Introduction
Health technology assessment (HTA) is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its life cycle (Reference O'Rourke, Oortwijn and Schuller1). This information is used for health system decision making regarding the allocation of health resources, for example, whether or not to incorporate a health technology in the benefits package or to buy new diagnostic equipment.
In general, HTA is more solidly established and has a longer history of use in high-income countries than in low- or middle-income countries. These include countries in Latin America where the level of HTA development and use is heterogeneous (2–Reference Pichon-Riviere, Soto, Augustovski and Garcia-Marti5).
All health systems face resource constraints and even rich countries do not have the capacity to assess or incorporate all potentially effective technologies (Reference Henshall, Oortwijn, Stevens, Granados and Banta6). For this reason, it is always necessary to prioritize which technologies to assess. This is particularly true for low- and middle-income countries where the resources to conduct HTAs are scarcer. In such contexts, the selection of technologies to be assessed and considered for coverage and/or inclusion in the benefits package can cause serious distortions in resource use decision making if there are no clear and explicit mechanisms to guide the prioritization process (Reference Drummond, Schwartz, Jönsson, Luce, Neumann and Siebert7). The initial selection of technologies to be assessed can be more impactful than the postassessment results. Additionally, it is necessary to ensure the efficient and cost-effective use of resources dedicated to the assessment, which means asking if the assessment has the potential to offer the greatest benefit in relation to the cost implicated in the assessment itself (Reference Henshall, Oortwijn, Stevens, Granados and Banta6;Reference Drummond, Schwartz, Jönsson, Luce, Neumann and Siebert7). For these reasons, the need to establish priorities for which technologies to be assessed is neither a new nor minor issue for the HTA community. Although there are some common aspects in the prioritization processes developed over time by HTA agencies around the globe, no two countries, agencies, or health systems do this in the same way, and the same criteria are not used in all cases (Reference Henshall, Oortwijn, Stevens, Granados and Banta6;Reference Specchia, Favale, Di Nardo, Rotundi, Favaretti and Ricciardi8;Reference Frutos Pérez-Surio, Gimeno-Gracia, Alcácera López, Sagredo Samanes, Pardo Jario and Salvador Gómez9). This situation is likely attributable to different health contexts that inform the process and the components of the prioritization mechanism in accordance with the values and characteristics of the health system.
For 16 years, Health Technology Assessment International (HTAi) has organized the Global HTA Policy Forum (Policy Forum) with the objective of creating a neutral space for strategic discussions about the current state of HTA, its development, and implications for the health system, industry, patients, and other stakeholders (10;11). In 2016, in Costa Rica, the first Latin American Forum (LatamPF) was held (Reference Pichon-Riviere, Soto, Augustovski and Garcia-Marti5). The topic of the Forum was good practice principles for HTA in the region, and one of the priority principles identified for improvement and strengthening was the need for clear mechanisms for the prioritization of assessments to be completed by HTA agencies in Latin America.
This paper presents the results of the fifth Latin American Policy Forum (2020). The objective of the Forum was to explore how to improve the way HTA agencies in Latin America identify and prioritize technologies for assessment. The paper does not represent a formal consensus statement by the participants, and it should not be interpreted as necessarily representative of the opinions of the participants or the organizations where they work.
Methods
The scientific secretariat prepared a background document on the mechanisms of prioritization for technology assessment that exist in other countries (Reference Pichon Riviere, Garcia Martí, Alfie and Alcaraz12). This document was informed by the scientific and gray literature through a rapid search for recent key publications and discussions with the members of the Forum organizing committee. Additionally, the Web sites of selected HTA agencies and governments were searched (Germany, Spain, England, and Thailand) to identify mechanisms used to identify and prioritize technologies for assessment. These countries were selected to provide examples from high- and middle-income countries with diverse prioritization systems. The background document served to build a common understanding among the Forum participants, to harmonize definitions and key terms, and to support the discussions that were held during the in-person meeting (Reference Pichon Riviere, Garcia Martí, Alfie and Alcaraz12). Additionally, to include information about current practice in the region, a survey was administered to the country representatives participating in the LatamPF to understand the characteristics of the prioritization processes used by HTA agencies in their respective countries.
This fifth Forum was held in virtual format in October 2020 over three days in 2.5-h sessions each day. There were forty-six participants; eleven representatives of HTA agencies; six representatives of payers of public, social security, and private sectors; fifteen representatives from the industry (pharmaceuticals, medical devices, and diagnostics); two representatives from the Pan American Health Organization; two representatives from patient groups; four representatives from HTAi; and six academics, organizers, and staff from the scientific secretariat for the event. In total, there were eleven countries from the region represented: Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, Mexico, Paraguay, Peru, and Uruguay. The Acknowledgments section contains a list of participants, their affiliations, and countries. The format of the meeting included keynote presentations, breakout groups, and plenary discussions.
The objective of the first day of the event was to introduce the topic of the identification and prioritization of technologies for HTA processes from an international and a regional perspective, including the views of different stakeholders. Presentations included: the system for prioritizing technologies for assessment in Spain, as an example of a country with regional HTA agencies where different prioritization schemes coexist; an international view of different country experiences in the world; and an analysis of an experience from a country in the region, Brazil. Additionally, perspectives from the medical device industry and patient group representatives were shared. During the two days that followed, a series of group activities tailored to the virtual format were conducted following a design thinking methodology, which consisted of a set of cognitive, strategic, and practical processes used to redefine problems and create innovative solutions (Reference Johansson-Sköldberg, Woodilla and Çetinkaya13;Reference Brown and Funk14). The participants were divided into groups, balanced in terms of countries and stakeholders represented. After each group activity, the results were presented and debated in plenary sessions.
The objective of the first group activity was to discuss and analyze the current situation in the region regarding the process to prioritize technologies for assessment by HTA agencies. The discussion started with a debate about the reasons why many countries in the region have not put into place, formally at least, a systematic process to identify and prioritize technologies for assessment. Next were explored the consequences to the decision-making process that could be produced as a result of the lack of an explicit prioritization process. Using a voting mechanism provided in the virtual platform, participants identified the most important consequences, and the main barriers and facilitators to strengthening and/or improving these prioritization processes.
The second breakout group activity, on the third day of the Forum, consisted of identifying the main criteria and dimensions that should be taken into account by HTA agencies in Latin America when prioritizing technologies for assessment. Participants worked on a list of twenty-five criteria previously identified in the literature that are used by different HTA agencies and health systems around the world (Supplementary Material 2). During the breakout groups, these criteria were discussed and clarified and participants could propose other potential criteria that were not accounted for in the initial list. Through group discussions and two rounds of voting, one in the breakout groups and the second in the plenary session, the most relevant criteria to be considered in the technology identification and prioritization process in the region were identified. Furthermore, in this group activity and the plenary discussion that followed, Forum participants discussed a series of potential recommendations and future actions for HTA agencies in Latin America.
The Forum was conducted under the Chatham House Rules and all materials were produced in Spanish and English (15). A Spanish version of this paper is also available: https://doi.org/10.1017/S0266462321000519.
Results
Current State in the Region
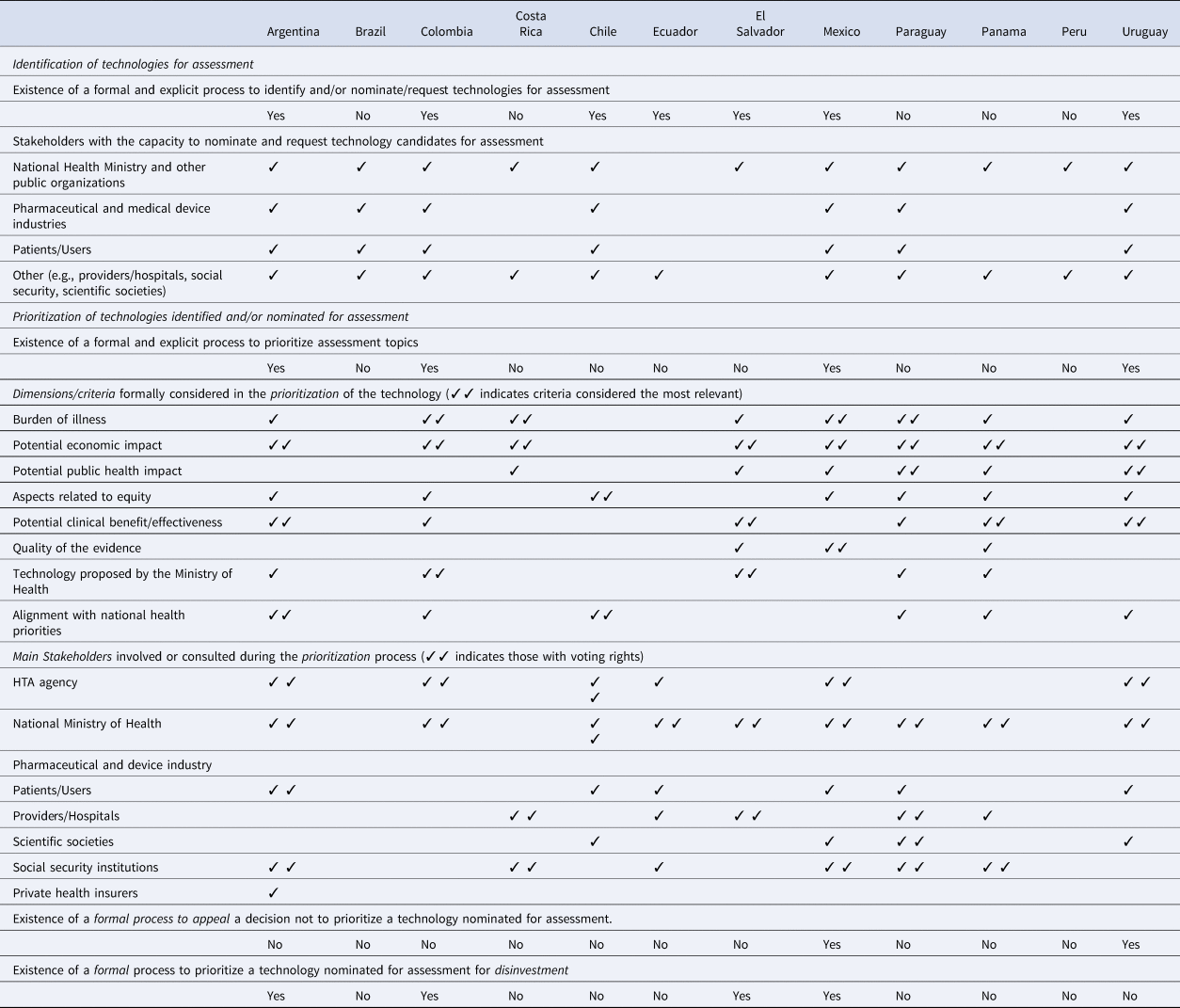
The results of the prioritization process characteristics survey of Latin American HTA agencies showed that seven of twelve countries currently have a formal process to identify and/or nominate candidate technologies for assessment and four of twelve countries have explicit, well-established processes to prioritize technologies and interventions for assessment by HTA agencies (Table 1). In four countries, the prioritization process included technologies considered for disinvestment. The burden of illness and the potential economic impact were the elements most frequently taken into account by agencies when prioritizing technologies for assessment. Two countries had mechanisms for stakeholder appeal or request for revision of the prioritization results. The complete results of the survey are presented in Supplementary Material 1.
Table 1. Main characteristics of the prioritization processes used by HTA agencies in Latin America: survey results (complete results of the survey in Supplementary Material 1)
Consequences of No Explicit Prioritization Process
The results of the breakout group discussions showed convergence in the observation that a lack of an explicit prioritization mechanism weakens the entire HTA and decision-making process. For example, decisions about the coverage or inclusion of technologies and interventions in the benefit package lose legitimacy if these technologies and interventions do not arise through a transparent prioritization process. The consequences can be, among others, that the whole HTA and decision-making process can be perceived as insufficiently transparent and excessively exposed to political or interest group pressure. A deficient or poor process to prioritize technologies for assessment can also carry consequences for the results of decisions, such as, for example, an increase in inequities in the health system, inefficient use of resources for the incorporation of interventions that are not a priority, or, on the other hand, delaying the coverage of interventions clearly beneficial and cost-effective. Finally, it was observed that where no clear procedure for prioritization exists, this could subsequently contribute to the judicialization of coverage decisions based on HTA. Figure 1 provides the main consequences identified by Forum participants.
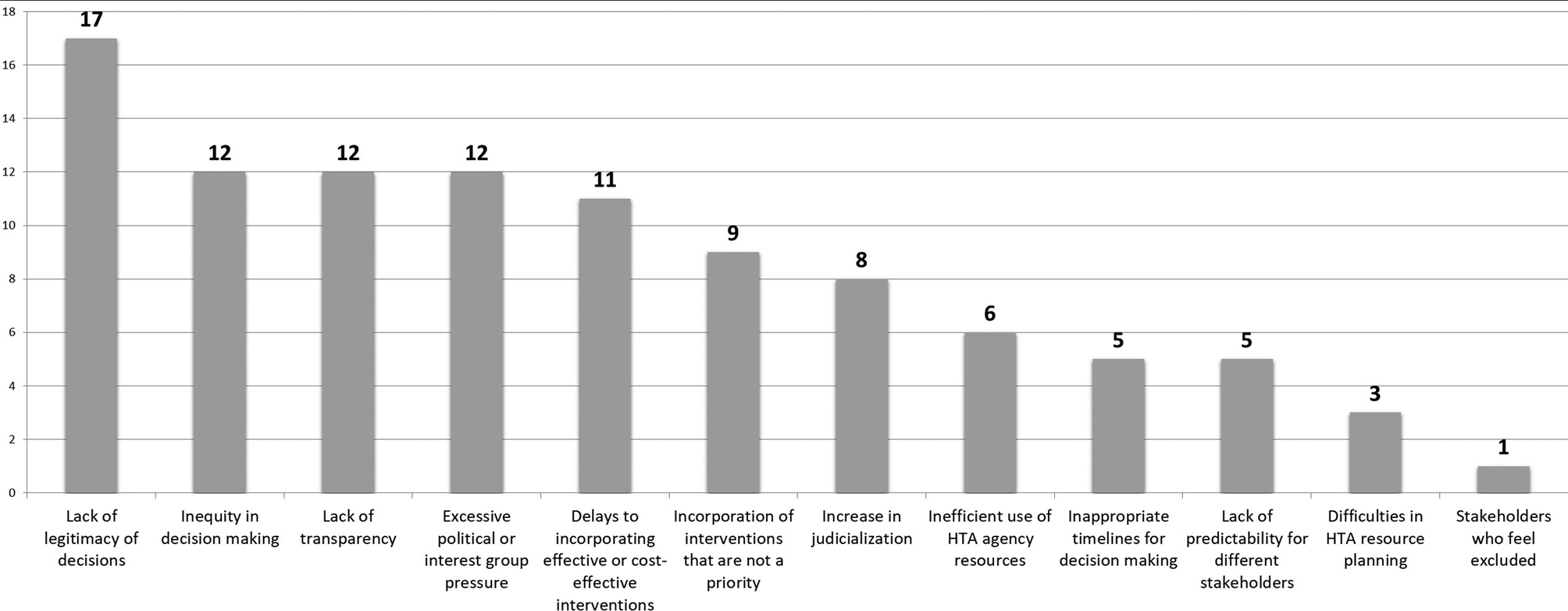
Figure 1. Consequences of having no explicit prioritization process identified by forum participants (the number indicates the frequency of mention). HTA, health technology assessment.
Barriers That Limit or Prevent the Establishment of Explicit Prioritization Processes
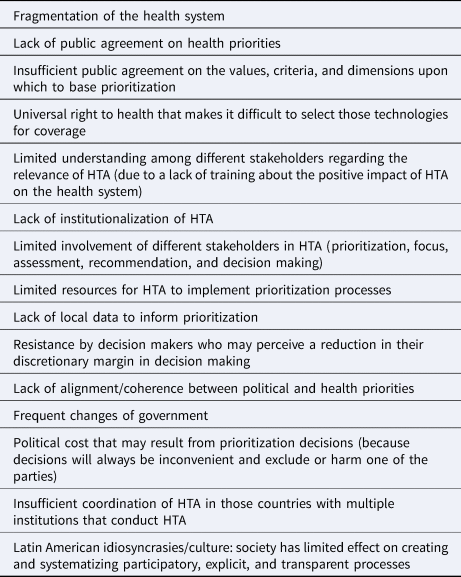
Two structural characteristics of health systems in the region were perceived as important elements that limit the establishment of improved technology assessment prioritization processes. These were: (i) the fragmentation of the health system, in other words, the existence of different organizations that make isolated decisions and that have distinct priorities and criteria regarding what is necessary and what is not; and (ii) the lack of institutionalization of HTA processes, which often implies a situation where the HTA agencies lack power within the system. Furthermore, other aspects at the macro level were considered to be barriers such as the lack of public agreement on health priorities, the low consensus about the values that should be considered for prioritization, and the universal right to health whose interpretation often makes it difficult to restrict access to technologies, even if they are not safe, effective, or cost-effective. At the HTA agency level, the barriers that were noted were the lack of resources and the challenges for some stakeholders to understand the process (typically due to limited/no training). These aspects make dialogue and participation of all potential stakeholders difficult. A barrier of special relevance is the lack of training for politicians and decision makers regarding the key role and positive impact that HTA can have on the road to universal health coverage. A lack of political will was also identified as a barrier, both in the political cost implicated in prioritization decisions and in the resistance of decision makers who may perceive an explicit prioritization process as a limit to their discretionary margin in decision making. Table 2 presents the barriers that were identified.
Table 2. Barriers that limit or impede the establishment of explicit prioritization processes
Facilitators for Advancing Toward More Explicit and Transparent Processes for Technology Assessment Prioritization by Agencies in Latin America
Despite the barriers identified, participants agreed that it is an appropriate moment for HTA agencies and countries in the region to move ahead in improving prioritization processes. There is an alignment of interests across all involved stakeholders (HTA agencies, patients and users, industry, and health professionals) who agree that a lack of prioritization processes can have negative consequences on the health system and there is a need to improve these processes. In recent years, Health Ministries in the region have begun to define lists of priority health conditions, often based on studies of burden of illness, and this information can be an important input for the HTA prioritization process. Moreover, there are varied experiences at the regional and international levels that can be taken as good examples and models for the design of prioritization mechanisms that are appropriate to the needs of each country. The involvement of different stakeholders in the HTA process and the mechanisms of early dialogue and horizon scanning were identified as additional potential facilitators. In addition, the generation of local evidence about the burden of disease to better inform health priorities and leveraging the work of regional networks and organizations can also support and facilitate this process.
Criteria and Dimensions for Prioritizing Technologies for Assessment
At the beginning of the breakout group work, participants identified some criteria that were not included on the original list provided beforehand (Supplementary Material 2): environmental impact, the opportunity cost, the benefits perceived by caregivers, potential cures, and technologies for prevention. After the breakout groups, where both the original list of criteria and those identified by participants were discussed, a total of eleven criteria were considered as the most relevant for consideration in the prioritization process. A vote was then conducted during the plenary session to identify the relative importance of these eleven criteria. The burden of illness of the condition that was to be treated by the intervention or technology, as well as the potential clinical benefit, were the two criteria considered the most important (see Figure 2). The alignment with national health priorities, the potential impact on equity, the lack of alternatives for patients (unmet need), the potential economic impact (cost-effectiveness and budget impact), and the organizational impact of the technology were also identified as priorities by a high proportion of participants. The disease severity, the quality of the evidence, and the potential impact on judicialization were other criteria identified as important.
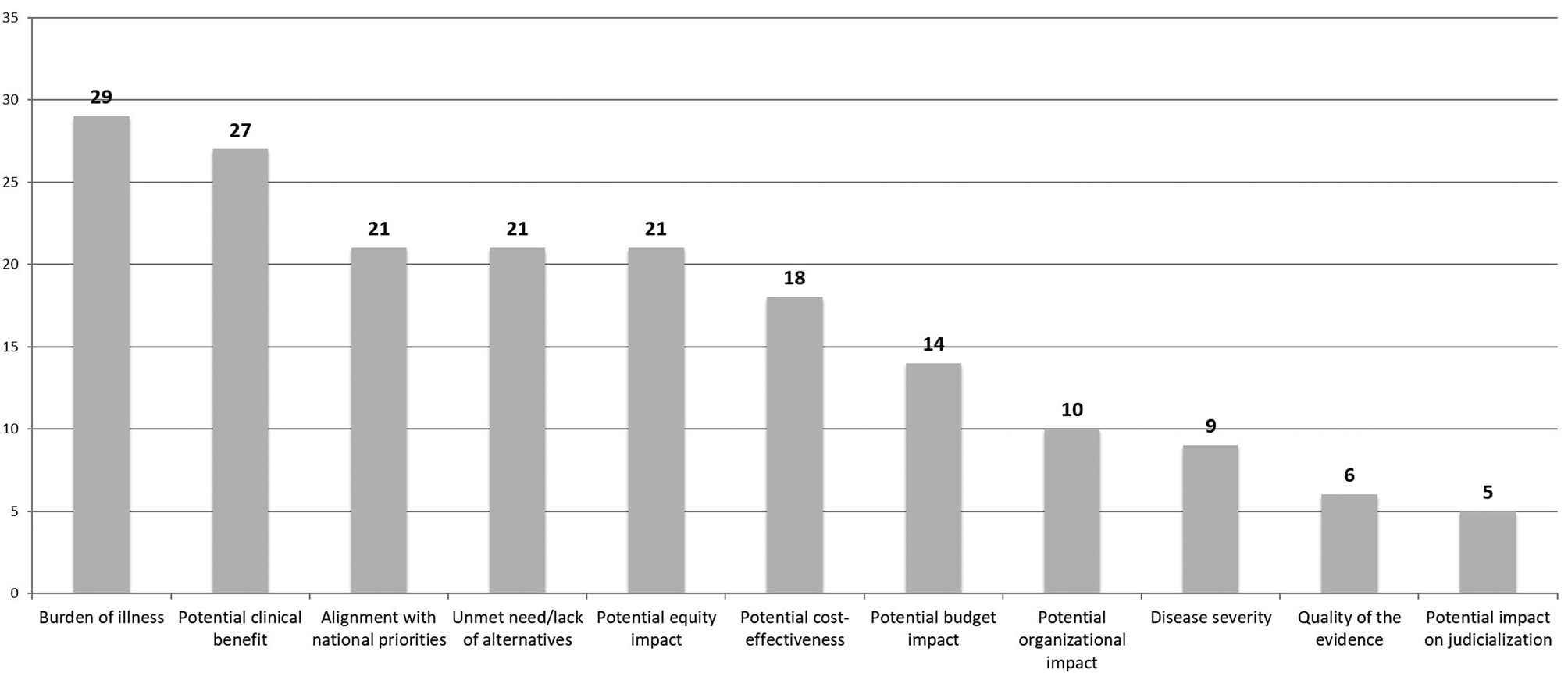
Figure 2. Principles, criteria, and dimensions to be taken into account during the prioritization of technologies and interventions for assessment by HTA agencies (the number indicates the number of votes received for each criterion). HTA, health technology assessment.
Policy Implications
Recommendations for Countries and Health Systems for the Establishment of More Formal Processes to Identify and Prioritize Technologies for Assessment
A published review of the criteria used by eleven agencies to prioritize technologies for assessment showed that the most frequently mentioned were clinical impact, the economic impact, the burden of illness, and the level of available evidence (Reference Noorani, Husereau, Boudreau and Skidmore16). An exhaustive review of the literature of the Web sites of leading HTA agencies published by Varela-Lema and colleagues (Reference Varela-Lema, Atienza-Merino and López-García17) in 2017 concluded that, although universal criteria were not identified nor were there standardized procedures for prioritization, the majority of agencies analyzed took into account eight domains of criteria: (i) the need for the intervention; (ii) health outcomes; (iii) the type of benefit of the intervention; (iv) economic consequences; (v) existing knowledge about the intervention/quality and uncertainty of the evidence; (vi) implementation and complexity of the intervention/feasibility; (vii) priority, justice, and ethics; and (viii) global context. Specchia et al. (Reference Specchia, Favale, Di Nardo, Rotundi, Favaretti and Ricciardi8) conducted a systematic review of the literature and of the Web sites of all European HTA agencies that are members of the International Network of Agencies for Health Technology Assessment (INAHTA). They found that the criteria most frequently cited for prioritization were the potential benefits of the technology, the cost and cost-effectiveness associated with the introduction of the technology, and the burden of disease. Those criteria identified in the international literature mirrored those identified by Forum participants as the most relevant criteria to guide the prioritization process in Latin America. In regard to the design of the prioritization process, the main recommendation of Forum participants was to start from a broad perspective, being both transparent and participatory, and involving all relevant stakeholders to ensure that the process is perceived as legitimate. With respect to the characteristics of the prioritization mechanism, the following recommendations were proposed, both for the prioritization process followed and for the process contents.
Characteristics of the Prioritization Process
1. Transparent, systematic, predictable, and sustainable
2. Defined times and clear rules for each of the stages
3. The simplest mechanism possible
4. The roles for all stakeholders clearly defined a priori
5. Established processes to ensure that the necessary information elements for the prioritization process are considered (e.g., disease prevalence, quality of the evidence)
6. Adaptable to the prioritization needs of different regions in a country or of different areas of the health sector
7. The mechanisms established relate well to different processes such as regulatory approval, nomination, prioritization, and assessment to be more efficient and reduce duplication of effort.
Contents of the Prioritization Process
1. Ideally, a mechanism that includes objective components (e.g., scores associated with the criteria and their weighting, to reduce discretion), but which also includes a deliberative component to allow the consideration of other factors.
2. It aims to include the fewest number of criteria possible in the prioritization design, while keeping these simple to define and evaluate.
3. The selected criteria reflect societal values and the stakeholders involved in the prioritization process.
4. The mechanism is strongly based on technical aspects (HTA as a “policy” tool) but thatalso takes into account political aspects (politics) that are implied in the prioritization process.
Suggested Actions to Advance in Improving the Prioritization Process
The proposed actions for initiating and driving improvements in the prioritization process were:
• Conduct an accurate diagnosis of the situation and sensitize different stakeholders about the benefits of HTA in the health system (showing clear examples in the region) and about the need for, and the favorable consequences of, establishing prioritization processes.
• Identify all relevant stakeholders who have a potential interest in the prioritization process and invite them to join in the participatory and inclusive design of these processes.
• Benefit from international and regional experiences (“benchmarking”; not “reinventing the wheel”), while avoiding copying foreign models without adequate contextualization.
• Benefit from regional networks to stimulate and support the improvement of these processes. A tangible action would be to conduct supranational horizon scanning where results are contextualized for each country in the region.
• Continue with and expand HTA training activities for different stakeholders (HTA agencies, patients and users, technology producers, and especially the health authorities/politicians after each change in government), noting that it is difficult to advance these processes without a common language and values.
• Promote the recording and collection of good quality local data.
• Promote a better institutionalization of the HTA process.
Conclusions
There is no country nor HTA agency that has the resources necessary to assess all possible technologies within the time frame required. Therefore, it is necessary for health systems and HTA agencies to establish a clear process to identify, prioritize, and select topics for assessment. Ultimately, the ideal objective is to prioritize and assess interventions and technologies with a high potential value for society. This is where HTA can bring forward the evidence relevant for decision making as a demonstration and justification of the value of the HTA effort and the resources invested in the assessment process.
Forum participants agreed that the establishment of transparent prioritization processes is a key element for all health systems, and there is now a window of opportunity to effect improvements to strengthen HTA in Latin America so as to provide greater legitimacy to decisions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266462321000416.
Acknowledgments
To the participants of the event for their valuable contributions:
Adriana María Robayo García, Instituto de Evaluación Tecnológica en Salud — IETS, Colombia; Alberto Salazar Ramirez, Johnson & Johnson, Mexico; Alexandre Lemgruber, PAHO — Pan American Health Organization, United States; Alicia Granados, Sanofi-Genzyme, Spain; Ana Eduviges Sancho Jimenez, Ministry of Health, Costa Rica; Andrea Alcaraz, Institute for Clinical Effectiveness and Health Policy (IECS), Argentina; Andrea Rappagliosi, Edwards Lifesciences, Switzerland; Andrés Pichón-Riviere, Institute for Clinical Effectiveness and Health Policy (IECS), Argentina; Aurelio Yamada, BioMarin, Brazil; Carlos Gonzalez Malla, Ministry of Health, Argentina; Cintia Parellada, MSD, Brazil; Debora Aligieri, Diabetes e Democracia, Brazil; Dino Sepúlveda Viveros, Ministry of Health, Chile; Eva María Ruiz de Castilla, Latin America Patient Academy (LAPA), United States; Fabiana Raynal Floriano, Ministry of Health, Brazil; Federico Augustovski, Institute for Clinical Effectiveness and Health Policy (IECS), Argentina; Fernanda Laranjeira, Johnson & Johnson, Brazil; Francisco Caccavo, PAHO — Pan American Health Organization, United States; Francisco Tellechea, F. Hoffmann-La Roche AG, Argentina; Gabriela Rodriguez, Institute for Clinical Effectiveness and Health Policy (IECS), Argentina; Graciela Fernandez, National Resources Fund, Uruguay; Héctor Castro, MSH Management Sciences for Health; HTAi United States; Homero Monsanto, MSD, Puerto Rico; Hugo Marín Piva, Costa Rican Social Security Fund (CCSSS), Costa Rica, Iñaki Gutierrez Ibarluzea, Head of the Organization and Innovation Management, Basque Government (Spain), HTAi, Spain; Johanna Elisabeth Guambo Coello, Ministry of Health, Ecuador; Joice Valentim, F. Hoffmann-La Roche AG, Brazil; José Thomaz, BioMarin, Brazil; Juan Luis Gerardo Durán Arenas, Mexican Social Security Institute, Mexico; Kariluz Maestre, Novartis Pharma AG, Colombia; Laura Sampietro-Colom, Hospital Clinic of Barcelona, HTAi, Spain; Leticia Perdomo, Ministry of Public Health of the Oriental Republic of Uruguay, Uruguay; Lizbeth Acuña Merchán, High Cost Account, Colombia; Manny Papadimitropoulos, Eli Lilly Interamerica Inc., Canada; Marcia Alves, Edwards Lifesciences, Brazil; Nora Reyes Puma, National Institute of Health, Perú; Paula Soledad Luque, Novartis Pharma AG, Argentina; Pedro Galván, Health Sciences Research Institute (IICS-UNA), Paraguay; Ricardo Humberto Ruano Arévalo, Ministry of Health, El Salvador; Santiago Torales, Ministry of Health, Argentina; Sebastián García Martí, Institute for Clinical Effectiveness and Health Policy (IECS), Argentina; Silvana Kelles, UNIMED, Brazil; Vania Cristina Canuto Santos, Ministry of Health, Brazil; Verónica Alfie, Institute for Clinical Effectiveness and Health Policy (IECS), Argentina; Wija Oortwijn, Radboud University Medical Centre, Department for Health Evidence, HTAi, Netherlands; William Dorling, Pfizer Inc., United States.
Thanks also to Tara Schuller for her assistance with the translation of the manuscript into English; to Iñaki Gutierrez-Ibarluzea and Wija Oortwijn for their presentations and participation in the Forum; and special thanks to Gabriela Rodriguez (IECS-Argentina) for her collaboration in the organization of the LatamPF.
Funding
The organization and attendance at the Forum were financed by the Health Technology Assessment International (HTAi). The preparation of the manuscript was undertaken by the Institute for Clinical Effectiveness and Health Policy (IECS) with funding support from the HTAi.






