal studies(Reference Bichler, Cavin, Simic, Chakraborty, Ferk, Hoelzl, Schulte-Hermann, Kundi, Haidinger, Angelis and Knasmuller282, Reference Ferk, Chakraborty, Simic, Kundi, Knasmüller, Gee and Fogliano381, Reference Akturk, Demirin, Sutcu, Yilmaz, Koylu and Altuntas384–Reference Pedraza-Chaverri, Granados-Silvestre, Medina-Campos, Maldonado, Olivares-Corichi and Ibarra-Rubio386), one exception being experiments with Ginkgo biloba extracts administered to ethanol fed rats, in which an increase was detected(Reference Yao, Li, Song, Zhou, Sun, Zhang, Nussler and Liu387). It can be seen in Table 3 that the results of catalase (CAT) measurements with different dietary the antioxidants are inconsistent. Antioxidant diets caused an induction of CAT in a human study(Reference Nelson, Bose, Grunwald, Myhill and McCord382) while garlic feeding to rats caused a decrease of its activity(Reference Pedraza-Chaverri, Granados-Silvestre, Medina-Campos, Maldonado, Olivares-Corichi and Ibarra-Rubio386).
NADPH-quinone reductase (NADPH-QR) was used as a marker enzyme for the general induction of phase I and phase II enzymes. Prochaska & Talalay(Reference Prochaska, Santamaria and Talalay388) developed a protocol for high throughput measurements of food derived enzyme inducers with a murine hepatoma cell line (Hepa 1c1c7), subsequently, an improved protocol was developed, which enables the discrimination between monofunctional and bifunctional inducers(Reference Prochaska and Talalay389) i.e. between compounds which cause an induction of both, phase I and phase II enzymes and those which only affect the latter group. In a large number of studies, induction of NADPH-QR was observed with a variety of different dietary compounds in this experimental system; one of the most potent inducers identified was sulforaphane, which was used as a model compound in numerous subsequent experiments(Reference Talalay and Fahey390). However, comparative measurements in human derived cells (Hep G2) showed, that the measurement of NADPH-QR does not correlate quantitatively with the induction of GST, a key enzyme involved in the detoxification of many carcinogens(Reference Hintze, Wald, Zeng, Jeffery and Finley391).
Gamma glutamylcysteine synthetase (GCS) can be regarded as an “indirect” AO enzyme. It catalyses the rate limiting step of glutathione (GSH) synthesis(Reference Wild and Mulcahy392) and its activity can be measured with the protocol of Nardi and coworkers(Reference Nardi, Cipollaro and Loguercio393). This mehtod is based on the formation of c-glutamylcysteine from cysteine and glutamic acid which is quantified by derivatization with bromobimane and fluorimetric detection after separation by high performance liquid chromatography. It was shown that coffee diterpenoids and also other antioxidants such as the phenolic food additive butylated hydroxyanisole and vitamin E induce GCS(Reference Huber, Scharf, Rossmanith, Prustomersky, Grasl-Kraupp, Peter, Turesky and Schulte-Hermann394–Reference Eaton and Hamel396). GSH itself is a potent antioxidant and scavenges predominantly ![]() , ∙OH, RO∙ and ROO∙(Reference Younes, Marquart and Schäfer397). In a number of dietary studies the ratio between oxidised and reduced GSH was monitored as a parameter of antioxidant effects. The method is based on spectrophotometric measurements with Ellmans reagent(Reference Boyne and Ellman398) and changes were observed in newer in vivo studies for example with kawheol/cafestol(Reference Huber, Scharf, Rossmanith, Prustomersky, Grasl-Kraupp, Peter, Turesky and Schulte-Hermann394), polyphenol rich cereals(Reference Alvarez, Alvarado, Mathieu, Jimenez and De la Fuente399) antioxidant (vitamins C and E, l-carnitine, and lipoic acid or additional bioflavonoids, polyphenols, and carotenoids) enriched diets(Reference Rebrin, Zicker, Wedekind, Paetau-Robinson, Packer and Sohal400) and vitamin E(Reference Lii, Ko, Chiang, Sung and Chen401). GSH is also the substrate of glutathione-S-transferases (GSTs), which are regulated by Nrf2 and catalyse the detoxification of a broad variety of xenobiotics including food specific carcinogens (for reviews see(Reference Landi402, Reference Tew and Ronai403)) and it has been postulated that GSTs are also involved in the inactivation of radicals(Reference Younes, Marquart and Schäfer397, Reference Landi402).
, ∙OH, RO∙ and ROO∙(Reference Younes, Marquart and Schäfer397). In a number of dietary studies the ratio between oxidised and reduced GSH was monitored as a parameter of antioxidant effects. The method is based on spectrophotometric measurements with Ellmans reagent(Reference Boyne and Ellman398) and changes were observed in newer in vivo studies for example with kawheol/cafestol(Reference Huber, Scharf, Rossmanith, Prustomersky, Grasl-Kraupp, Peter, Turesky and Schulte-Hermann394), polyphenol rich cereals(Reference Alvarez, Alvarado, Mathieu, Jimenez and De la Fuente399) antioxidant (vitamins C and E, l-carnitine, and lipoic acid or additional bioflavonoids, polyphenols, and carotenoids) enriched diets(Reference Rebrin, Zicker, Wedekind, Paetau-Robinson, Packer and Sohal400) and vitamin E(Reference Lii, Ko, Chiang, Sung and Chen401). GSH is also the substrate of glutathione-S-transferases (GSTs), which are regulated by Nrf2 and catalyse the detoxification of a broad variety of xenobiotics including food specific carcinogens (for reviews see(Reference Landi402, Reference Tew and Ronai403)) and it has been postulated that GSTs are also involved in the inactivation of radicals(Reference Younes, Marquart and Schäfer397, Reference Landi402).
Increased levels of the antioxidant enzymes listed in Table 3 are also seen under oxidative stress conditions and in inflammatory and ROS-related diseases and in tumour tissues(Reference Soini, Kaarteenaho-Wiik, Paakko and Kinnula404, Reference Wenzel, Herzog, Kuntz and Daniel405). This holds also true for GCS which is increased for example by NO and in meta- and dysplastic cells(Reference Soini, Kaarteenaho-Wiik, Paakko and Kinnula404). Therefore the induction of these enzymes cannot be taken as a firm evidence for protective properties of dietary constituents and results of such measurements should be supported by additional data.
A plethora of methods has been developed to monitor the impact of antioxidants on proteins involved in cell signalling and on the activation of transcription factors. A detailed description of the currently used techniques is beyond the scope of the present article. In general, biochemical standard methods, such as Western blots, Northern blots, real time PCR and ELISA-based techniques are employed. For the measurement of kinases, e.g. mitogen activated protein kinases (MAPKs) and others, like protein kinase C (PKC) as well as phosphoinositide 3 kinase (PI3K), which are also important targets of phytochemicals(Reference Surh, Kundu, Na and Lee121, Reference Surh406) mainly Western blots with phosphorylation specific antibodies(Reference Favata, Horiuchi, Manos, Daulerio, Stradley, Feeser, Van Dyk, Pitts, Earl, Hobbs, Copeland, Magolda, Scherle and Trzaskos407, Reference Fukazawa and Uehara408) and more recently also more expensive ELISA techniques are used (for commercially available test kits and description of methods see also www.biocompare.com or www.cellsignal.com). Transcription factors like the nuclear factor kappa B (NFκB) can be determined by the luciferin/luciferase system, which is a sensitive reporter assay for gene expression. d-Luciferin is the substrate for firefly luciferase catalyzing the oxidation of luciferin to oxyluciferin in presence of ATP and magnesium, resulting in bioluminescence(Reference Bronstein, Fortin, Stanley, Stewart and Kricka409). By use of this assay, expression of NFκB can be measured in vivo simultaneously in different organs(Reference Mann410) or in stable cell cultures(Reference Hintze, Wald, Zeng, Jeffery and Finley391, Reference Lai, Jiang and Li411).
Two other enzymes, which are often monitored in antioxidant studies are cyclooxygenase 2 (COX 2) and ornithine decarboxylase (ODC). They are regarded as important makers of inflammation and tumour development.
COX 2 is an inducible enzyme which can be activated by NFκB and catalyses the cyclooxygenase pathway of prostaglandin synthesis from arachidonic acid. It is abundant in activated macrophages and other cells at sites of inflammation. COX 2 overexpression has been found in various types of human cancers(Reference Sarkar, Adsule, Li and Padhye412). Protein and m-RNA levels of COX 2 are measured with Western blots, SDS-PAGE, and Northern blots. COX 2 activity can be determined in various ways; a) oxygen consumption can be monitored using an oxygen electrode(Reference Kulmacz, Lands, Benedetto, McDonald-Gibson and Nigam413), b) by measurement of COX 2 produced prostaglandines via enzyme immunoassays (EIA)(Reference Pradelles, Grassi and Maclouf414), c) In addition, several methods for the determination of peroxidase activity of COX 2 with luminescent detection have been developed (for a detailed description of methods and commercially available test kits see e.g. www.caymanchem.com/app/template/cur-rents,010.vm/a/z; ELISA kits also can be found on www.calbiochem.com). The results obtained in experiments with dietary antioxidants on COX 2 inhibition are reviewed in the article of Surh and coworkers(Reference Surh, Chun, Cha, Han, Keum, Park and Lee415).
ODC catalyses the decarboxylation of ornithine into putrescine and is involved in cell proliferation. Its activity is rapidly modified in response to various chemical or physical stimuli(Reference Gaboriau, Havouis, Groussard, Moulinoux and Lescoat416). ODC is a marker enzyme for tumour promotion and is upregulated in transformed cells(Reference Pegg417). The reduction of its activity as well as reversal of the malignant state of cells by antioxidants has been shown in many studies(Reference Hunt and Fragonas418, Reference Friedman and Cerutti419). Various methods for ODC activity measurements were developed, including radiometric assays based on trapping the [14C]O2 generated from the decarboxylation of [1− 14C]ornithine(Reference Russell and Snyder420), the retention of 3H-putrescine from tritiated ornithine on a strong cation-exchange paper(Reference Djurhuus421) and the determination of the radiolabelled form of the enzyme-activated suicide inhibitor of ODC, difluoro-methylornithine (DFMO)(Reference Seely, Poso and Pegg422). A radio- immunoassay using purified polyclonal antibodies has also been employed in some experiments(Reference Seely and Pegg423). More recently, chemiluminescence and spectrophotometry based methods have became available(Reference Gaboriau, Havouis, Groussard, Moulinoux and Lescoat416, Reference Wang and Bachrach424).
Use of -omics based approaches for the investigation of oxidant and antioxidant effects
During the last decade, a number of new techniques has been developed and optimised which enable the simultaneous detection of a large number of alterations of biological functions. The use of these approaches in nutritional sciences has led to the formation of a new discipline termed “Nutrigenomics”(Reference Ordovas and Mooser425). The overall aim of this new field of research is to find out how dietary factors alter gene transcription, protein expression and metabolism(Reference Ordovas and Corella426) in regard to health effects. The area covers three interrelated main areas namely transcriptomics (gene expression analyses), proteomics (global protein analysis) and metabolomics (metabolite profiling). Details on the basic concepts can be found in the article of Kussmann et al. (Reference Kussmann, Affolter and Fay427).
In the following chapters we will focus on the description of results which have been obtained in studies concerning the effects of oxidative stress and with dietary antioxidants in transcriptomics, proteomics and metabolomics. The latter approach are based on the chemical characterisation of metabolites by use of nuclear magnetic resonance and/or mass spectrometry(Reference Gavaghan, Holmes, Lenz, Wilson and Nicholson428, Reference Glassbrook and Ryals429). The use of these techniques for dietary studies is described in recent reviews, e.g.(Reference Scalbert, Milenkovic, Llorach, Manach, Leroux and Ortigues-Marty430, Reference Ovesná, Slabý, Toussaint, Kodíček, Maršík, Pouchová and Vaněk431). Of particular interest for the detection of oxidant/antioxidant effects are analyses of the lipidome (i.e. of the lipids in biological liquids).
Only few dietary studies have been carried out in which the effects of plant foods, beverages and phytochemicals were investigated. Urinary patterns of three types of diets – vegetarian, low meat and high meat consumption in humans were compared, and a specific vegetarian metabolic signatures (i.e. a metabolite of microbial metabolism) were found(Reference Stella, Beckwith-Hall, Cloarec, Holmes, Lindon, Powell, van der Ouderaa, Bingham, Cross and Nicholson432). In other dietary studies with humans, e.g. with camomile tea(Reference Wang, Tang, Nicholson, Hylands, Sampson and Holmes433) and also with black and green teas(Reference Van Dorsten, Daykin, Mulder and Van Duynhoven434) changes were registered; e.g. in the latter trial a change of the excretion of dihydroxyphenylsulphates (end products of flavonoid degradation by colonic bacteria) was seen.
Other studies which are notable are the one of Solanky et al. (Reference Solanky, Bailey, Beckwith-Hall, Davis, Bingham, Holmes, Nicholson and Cassidy435) who investigated the impact of isoflavones on plasma profiles, and the investigations of Ito et al. (Reference Ito, Gonthiera, Manach, Morand, Mennen, Remesy and Scalbert436, Reference Ito, Gonthier, Manach, Morand, Mennen, Remesy and Scalbert437) who studied metabolites of dietary polyphenolics. Fardet et al. studied the excretion profiles after consumption of whole-grain and refined wheat flours(Reference Fardet, Canlet, Gottardi, Lyan, Llorach, Remesy, Mazur, Paris and Scalbert438). Overall, none of these investigations is clearly indicative for antioxidant properties of the food factors studies and the changes detected concern alterations of the composition of the gut microflora and of energy metabolism, or the identification of compound specific metabolites.
Transcriptomics
Basic principles
Subtractive and differential hybridization of messenger RNAs (mRNAs) have been the most frequently employed techniques to determine disparities in the steady state expression levels of transcripts in cells at a given time, the latter in general referred to as the transcriptome. Starting in the early eighties, cDNA (complementary DNA to mRNA) libraries have been used to identify clones representative of genes whose changes in transcription and/or mRNA stability result from variations in the proliferation state(Reference Mohn, Melby, Tewari, Laz and Taub439), the stage of development and disease(Reference DeRisi, Penland, Brown, Bittner, Meltzer, Ray, Chen, Su and Trent440, Reference Rothstein, Johnson, DeLoia, Skowronski, Solter and Knowles441), or the consequences of an agent(Reference Friedman and Weissman442). Although these techniques allow to reveal the whole transcriptome and to select for both, known and unknown differentially expressed mRNAs characteristic to open systems, these methods showed limitations due to the preferential identification of highly to moderately abundant mRNAs(Reference Liang and Pardee443). Subsequently, several more efficient procedures emerged, among them the reverse transcription-polymerase chain reaction (RT-PCR)-based representational difference analysis (RDA)(Reference Lisitsyn and Wigler444), the differential display (DD) technique and the serial analysis of gene expression (SAGE)(Reference Liang and Pardee445, Reference Velculescu, Zhang, Vogelstein and Kinzler446), which both represent open screening techniques even allowing to detect and quantify differentially expressed mRNA species of low abundance. Yet, these methods are subject to the criticism that they require considerable sequencing efforts and may yield false positive signals which demand additional experimental arrangements to get rid of them. In the mid to late nineties, DNA microarrays have been developed for the high throughput expression profiling of the transcriptome which facilitates to dissect the genetic flow of information upon various (patho)physiological stages and to identify suitable drug targets(Reference Brown and Botstein447, Reference Duggan, Bittner, Chen, Meltzer and Trent448).
DNA microarrays are currently widely used and provide the unique opportunity to detect hybridization signals to sequenced clones and collections of partially sequenced cDNAs known as expressed sequence tags (ESTs)(Reference Adams, Kelley, Gocayne, Dubnick, Polymeropoulos, Xiao, Merril, Wu, Olde and Moreno449, Reference Greely450). A variety of different cDNA microarrays with high density hybridization targets immobilized on nitrocellulose or nylon surfaces are available and frequently applied in basic research and clinical settings(Reference Mikulits, Pradet-Balade, Habermann, Beug, Garcia-Sanz and Mullner451). Advances in the microarray technology are associated with the development of different techniques to synthesize DNA probes and to deliver them by immobilization on solid surfaces(Reference Schena, Heller, Theriault, Konrad, Lachenmeier and Davis452). Beside mechanical microspotting and ink jetting of non-synthetic cDNA probes with various lengths on coated glass slides which were prepared by biochemical methods such as PCR, the photolithography technologies employs in situ synthesis of oligonucleotides.
The isolation of total RNA or mRNA by standard biochemical methods requires quality control by analyzing the respective integrity. Solid techniques are available to amplify even low amounts of the extracted RNA for subsequent labeling and hybridization on microarrays. Particular efforts have been made to separate RNA in polysome-associated species which are involved in protein synthesis and to profile translated mRNA in order to more closely depict the proteome and to identify both transciptionally and translationally controled mRNAs(Reference Pradet-Balade, Boulme, Beug, Mullner and Garcia-Sanz453–Reference Petz, Kozina, Huber, Siwiec, Seipelt, Sommergruber and Mikulits455). In all experimental settings, standard operating procedures are recommended for the RNA extraction and labeling, the hybridization procedure and the subsequent monitoring of hybridization signals to obtain reproducible and comparable microarray data. Cross-analysis of hybridization signals from independent experiments performed in triplicate ensures a high reliability of results. For routine applications, e.g. GeneSpring™ provides a user-friendly solution for the computational analysis of raw data allowing hierarchical clustering of data sets. However, evaluation of whole-genome microarrays requires particular efforts with regard to the management of data. Changes in the transcriptome indicated by microarray assays depend finally on the confirmation of data by quantitative RT-PCR which is the most sensitive method for the detection of rarely expressed transcripts(Reference Bustin456). The incorporation of SYBR Green dye or the disruption of the Taqman probe exhibit fluorescence during RT-PCR, and are frequently using techniques for the quantitative analysis of mRNA expression. Together, cDNA or oligonucleotide microarrays are regarded as powerful tools for the profiling of the transcriptome, and being greatly versatile in gene throughput by customization and applicability in various experimental approaches.
Results of microarray studies concerning the transcription of genes by oxidative stress
Several reviews on the impact of oxidative stress on gene regulation have been published(Reference Allen and Tresini80, Reference Kunsch and Medford457–Reference Finkel and Holbrook459), probably the most comprehensive overview is the one by Allen & Tressin(Reference Allen and Tresini80). However, most of the data contained in these surveys are derived from experiments with conventional methods (including RT-PCR). The present paper is confined to newer findings obtained with arrays which are increasingly used in the last years.
The investigations can be grouped into three categories namely in vitro experiments with stable cell lines or primary cells; animal studies and gene expression analyses with humans. Table 4 gives an overview of groups of genes which were measured in a number of studies.
Table 4 Examples for genes which are transcriptionally regulated by oxidative stress

* OS, Organ specificity.
† LO, Location.
In vitro studies: An interesting and promising approach has been published by Scherf and coworkers(Reference Scherf, Ross, Waltham, Smith, Lee, Tanabe, Kohn, Reinhold, Myers, Andrews, Scudiero, Eisen, Sausville, Pommier, Botstein, Brown and Weinstein460) who assessed gene expression profiles in 60 human derived cancer cell lines for a drug discovery screen. They used relatively large arrays (8000 genes) and tested approximately 70 000 compounds; subsequently the results were clustered according to different parameters including known mechanisms of action of the different compounds. The database which emerged from this large study is available from NCI (http//dtp.nci.nih.gov) and provides a valuable source of information. It was employed for example in a study by Efferth & Oesch(Reference Efferth and Oesch461) to characterize the molecular mechanisms of the toxicity of two antimalarial drugs; the authors selected from the database 170 genes involved in oxidative defence and metabolism which they used to design a custom made array and tested the acute toxic effects of the compounds in the individual cell lines; on the basis of the patterns they found distinct differences in the mode of action of the two drugs.
All other studies with human derived cells comprised a lower number of cell lines and chemicals. Murray et al. (Reference Murray, Whitfield, Trinklein, Myers, Brown and Botstein462) compared the effects of different types of stress (heat shock, ESR stress, oxidative stress and crowding) in human fibroblasts and HeLa cells and found clear differences in the responses. On the contrary, similar transcription patterns were identified by Chuang et al. (Reference Chuang, Chen, Gadisetti, Chandramouli, Cook, Coffin, Tsai, DeGraff, Yan, Zhao, Russo, Liu and Mitchell463) who analysed the effects of three ROS generating chemicals namely H2O2, 4-HNE and tBOH in human retinal cells. Other papers with human derived cells were published by Yoneda et al. (Reference Yoneda, Chang, Chmiel, Chen and Wu464), who studied the effects of H2O2 and tobacco smoke in bronchial epithelial cells and Morgan and coworkers(Reference Morgan, Ni, Brown, Yoon, Qualls, Crosby, Reynolds, Gaskill, Anderson, Kepler, Brainard, Liv, Easton, Merrill, Creech, Sprenger, Conner, Johnson, Fox, Sartor, Richard, Kuruvilla, Casey and Benavides465) who used the human derived liver cell line HepG2 to investigate the effects of a variety of ROS generating chemicals. Except in the later study, relative large arrays (>1000 genes) were used in these investigations. In some experiments, ionising radiation was used to induce stress responses; for example Amundson et al. (Reference Amundson, Bittner, Chen, Trent, Meltzer and Fornace466) analysed the expression of genes caused by γ-radiation in twelve different human cell lines.
Another topic which was addressed in in vitro gene expression studies concerns the impact of specific cell functions (transcription factors, enzymes involved in signalling pathways) which are regulated by ROS. Investigations which fall into this category are for example analyses of the impact of PI3K in tBOH induced gene expression in IMR3 cells(Reference Li, Lee and Johnson467), experiments on the role of the BRCA1 gene in breast cancer cells(Reference Bae, Fan, Meng, Rih, Kim, Kang, Xu, Goldberg, Jaiswal and Rosen468), as well as comparative studies with cell lines differing in their p53 status, Nrf2 functions and SOD activity(Reference Amundson, Do, Vinikoor, Koch-Paiz, Bittner, Trent, Meltzer and Fornace469–Reference Kirby, Menzies, Cookson, Bushby and Shaw471).
Animal experiments: Only a few studies have been carried out in which the effects of ROS were investigated in laboratory rodents have been published so far. Examples for experiments with different oxidants are described in the articles of McMillian et al. (Reference McMillian, Nie, Parker, Leone, Kemmerer, Bryant, Herlich, Yieh, Bittner, Liu, Wan, Johnson and Lord472, Reference McMillian, Nie, Parker, Leone, Bryant, Kemmerer, Herlich, Liu, Yieh, Bittner, Liu, Wan and Johnson473) who analysed gene expression patterns of oxidants in rat livers and attempted to establish compound specific expression signatures. Examples for the use of knockout animals are the investigations of Yoshihara et al. (Reference Yoshihara, Ishigaki, Yamamoto, Liang, Niwa, Takeuchi, Doyu and Sobue474) and Thimmulappa and coworkers(Reference Thimmulappa, Scollick, Traore, Yates, Trush, Liby, Sporn, Yamamoto, Kensler and Biswal475) who studied comparatively gene expression patterns in normal, SOD and Nrf2 deficient animals.
Another topic which has been addressed in a number of in vivo array studies concerns the impact of ageing on gene transcription and oxidant responses. As mentioned above, it is assumed that several age related diseases are due to increased oxidative damage and it was shown by Lee et al. (Reference Lee, Allison, Brand, Weindruch and Prolla476–Reference Lee, Klopp, Weindruch and Prolla478) that transcription patterns in muscles, brain and heart of mice are age dependent. Edwards et al. (Reference Edwards, Sarkar, Klopp, Morrow, Weindruch and Prolla479) found subsequently, that paraquat induced expression patterns differ significantly depending on the age of the animals.
Human studies: Only few articles have appeared in the last years. An example for an occupational study is the one published by Wang et al. (Reference Wang, Neuburg, Li, Su, Kim, Chen and Christiani480) who investigated the responses of blood cells in metal fume exposed workers. It is assumed that ROS are at least partly involved in the toxic effects of metals and indeed the authors found that oxidative damage responsive genes were altered in their expression. Microarrays were also used in a number of studies with patients who suffered from ROS related diseases such as rheumatoid arthritis, inflammatory bowel disease, arterial fibrillation and sickle cell anaemia(Reference Kim, Lim, Lee, Shim, Ro, Park, Becker, Cho-Chung and Kim481–Reference Jison, Munson, Barb, Suffredini, Talwar, Logun, Raghavachari, Beigel, Shelhamer, Danner and Gladwin483).
The results of all these investigations aimed at identifying genes which are transcriptionally regulated by ROS depend largely on the experimental model used and the most important parameters are the origin of the cells, the time schedule as well as the mechanisms by which oxidative damage is induced; also the design and size of array technique plays an important role.
The results of the in vitro experiments described above show that the patterns of gene alterations caused by oxidants depends primarily on the type of indicator cells used and to a lesser extent on the chemicals(Reference Murray, Whitfield, Trinklein, Myers, Brown and Botstein462, Reference Weigel, Handa and Hjelmeland484). On the basis of the current state of knowledge one may expect that genes which are predominately altered in their transcription are those which are regulated by ROS dependent transcription factors. Indeed, it was found in some studies that Nrf2/ARE, apoptosis and p53 regulated genes are affected(Reference Li, Lee and Johnson467, Reference Bae, Fan, Meng, Rih, Kim, Kang, Xu, Goldberg, Jaiswal and Rosen468, Reference McMillian, Nie, Parker, Leone, Kemmerer, Bryant, Herlich, Yieh, Bittner, Liu, Wan, Johnson and Lord472, Reference McMillian, Nie, Parker, Leone, Bryant, Kemmerer, Herlich, Liu, Yieh, Bittner, Liu, Wan and Johnson473). However, in some investigations only little evidence for the up regulation of such genes was detected(Reference Bae, Fan, Meng, Rih, Kim, Kang, Xu, Goldberg, Jaiswal and Rosen468, Reference Suzuki, Spitz, Gandhi, Lin and Crawford485, Reference Weindruch, Kayo, Lee, Prolla, Rosenberg and Sastre486) and often genes were identified which are involved in cell cycle regulation, cellular communication, biosynthesis and metabolism. Some of them are controlled by ROS dependent signalling pathways and transcription factors but the patterns seen in the different studies are highly divergent and it is not possible to identify specific marker genes(Reference Li, Lee and Johnson467, Reference Bae, Fan, Meng, Rih, Kim, Kang, Xu, Goldberg, Jaiswal and Rosen468, Reference Weigel, Handa and Hjelmeland484, Reference Yoneda, Peck, Chang, Chmiel, Sher, Chen, Yang, Chen and Wu487).
What are the reasons for the strong inconsistencies? As mentioned above, one of the important parameters is the origin of the cells. For example, Murray et al. (Reference Murray, Whitfield, Trinklein, Myers, Brown and Botstein462) compared the effects of ROS generating chemicals in human fibroblasts and HeLa cells under identical experimental conditions and found the former cells by far more responsive. The reasons for these discrepancies may be due to the fact that signalling pathways and transcription factors are partly tissue specific and may be impaired in cancer cells lines which are frequently used in array studies. Nrf2 and p53 are found in a broad variety of different organs(Reference Lee, Li, Johnson, Stein, Kraft, Calkins, Jakel and Johnson488), while NFκB is lacking in a number of tissues(Reference Allen and Tresini80). It is known that p53 is frequently mutated in tumours in many organs(Reference Ivyna Bong, Patricia, Pauline, Edmund and Zubaidah489–Reference Stewart, Querec, Ochman, Gruver, Bao, Babb, Wong, Koutroukides, Pinnola, Klein-Szanto, Hamilton and Patriotis491), and it was shown in a comparative study with p53+ and p53 − cell lines that loss of this function has a substantial impact on oxidative stress induced gene transcription(Reference Amundson, Do, Vinikoor, Koch-Paiz, Bittner, Trent, Meltzer and Fornace469). In addition, it is so that xenobiotic drug metabolising as well as antioxidant enzymes, which are controlled by the ARE element are highly organ specific (see Table 3). It is also well documented that stable cell lines used routinely in toxicological studies lack the activities of phase I, phase II and antioxidant enzymes which are regulated by ARE via Nrf2(Reference Knasmüller, Mersch-Sundermann, Kevekordes, Darroudi, Huber, Hoelzl, Bichler and Majer299), therefore strong efforts have been made to establish cell lines which have retained the activities of these enzymes in an inducible form. Also other factors may account for the inconsistency of the results(Reference Murray, Whitfield, Trinklein, Myers, Brown and Botstein462) found strong differences between the results of array studies conducted in vitro and in rodents, and the authors hypothesized that the fact that substantially more genes were transcriptionally regulated in vivo may be due to the inadequate representation of cell communication in cultures.
Another important parameter which has been often neglected is the time dependency of AO responses(Reference Murray, Whitfield, Trinklein, Myers, Brown and Botstein462, Reference Yoneda, Chang, Chmiel, Chen and Wu464, Reference Yoneda, Peck, Chang, Chmiel, Sher, Chen, Yang, Chen and Wu487). For example, three distinct phases could be discriminated in experiments with human bronchial epithelial cells. The first was characterised by upregulation of apoptosis related genes and MAPKs, the second by activation of proteins involved in the turnover of damaged proteins and only in the third (10 h after the challenge) activation of genes was observed which are involved in the detoxification of ROS(Reference Yoneda, Chang, Chmiel, Chen and Wu464, Reference Yoneda, Peck, Chang, Chmiel, Sher, Chen, Yang, Chen and Wu487).
Despite these inconsistencies, it is possible to define a number of genes which are typical markers of oxidative stress under experimental conditions (Table 3) and it was possible to establish distinct signatures of gene expression for different types of oxidative tress in specific experimental models such as macrophage activation, peroxisomal proliferation and ROS releasing chemicals in rat hepatocytes in vivo (Reference McMillian, Nie, Parker, Leone, Kemmerer, Bryant, Herlich, Yieh, Bittner, Liu, Wan, Johnson and Lord472, Reference McMillian, Nie, Parker, Leone, Bryant, Kemmerer, Herlich, Liu, Yieh, Bittner, Liu, Wan and Johnson473). Also in in vitro studies with a human derived liver cell line a clear difference was observed between oxidative and non-oxidative stress responses(Reference Morgan, Ni, Brown, Yoon, Qualls, Crosby, Reynolds, Gaskill, Anderson, Kepler, Brainard, Liv, Easton, Merrill, Creech, Sprenger, Conner, Johnson, Fox, Sartor, Richard, Kuruvilla, Casey and Benavides465).
It is known for other areas of genomic research that studies interrogating gene transcription in similar contexts often resulted in poor overlapping lists of regulated genes (for a general review see(Reference Jarvinen, Hautaniemi, Edgren, Auvinen, Saarela, Kallioniemi and Monni492)); are a typical example are the strongly divergent results obtained in investigations aimed at identifying retinoic acid responsive genes(Reference van der Spek, Kremer, Murry and Walker493). These inconsistencies have lead to strong efforts to elucidate the methodological reasons for these variations and it became clear that correlations are improved when low abundant genes are excluded(Reference Shippy, Sendera, Lockner, Palaniappan, Kaysser-Kranich, Watts and Alsobrook494). Different platforms were established which attempted to standardise the techniques of array based approaches. The outcome of these efforts have been published in a number of recent articles(Reference Brazma, Hingamp, Quackenbush, Sherlock, Spellman, Stoeckert, Aach, Ansorge, Ball, Causton, Gaasterland, Glenisson, Holstege, Kim, Markowitz, Matese, Parkinson, Robinson, Sarkans, Schulze-Kremer, Stewart, Taylor, Vilo and Vingron495–Reference Bammler, Beyer, Bhattacharya, Boorman, Boyles, Bradford, Bumgarner, Bushel, Chaturvedi, Choi, Cunningham, Deng, Dressman, Fannin, Farin, Freedman, Fry, Harper, Humble, Hurban, Kavanagh, Kaufmann, Kerr, Jing, Lapidus, Lasarev, Li, Li, Lobenhofer, Lu, Malek, Milton, Nagalla, O'Malley, Palmer, Pattee, Paules, Perou, Phillips, Qin, Qiu, Quigley, Rodland, Rusyn, Samson, Schwartz, Shi, Shin, Sieber, Slifer, Speer, Spencer, Sproles, Swenberg, Suk, Sullivan, Tian, Tennant, Todd, Tucker, Van Houten, Weis, Xuan and Zarbl498).
Results of microarray studies with dietary antioxidants
In total, results from approximately 60 studies are available at present in which the impact of dietary antioxidants on gene expression patterns was investigated. The number of publication has almost doubled in the last two years and it is also interesting that many of the newer studies were conducted with laboratory rodents; nevertheless in vitro experiments with stable cell lines are still dominating. It is also notable, that research focused predominantly on specific types of compounds such as resveratrol, a phenolic compound from red wine, epigallocatechingalleate (EGCG), the main catechin in green tea, cucurmin contained in the spice tumeric, sulphoraphane the breakdown product of glucobrassicin (a glucosinolate found in cruciferous vegetables) and the phytoestrogen genistein which is contained in soy beans. Several reviews have been published which contain results of microarray studies with phytochemicals. The most comprehensive one by Narayanan(Reference Narayanan31) describes data obtained in twenty studies (3 in vivo, 17 in vitro) with various dietary components, the findings with EGCG are summarised in the paper of Mariappan et al. (Reference Mariappan, Winkler, Parthiban, Doss, Hescheler and Sachinidis499), results with carotenoids can be found in the overview of Elliott(Reference Elliott500).
Table 5 summarises the results of newer studies which have not been evaluated in the aforementioned articles. The first part describes results of in vitro experiments with cells lines, the second contains in vivo experiments with laboratory rodents.
Table 5 Examples for results obtained with dietary antioxidants in microarray experiments

* AS, Arrays size (genes number); SD, Sprague–Dawley
† ↓ , downregulation; ↑ , upregulation; ↔ , no changes; CDKN, cyclin-dependent kinase inhibitor; COX, cyclooxygenase; FGFR2, fibroblast growth factor receptor 2; GAPDH, glyceraldehyde-3-phosphatase dehydrogenase; LOXL, lysyl oxidase-like genes; MAPK2, mitogen-activated kinase 2; MCP-1, monocyte chemoattractant protein 1; MPK6, mitogen-activated protein kinase 6; NAD(P)H, nicotinamide adenine dinucleotide phosphate; NQO1, NAD(P)H dehydrogenase, quinone 1; TNF, tumour necrosis factor; TGFB, transforming growth factor beta; TF, transcription factor; SOD, superoxide dismutase; n.s., not statistically significant.
Apart from the in vitro and animal studies, also a few human intervention trials have been carried out with antioxidants. For example Majewitz et al. (Reference Majewicz, Rimbach, Proteggente, Lodge, Kraemer and Minihane501) conducted a study with normal and apoE smokers (which carry a specific mutation in apolipoprotein E and are at increased risk for coronary heart disease). Before and after vitamin C supplementation (60 mg/P/4 weeks) they analysed monocytes by use of a small array (225 genes). After the intervention, the expression of 22 of the genes was altered in normal smokers, 71 were altered in apoE individuals. The most interesting observation was the down regulation of TNF-β and its receptor, also genes encoding for the neutrophil growth factor receptor and the monocyte chemoattractant protein receptor were affected. These genes are involved in inflammatory responses and it is known that TNF-β is regulated by NFκB. Another human study was published by Hoffmann and coworkers(Reference Hofmann, Liegibel, Winterhalter, Bub, Rechkemmer and Pool-Zobel502) who analysed the effects of consumption of a polyphenolics mix in lymphocytes of healthy individuals by use of a small targeted array (containing 96 transcripts related to drug metabolism). Fifty six genes were found expressed in the target cells and seven of them were altered after the intervention, three of them encoded for cytochrome P450 isozymes (2F1, 3A4 and 3A5), two for them for sulfotransferases (SULT 2A1 and 1C2) the others were associated with microsomal GST, nicotinamide–N-methyltransferase and the ATP binding cassette.
All of the compounds listed in the table are potent antioxidants and it is apparent that many of the transcriptional changes which were detected concern genes which encode for AO defence, inflammatory responses, apoptosis and cell signalling and division. Nevertheless, it is not possible, to define a general signature of AO effects. The lack of a common pattern may be due to the fact that the chemical structures of the antioxidants as well as their mode of action and tissue specificity differs substantially. For example vitamins C and E differ strongly in their organ distribution and it their ability to inactive ROS species(Reference Cotgreave, Moldeus and Orrenius503). Furthermore, also the experimental design of the studies plays an important role. As described above, the expression patterns seen with resveratrol were highly sex specific, in female mice the number of genes which were transcriptionally altered in hepatic tissue was approximately 3 times higher in females as compared to males(Reference Hebbar, Shen, Hu, Kim, Chen, Korytko, Crowell, Levine and Kong504). Also organ differences have an impact on the results of array studies, for example EGCG affected in the liver of mice three times more genes as compared to the colon(Reference Funk, Frye, Oyarzo, Kuscuoglu, Wilson, McCaffrey, Stafford, Chen, Lantz, Jolad, Solyom, Kiela and Timmermann505). Another important parameter affecting the outcome is the time dependency of the expression patterns and also the dose dependency of the responses (see Table 5).
The results of microarray analyses do at present not allow to draw firm conclusions if a compound elicits ROS protective effects. For example, findings obtained with moderate doses of vitamin C in mice(Reference Selman, McLaren, Meyer, Duncan, Redman, Collins, Duthie and Speakman506) indicate that it rather induces inflammation (up regulation of COX 2) and reduces ROS protection (down regulation of SOD). Also the results obtained with diallyldisulfide in HepG2 cells were unspecific and not indicative for antioxidant properties which are well documented for this compound(Reference Belloir, Singh, Daurat, Siess and Le Bon507). However, it may be possible that general antioxidant gene expression patterns can be defined on the basis of comparative standardised experiments with a variety of compounds which are lacking at present. The currently available results show that microarray studies nevertheless provide highly useful information concerning the molecular mechanisms of prevention of ROS related diseases. Typical examples are the results of studies with prostate cancer cell lines and compounds such as resveratrol and sulforaphane which showed that these compounds interact with the transcription of genes involved in cell cycle regulation and apoptosis; these findings provide a possible explanation for protective effects of these dietary components towards prostate cancer cell line LNCaP (for review see(Reference Narayanan31)); also the assumption of cancer protective effects of EGCG and retinoids could be enhardened by results of array studies(Reference Mariappan, Winkler, Parthiban, Doss, Hescheler and Sachinidis499, Reference Elliott500).
Proteomics
Basic principles
Proteomics is a screening technology based on the separation, quantification and identification of proteins from biological samples and allows to directly identify proteins bearing oxidative modifications. Adaptive cell responses in order to cope with the cell damage imposed by oxidative stress may as well be investigated by transcriptomics. As proteins are translated by ribosomes based on the information provided by mRNA molecules, no protein can be synthesised without the appropriate RNA. On the other hand, the mere existence of mRNA does not mean that the corresponding protein gets translated. It only means that the protein might get translated upon demand. This defines one main difference between proteomics and transcriptomics. Transcriptomics shows which genes are expressed, but does not tell which proteins actually get translated. The picture gets even more complicated, when time is considered. Some proteins such as histones are almost exclusively translated during the S-phase of the cell cycle, but not in G0(Reference Schumperli508). A poor correlation of proteomics to transcriptomics data may be the consequence, because these proteins may be abundant in G0, but no corresponding mRNA may be detectable at this time. On the other hand, several regulatory proteins such as p53 may be subject to continuous protein degradation by proteasomes. Here, protein amounts may accumulate to minute and undetectable amounts only, while the mRNA may be easily detectable. This explains why proteomics may give very different results when compared to transcriptomics.
In order to assign the identity of the modified molecules, separation of proteins may be applied before the detection of oxidative modification. 2D-PAGE is still the most important technique to separate proteins according to electric charge and molecular weight(Reference Nordhoff, Egelhofer, Giavalisco, Eickhoff, Horn, Przewieslik, Theiss, Schneider, Lehrach and Gobom509). Protein spots on 2D gels can be identified by Western analysis or mass spectrometric analysis of tryptic digests and quantified by a variety of techniques including silver staining, fluorescence detection or autoradiography(Reference Gerner, Vejda, Gelbmann, Bayer, Gotzmann, Schulte-Hermann and Mikulits510). This approach is used to perform comparative analysis of protein fractions isolated from e.g. treated and untreated cells. Spot patterns can be compared in order to detect alterations in relative amounts or, quite relevant to oxidative stress, protein modifications accompanied by changes of the molecular charge of the protein. Application of immunological detection of carbonyl groups or nitro-tyrosine by Western analysis may subsequently identify the modified proteins. Thus, application of 2D-PAGE may enable the detection of upregulated and/or modified proteins consequent to oxidative stress. The main draw-back of 2D-PAGE is its limited resolution and sometimes poor reproducibility concealing many important effects(Reference Rabilloud511). The number of proteins accessible via 2D-PAGE is usually in the order of several hundreds and hardly exceeds thousand different proteins(Reference Nordhoff, Egelhofer, Giavalisco, Eickhoff, Horn, Przewieslik, Theiss, Schneider, Lehrach and Gobom509).
Another essential technique for proteome analysis is mass spectrometry. Upon tryptic digestion, peptides can be obtained from isolated proteins which can be forwarded to mass analysis. Determination of the molecular weight of the peptides by e.g. MALDI-TOF provides a fingerprint of peptide masses for each protein(Reference Gras, Muller, Gasteiger, Gay, Binz, Bienvenut, Hoogland, Sanchez, Bairoch, Hochstrasser and Appel512). As tryptic peptides may be predicted from databases for all known proteins, a best-fit search can be performed resulting in candidates which show theoretical peptide masses comparable to those identified in the experiment. This technique is called fingerprint analysis and can be applied only to well-purified proteins as potentially obtained from 2D-PAGE. Minimal contamination of peptide preparations may render fingerprint data not accessible for meaningful interpretation and are a main drawback of this method.
Much more robust and powerful is the identification of peptide sequences obtained from peptide fragmentation analysis. Peptides can be fragmented specifically in a mass spectrometer resulting in the breakage of a single peptide bond per molecule(Reference Fountain, Lee and Lubman513). Ordering of the resulting peptide fragments may generate mass differences according to single amino acids in the peptide. As a result, not only total masses of peptides are determined as in case of fingerprint analysis, but complete amino acid sequences are obtained, which allow identification of proteins with much higher confidence. Furthermore, amino acid modifications may be determined by fragmentation analysis. When applied to isolated spots from 2D-gels, proteins can be identified with very high confidence. Furthermore, specific modifications such as oxidation of methionine, cysteine, tryptophan or others can be determined(Reference Spickett, Pitt, Morrice and Kolch514). On the other hand, the determination of amino acid sequences renders separation of proteins before tryptic digest unnecessary. Protein mixtures may be digested and then peptide mixtures separated by e.g. nano-flow chromatography(Reference Wolters, Washburn and Yates515). Peptide sequences may be determined successively and peptides derived from one protein sorted after the experiment. This powerful technique is called shotgun analysis and enables the identification of more proteins as 2D-PAGE/MS(Reference Rabilloud511). As long as peptide modifications are detected, protein modification can also be assigned. The major drawback of this approach is the limited sequence coverage rendering a lot of information missing and the inapplicability of immunological detection methods(Reference Bodnar, Blackburn, Krise and Moseley516).
As a consequence, the experimental design studies has to be carefully prepared for proteome. First of all, it should be clear what to look at. In case of assessment of the extent of damage in response to a defined challenge, a bulk analysis summarizing the extent of e.g. carbonyl formation with chemical or immunological methods may be appropriate. When the identity of target proteins appears desirable, 2D-PAGE can be used to assign the identity to the modified proteins. Here, the poor access of e.g. membrane proteins by most proteome analysis techniques poses(Reference Watarai, Inagaki, Kubota, Fuju, Nagafune, Yamaguchi and Kadoya517) a severe limitation. Mass analysis of the tryptic digest of an isolated 2D-spot may easily identify the corresponding protein because a few peptide fragmentation data may be sufficient for unequivocal protein identification. When the target amino acids of a modified protein are desired, full sequence coverage of the identified peptides is desired, but hard to realise. Peptides have to be ionised before they can be analysed by mass spectrometry. Ionisation is an unpredictable event and may be strongly dependent on chemical properties of the peptide. Consequently, only a subset of the peptides generated by proteolytic digests can usually be detected by mass spectrometry(Reference Zhu, Kim, Yoo, Miller and Lubman518).
Results of proteomic studies concerning oxidative stress
In order to identify proteins related to oxidative stress, several strategies were employed. A direct consequence of ROS-mediated damage may be amino acid modifications such as oxidation or nitration. Such modifications are accessible via peptide fragmentation analysis by mass spectrometry. While this method is very exact providing direct proof of oxidation together with the identification of the target protein and target amino acid, it has several draw-backs. First, it is almost impossible to survey the total amino acid sequence of proteins. Consequently, a negative result is hardly a proof for the absence of modifications. Second, proteins are very sensitive to artifacts introduced by sample handling procedures. For the proteome analysis of cells, they have to be lysed to extract the proteins for further analyses. Upon cell lysis, organelles get ruptured, calcium is set free, enzymes may get activated and many biochemical reactions including oxidations may get out of control. Also separation of proteins by 2D-PAGE exposes proteins to oxygen. This can be directly observed by the detection of oxidized methionine in samples from the same cell lysis, which were processed by 2D-PAGE and shotgun analysis in parallel. The proteins separated by 2D-PAGE generally show more methionine oxidation compared to the same proteins analysed by shotgun proteomics(Reference Manzanares, Rodriguez-Capote, Liu, Haines, Ramos, Zhao, Doherty-Kirby, Lajoie and Possmayer519). Thus, it is essential to most accurately control all experimental conditions and run comparative experiments strictly in parallel. Actually, several amino acid modifications consequent to oxidative stress are detectable by mass spectrometry, including oxidation of cysteine(Reference Paron, D'Elia, D'Ambrosio, Scaloni, D'Aurizio, Prescott, Damante and Tell520, Reference Rabilloud, Heller, Gasnier, Luche, Rey, Aebersold, Benahmed, Louisot and Lunardi521), methionine and tryptophan(Reference Manzanares, Rodriguez-Capote, Liu, Haines, Ramos, Zhao, Doherty-Kirby, Lajoie and Possmayer519), and nitration of tyrosine(Reference Koeck, Fu, Hazen, Crabb, Stuehr and Aulak522). Beside direct amino acid modification, the formation of disulfide bridges by oxidation may form protein adducts(Reference Brennan, Wait, Begum, Bell, Dunn and Eaton523) or protein glutathione adducts(Reference Fratelli, Demol, Puype, Casagrande, Eberini, Salmona, Bonetto, Mengozzi, Duffieux, Miclet, Bachi, Vandekerckhove, Gianazza and Ghezzi524). Amino-acid adducts can also be detected with antibodies specific for e.g. nitrotyrosine and carbonyl adducts(Reference Pocernich, Poon, Boyd-Kimball, Lynn, Nath, Klein and Butterfield525, Reference Stagsted, Bendixen and Andersen526). As mentioned above, the assignment of the target protein identity might be a daunting task and the assignment of the modified amino acid within the sequence is impossible. On the other hand, modified peptides which may be missed by MS-based analysis might be easily detected with antibodies.
In contrast to the identification of proteins directly targeted by oxidative stress, an alternative approach is the investigation of the response of cells via up-regulation and down-regulation of regulatory proteins(Reference Mathers, Fraser, McMahon, Saunders, Hayes and McLellan82). Here, rather indirect effects are observed, which may be consequent to transcriptional alterations of genes helping the cell to manage the stress situation and, on the other hand, proteasomal degradation of damaged proteins. As a consequence, the readout of such an experiment may become very complex, because we can see a lot of protein alterations, but we do not know where they are coming from. To reduce the complexity of the biological system, prokaryotes and yeast were used to investigate the basic responses to oxidative stress(Reference Godon, Lagniel, Lee, Buhler, Kieffer, Perrot, Boucherie, Toledano and Labarre527, Reference Mostertz, Scharf, Hecker and Homuth528). These works indicate that the expected up-regulation of antioxidant proteins and chaperones may be accompanied by substantial alterations in the metabolic state of the cells. Again, several approaches are feasible to investigate cellular responses. First, cells can be treated with sub-lethal concentrations of agents mediating oxidative stress such as H2O2. After a defined period of time, treated and untreated samples are forwarded to proteome analysis and the results compared in a quantitative fashion. Several studies with human monocytes and epithelial cells demonstrated that again, besides the obvious target proteins such as redox regulators and chaperones, a broad variety of other protein families seems to be involved in regulatory processes consequent to oxidative stress including cytoskeletal proteins and glycolytic proteins(Reference Chan, Gharbi, Gaffney, Cramer, Waterfield and Timms529, Reference Seong, Kim, Choi, Oh, Kim, Lee and Um530).
Second, cells which are very sensitive to oxidative stress may be compared to others which were made resistant e.g. by chronic exposure with increasing H2O2 concentrations(Reference Keightley, Shang and Kinter531). Here, differentially expressed proteins may include those which actually mediate resistance. Obviously, proteins differentially expressed due to different differentiation or activation states of the investigated cells may repress the identification of the true players related to oxidative stress control. Rather multiple comparisons of various cell systems or additional functional assays may allow to identify the relevant proteins. For example, it was demonstrated that enzyme aldose reductase identified by comparative analysis indeed mediates protection in Chinese hamster fibroblasts(Reference Keightley, Shang and Kinter531).
Finally, the involvement of oxidative stress in human diseases has been investigated by analysis of clinical material and it was shown that proteomics may help to understand patho-physiological processes relevant to the disease state. The involvement and relevance of protein oxidation in brain tissue in Alzheimer's disease has been clearly demonstrated(Reference Ding, Dimayuga and Keller532, Reference Sultana, Perluigi and Butterfield533). Specific oxidative modifications may also be involved in obesity(Reference Grimsrud, Picklo, Griffin and Bernlohr534), asthma(Reference Ghosh, Janocha, Aronica, Swaidani, Comhair, Xu, Zheng, Kaveti, Kinter, Hazen and Erzurum535), and various forms of inflammatory diseases(Reference Dalle-Donne, Scaloni, Giustarini, Cavarra, Tell, Lungarella, Colombo, Rossi and Milzani536).
Results of proteomic studies with dietary antioxidants
Although numerous position papers emphasized the importance of proteomics techniques in the investigations of health effects of dietary factors(Reference Go, Butrum and Wong537–Reference Opii, Joshi, Head, Milgram, Muggenburg, Klein, Pierce, Cotman and Butterfield546), the number of studies which contain data on the effects of food related compounds on protein patterns is strikingly low. One of the reasons may be that proteomics requires, unlike other techniques described in this article, a sound financial background to establish a proteomics facility. Most of the existing labs are specialised in the analysis of specific cells and tissues. Table 6 summarizes examples for results obtained in recent studies.
Table 6 Examples for results obtained with dietary antioxidants in recent proteomics studies

SwissProt Accession numbers: VDAC-1, P21796; VDAC-2, P45880; HSPBP1, Q9NZL4; GrpE, Q9HAV7; DEAD, Q92499; TGFβ, PO1137; Rho GDP, P52565; forkhead TF, Q08050.
* AO, antioxidant; bw, body weight; CE, capillary electrophoresis; 2 DE, two-dimensional electrophoresis; 2DE-SDS-PAGE, two-dimensional electrophoresis-sodium dodecylsulfate polyacrylamide gel electrophoresis; ESI, electro spray ionisation; h, hour; LC–MS/MS, liquid chromatography ion trap tandem mass spectrometry; MALDI-TOF MS, matrix assisted laser desorption ionization time-of-flight mass spectrometry; n.i., not indicated; ox-LDL, oxidised low density lipoproteins; s.c., subcutaneous.
† SD, Sprague–Dawley, pts, patients, t.o., target organ.
‡ ↓ , downregulation; ↑ , upregulation; ↔ , no changes; TF, transcription factor; SOD, superoxide dismutase; GAPDH, glyceraldehyde-3-phosphatase dehydrogenase; n.s., not significant.
It is quite clear that in most in vitro studies more drastic effects were observed than in human trials. This discrepancy may be not only due to the differences in the effects of the compounds tested, but also to the use of high doses in the experiments with isolated cells. Unphysiological test conditions my lead to secondary effects which may be due to cell death and not caused by the test compound itself. Furthermore, in some of the studies high abundant proteins (α-2 HS glycoprotein) with high inter-individual variations were postulated to be biomarkers for dietary effects. Many of the compounds/diets altered the expression of proteins, which play a role in apoptosis, cytoskeleton structure and energy metabolism, while no substantial alterations of enzymes were seen which are involved in ROS defence, except SOD, which was found upregulated in some of the studies.
Metabolomics
Impact of the experimental design on the outcome of antioxidant studies
In the last chapters we described extensively the technical difficulties of AO studies and the limitations of the reliability of different methods. Also the experimental design has a strong impact on the outcome and on the predictive value of investigations concerning antioxidant properties of dietary compounds which are conducted to find out if beneficial effects can be expected in humans.
General problems of the experimental designs of AO studies
Fig. 4 summarises the advantages and disadvantages of different experimental models. At present around 90 % of AO experiments are conducted in vitro with stable cell lines or with subcelluar fractions.
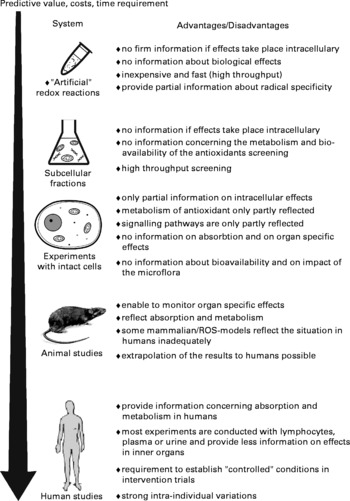
Fig. 4 Advantages and disadvantages of different experimental used to investigate ROS – protective effects of phytochemicals.
The classical strategy used to identify dietary antioxidants is the screening of a large number of potential candidates in high throughput systems and in vitro by use of stable cell lines and subsequently investigate the most promising compounds in more reliable models (e.g. in animals studies and in human intervention trials). This approach is justified on the basis of the assumption that the key mode of action of dietary antioxidants is the direct (non-enzymatic) inactivation of ROS which can be easily detected in this simple systems. However, it became clear over the last years that the mechanisms by which dietary compounds protect against ROS are by far more complex and that indirect modes of action such as induction of enzymes and other defence mechanisms play an important role. For example, the coffee diterpeonids cawheol and kafestol do not protect human lymphocytes under in vitro conditions against ROS mediated DNA damage(Reference Bichler, Cavin, Simic, Chakraborty, Ferk, Hoelzl, Schulte-Hermann, Kundi, Haidinger, Angelis and Knasmuller282) while Huber et al. (Reference Huber, Scharf, Rossmanith, Prustomersky, Grasl-Kraupp, Peter, Turesky and Schulte-Hermann394) found in experiments with rats that they are potent inducers of the enzyme gamma-glutamylcysteine synthetase (GCS) which catalyses the rate limiting step of the synthesis of glutathione which is a potent antioxidant(Reference Seifried, Anderson, Fisher and Milner547). It is conceivable that the increased GSH levels seen in the plasma of coffee drinkers(Reference Grubben, Nagengast, Katan and Peters548) and the protection against oxidative DNA damage observed in a human intervention trial and in animal experiments(Reference Bichler, Cavin, Simic, Chakraborty, Ferk, Hoelzl, Schulte-Hermann, Kundi, Haidinger, Angelis and Knasmuller282, Reference Hoelzl, Bichler, Ferk, Ehrlich, Cavin, Simic, Edelbauer, Grasl-Kraupp, Knasmüller, Ďuračková and Knasmüller549) with coffee are due to this mechanism.
AO enzymes and also transcription factors which regulate cellular defence mechanisms may be not represented adequately in stable cancer cell lines which are used in AO studies. For example, it is known that that p53 controls the transcription of a variety of genes which encode for responses towards antioxidants(Reference Li and Prives550). Since >50 % of human tumours are p53 deficient due to mutations(Reference Levesque and Eastman551), cell lines derived from them will not adequately reflect AO responses. Also the origin of the cells is important, while p53 and NrF2 are found in many organs, NFκB is not or only weakly expressed in certain tissues(Reference Allen and Tresini80); also the activities of AO enzymes show strong organ specificities (see Table 4). As mentioned above, the differences in the transcription pattern of genes in studies with oxidants and antioxidants support the assumption that the cellular responses depend largely on the type of indicator cells used.
Another important issue concerns the representation of phase I and phase II enzymes which metabolise dietary antioxidants and therefore may alter their protective properties; furthermore also reactions catalysed by the intestinal microflora are not reflected under in vitro conditions. Polyphenolics are extensively metabolised in the gut and in the body and non-conjugated metabolites most often account for a minor fraction of the circulating metabolites(Reference Manach, Williamson, Morand, Scalbert and Remesy552). In the case of isoquercitrin it was shown that completely different conjugates are formed under in vitro conditions (i.e. after exposure to colon cell lines) as in the serum of rats(Reference Depeint, Gee, Williamson and Johnson553, Reference Depeint554). Hydroxycinnamic acids which are potent oxidants in coffee are glucuronated in the body(Reference Nardini, Cirillo, Natella and Scaccini555, Reference Clifford556), but stable cell lines and peripheral lymphocytes which are used in in vitro experiments lack glucuronosyltransferase (UGT) activity which catalyses this reaction(Reference Mitoma and Fukuda557, Reference Hu and Wells558). In most cases, it is not known if and to which extent the AO properties of conjugates differ from that of the parent compounds, but for glucuronides of isoflavones and epicatechin it has been shown that they provide no protection against oxidative stress(Reference Zhang, Song, Cunnick, Murphy and Hendrich559, Reference Spencer, Schroeter, Crossthwaithe, Kuhnle, Williams and Rice-Evans560) while the parent compounds are potent antioxidants. The inadequate representation of drug metabolising enzymes is a general problem which is also encountered in acute toxicity and genotoxicity experiments with stable cells lined and various attempts were made to solve it. For example, the cultivation conditions of primary hepatocytes which possess a broad spectrum of xenobiotic drug metabolising enzymes have been improved(Reference Eckl and Raffelsberger561, Reference Fahrig, Rupp, Steinkamp-Zucht and Bader562), other solutions may be the establishment of hepatoma cell lines which have retained the activities of several enzymes in an inducible form, (for review see(Reference Knasmüller, Mersch-Sundermann, Kevekordes, Darroudi, Huber, Hoelzl, Bichler and Majer299)) and the construction of genetically engineered lines which express human phase I and phase II enzymes(Reference Dogra, Doehmer, Glatt, Molders, Siegert, Friedberg, Seidel and Oesch563, Reference Muckel, Frandsen and Glatt564).
The cultivation conditions of the cells may have a strong impact on the outcome of antioxidant studies. It is known that the composition of the medium affects the sensitivity of cells towards ROS. For example when lymphocytes are treated with ROS-generating chemicals in serum, they are up to five times less sensitive as compared to treatment in regular medium (L. Elbling, personal communication). When coffee or coffee specific compounds (chlorogenic and caffeic acid) were tested at high concentrations in genotoxicity tests with bacteria or human lymphocytes in vitro, strong effects were observed which were attributed to formation of H2O2, while with lower doses clear antioxidant effects were detectable and it was postulated by Aeschbacher et al. (Reference Aeschbacher, Wolleb, Loliger, Spadone and Liardon565) that adverse effects do not take place under realistic in vivo conditions.
On the basis of the results of in vitro experiments it was possible to identify a number of highly potent antioxidants such as chlorophylls(Reference Kumar, Shankar and Sainis566), curcumin(Reference Su, Chen, Lin, Wu and Chung567), EGCG(Reference Ogasawara, Matsunaga and Suzuki568) and anthocyanins(Reference Heinonen569) which are currently sold as food supplements and/or used for the production of functional foods(Reference Shahidi, Remacle and Reusens570). However, the results of experiments in which protection of oxidative DNA damage was monitored with foods containing these compounds are not promising, i.e. negative results were for example obtained with green vegetables, EGCG supplemented products(Reference Moller and Loft289, Reference Moller, Vogel, Pedersen, Dragsted, Sandstrom and Loft298, Reference Watzl and Leitzmann571) and anthocyan rich blueberries(Reference Duthie, Jenkinson, Crozier, Mullen, Pirie, Kyle, Yap, Christen and Duthie348). The reasons for these disappointing results are probably due to the fact that the active compounds which have a large molecular configuration are only poorly absorbed, therefore protective effects can be expected in the digestive tract but not in inner organs. In recent investigations, we found in a human intervention trial (SCGE experiments) with lymphocytes that gallic acid (GA), a small phenolic molecule, contained in specific plant foods reduces the formation of oxidised bases in peripheral lymphocytes with 30–40 fold higher efficiency as vitamins E and C while under in vitro conditions similar protective effects were observed(Reference Ferk, Knasmüller, Dusinska, Brantner, Uhl, Chakraborty, Bronzetti, Ferguson and De Flora296, Reference Ferk, Chakraborty, Simic, Kundi, Knasmüller and Schulte-Herman572). One of the reasons for the strong antioxidant properties of GA may be its high absorption rate(Reference Shahrzad, Aoyagi, Winter, Koyama and Bitsch573).
Overall, the predictive value of results obtained in in vivo experiments with rodents are higher as those of in vitro findings but one of the problems encountered in the interpretation of animal derived results is due to the fact that putative antioxidants were often administered at high doses which are irrelevant for humans.
The major problem of human studies concerns the fact that most experiments are carried out with peripheral blood cells and plasma and it remains unclear if and to which extent protection against ROS mediated damage can be expected in inner organs. In the case of GA we observed significant reduction of radical induced damage in a variety of inner organs in animal experiments(Reference Ferk, Chakraborty, Simic, Kundi, Knasmüller, Gee and Fogliano381, Reference Ferk, Chakraborty, Simic, Kundi, Knasmüller and Schulte-Herman572), also for a number of other compounds such as lycopene and vitamins and E it is known that the reduction of damage seen in blood cells in humans is paralleled by protective effects in a variety of tissues(Reference Ellinger, Ellinger and Stehle574, Reference Domenici, Vannucchi, Jordao, Meirelles and Vannucchi575).
An important question which has been neglected in human intervention trials concerns the impact of the overall antioxidant status of the participants on the outcome of protection studies. It can be expected that individuals which consume AO rich diets respond less sensitive. In the case of folic acid it has been shown that supplementation is only effective in regard to improvement of the DNA stability in individuals with low intake levels(Reference Beetstra, Thomas, Salisbury, Turner and Fenech576). On the basis of these findings Fenech(Reference Fenech577) developed the “genome health clinic and genome health nutrigenomics“ concept which postulates individualised supplementation strategies on the basis of biochemical and DNA-stability measurements and can be also applied to antioxidants(Reference Kazimirova, Barancokova, Krajcovicova-Kudlackova, Volkovova, Staruchova, Valachovicova, Paukova, Blazicek, Wsolova and Dusinska578, Reference Fenech579).
Justification of heath claims for antioxidant properties of dietary factors?
Growing consumer concerns about the health attributes of food products and supplements and the increased production of health foods have led to extensive discussions of the scientific evidence required for health claims(Reference Katan and de Roos580). Already in 2001, the global sales of functional foods were estimated USD 47,6 billion worldwide(Reference Sloan581). As a consequence, authorities such as the US Food and Drug Administration and the European Commission have issued new rules directed at inaccuracies, confusion and false information related to functional and dose related risk reduction claims(Reference Derby and Levy582–Reference Nickelson584). The EU regulation on nutrition and health claims made on foods (No. 1924/2006) entered into force on July 1st, 2007 and it is clearly stated in article 6 that claims have to be “based on and substantiated by generally accepted scientific evidence”.
The evaluation of the methods which are currently used to investigate the antioxidant properties of foods and food constituents show that not all approaches provide reliable results. Data from in vitro experiments cannot be extrapolated to the human situation in general, while the value of results obtained in animal and human studies depends largely on the experimental design and on the techniques employed. As mentioned above, frequently used methods such as TBARS measurements and the quantification of protein bound carbonyls and breath hydrocarbons do not provide firm evidence for AO effects, also some TAC measurements, which are conducted under unphyiological conditions (e.g. TOSC, CUPRAC), may not be reliable(Reference Aruoma171, Reference Hoelzl, Bichler, Ferk, Simic, Nersesyan, Elbling, Ehrlich, Chakraborty and Knasmuller257). Despite the uncertainities concerning the reliability of TAC measurements, results of such experiments can support the overall evidence for ROS protective effects but claims should be not solely based on such studies. Probably more relevant information can be expected from HPLC based isoprostane measurements as a marker for lipid peroxidation and from results of experiments concerning prevention of oxidative DNA-damage (HPLC based determination of oxidised bases, SCGE experiments with restriction enzymes). In this context it is notable that there is strong evidence that a number of ROS related diseases (including cancer) are causally directly related to damage of the genetic material (see introduction section).
As mentioned above, measurements of the induction of antioxidant enzymes do not provide reliable information for protective effects as they can also be induced by ROS themselves and their up regulation may reflect prooxidant effects.
Although the results obtained with -omics techniques no not allow to establish antioxidant specific patterns of gene transcription and protein alterations they can provide evidence for protective effects of antioxidants in specific experimental settings.
For example, it was found in a few studies that the transcription of genes caused by oxidative stress or by ROS related diseases can be normalised by dietary antioxidants which strongly supports the assumption of protective properties.
A promising strategy for the justification of health claims may be the combination of different methods to a test battery, but the selection of the individual approaches and techniques to be included will be a matter of intense debate.
Acknowledgements
The publication of this paper was made possible by the financial support of the European Co-operation in the field of Scientific and Technical (COST) Research Action 926 “Impact of new technologies on the health benefits and safety of bioactive plant compounds” (2004–2008). The authors had no conflicts of interest to disclose.