There have been scant reports of hemicrania continua (HC) following head trauma resulting in concussion or traumatic brain injury (TBI). Even more rare are reports of HC resulting from lesional pathology. A review from 2004 found five documented cases of HC secondary to head trauma including a 38-year-old female with onset following a motor vehicle accident (MVA), a 48-year-old female with onset after a syncopal episode causing head injury, a 32-year-old female with onset after a fall resulting in TBI, a 30-year-old male with onset after a head injury on a bicycle, and a 34-year-old female (Trucco et al.). Reference Trucco, Mainardi, Maggioni, Badino and Zanchin1 All of these cases had normal neuroimaging. Prakash et al. reported two more cases in 2009, namely a 52-year-old male with a closed head injury ten years prior secondary to a MVA and a 44-year-old female with onset after MVA that caused a left-sided parietal hematoma requiring surgical evacuation. Finally, Finkel et al. (2017) reported 12 military personnel who developed HC following mild TBI. Reference Finkel, Yerry, Klaric, Ivins, Scher and Choi2
We encountered a young adult, right-hand dominant, male who presented for the assessment of headache. He was otherwise healthy and taking no medications. Fifteen years prior, he sustained a blunt force trauma to the head in South America. He developed a headache the following day, and workup revealed intracranial hemorrhage for which surgery was suggested but the patient declined.
Since his injury, there was mild continuous headache and weekly exacerbations. Exacerbations had moderate intensity (rated as a 4/10), but became severe (10/10) when periorbital edema was present. Headaches were strictly left-sided and had a pulsatile and pressure-type quality in the periorbital area. Exacerbations were associated with nausea and vomiting, diplopia, left-sided periorbital edema, conjunctival injection, and tearing. Red discoloration of his vision also occurred during these episodes. There was no photo- or phonosensitivity. Headaches were not exacerbated by activity. There was no relief with oral naproxen. Physical examination was unremarkable.
Magnetic resonance imaging of the brain revealed multiple abnormalities (Figure 1) including left mesial temporal encephalomalacia and T2-weighted imaging hyperintensities scattered throughout the supratentorial white matter. There was no abnormal enhancement.
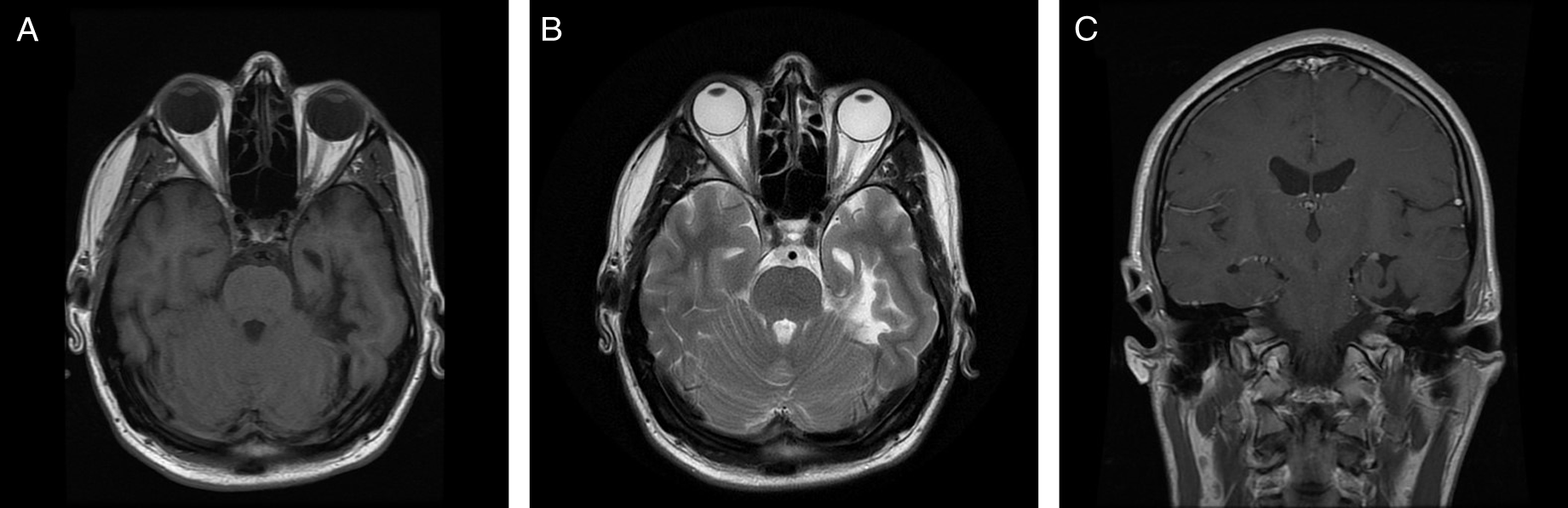
Figure 1: Neuroimaging findings. Axial T1-weighted image (A), axial T2-weighted image (B), and coronal T1-weighted image with gadolinium (C) demonstrate left mesial temporal encephalomalacia and atrophy.
He was diagnosed with persistent headache attributed to moderate or severe traumatic injury to the head with clinical features consistent with HC, unremitting type, and indomethacin was trialed at a dose of 75 mg PO TID. Within two weeks, he had complete resolution of headaches. Consumption of alcohol no longer triggered headaches. He gradually decreased the dose of indomethacin, and it was discontinued after 6 months. The patient remained headache-free.
HC is a rare primary headache disorder. It presents as a strictly unilateral, ‘side locked’ headache with ipsilateral autonomic features. There is typically a mild-to-moderate continuous headache with episodic exacerbations; however, the robust response to indomethacin is required for diagnosis.
Headache attributed to traumatic injury to the head, previously called post-traumatic headache, is a common cause of secondary headache and can have numerous clinical phenotypes that may mimic primary headache disorders.
Classically, there is no identifiable underlying pathology of HC; however, increasing numbers of symptomatic cases have been reported. Head trauma is a rare cause of HC, and this represents the first case due to a temporal lobe lesion. Such cases have been referred to as ‘secondary HC’ in the literature Reference Prakash, Shah and Soni3,Reference Prakash and Patel4 ; however, a more accurate diagnosis in accordance with The International Classification of Headache Disorders, 3rd edition (ICHD-3) is persistent headache attributed to moderate or severe traumatic injury to the head with clinical features consistent with HC. 5 While the relationship between TBI and onset of headache with features of HC may be spurious, the temporal relation in this case suggests causation. The authors hypothesize that the connections between the mesial temporal lobe and the posterior hypothalamus were disrupted, resulting in clinical HC.
Funding
The authors have no relevant funding to this article.
Conflicts of Interest
No conflicts of interest was reported by the authors.
Statement of Authorship
CRC drafted the manuscript and wrote the figure legend. CCL performed the patient’s clinical assessment, edited the manuscript, and created the figure. MWH assisted in the patient’s clinical assessment and performed critical revisions of the manuscript for intellectual content.