Folate, as an essential water-soluble B-complex vitamin, plays a critical role in many cellular reactions, including DNA, RNA, and protein methylation as well as DNA synthesis and maintenance( Reference Crider, Yang and Berry 1 ). Based on conclusive evidence that folic acid supplementation can prevent the occurrence of neural tube defects, women planning to become pregnant around the world are recommended to take 400 μg/d folic acid during the periconceptional period( Reference Gomes, Lopes and Pinto 2 ). To further increase folate intake, mandatory folic acid fortification programmes have also been implemented in numerous countries, including the USA, Canada, Chile and South Africa, but programmes have not been implemented in China( 3 ).
In 2009, the Ministry of Health of China issued a policy that free folic acid supplements (400 μg/d) were to be distributed to women of childbearing age in rural areas( 4 ). To ensure maternal and child health, the Chinese Nutrition Society advises women to take folic acid supplements throughout pregnancy( 5 ). However, to date, except for neural tube defects, the protective effects of folic acid on other adverse pregnancy outcomes are not generally recognised. While some epidemiological studies have observed an inverse association between folic acid supplementation and the risk of adverse outcomes( Reference Timmermans, Jaddoe and Hofman 6 – Reference Lamers, MacFarlane and O’Connor 9 ), others studies have found either no association or even a positive association( Reference Nilsen, Vollset and Monsen 10 , Reference Pastor-Valero, Navarrete-Munoz and Rebagliato 11 ). Furthermore, as the majority of these studies only focus on the periconceptional period, the evidence of the effect of taking folic acid supplements in later pregnancy on birth outcomes is limited( Reference Lamers, MacFarlane and O’Connor 9 , Reference Nilsen, Vollset and Monsen 10 ).
Small-for-gestational-age (SGA) is commonly defined as birth weight below the 10th percentile for gestational age and sex. Compared with low birth weight, SGA is a more comprehensive indicator for evaluating the growth and development of newborns( Reference Martinez-Galiano, Olmedo-Requena and Barrios-Rodriguez 12 ). It has been reported that SGA is associated with increased neonatal mortality and long-term health problems( Reference Werner, Savitz and Janevic 13 ). The fetal origins of health and disease framework suggests that it is imperative to improve maternal nutritional status and therefore prevent the occurrence of fetal growth restriction( Reference McGee, Bainbridge and Fontaine-Bisson 14 ). To our knowledge, few studies have investigated the association between maternal dietary folate intake during pregnancy and the risk of SGA births( Reference Nilsen, Vollset and Monsen 10 ).
In 2013, a population-based cross-sectional survey investigating the risk factors for adverse pregnancy outcomes and maternal nutritional status was conducted in Shaanxi Province of Northwest China. Using these data, the objective of the present study was to examine the influence of maternal folate intake from diet and supplements on the risk of SGA births.
Methods
Study design and population
The study design and methodology have been described in detail previously( Reference Yang, Dang and Cheng 15 , Reference Yang, Cheng and Pei 16 ). Briefly, a population-based cross-sectional survey was conducted in Shaanxi Province of Northwest China from August to November 2013. The target population for this survey was women who had been pregnant after 2010 and had a pregnancy outcome before enrolment. A stratified multistage random sampling method was adopted to determine the sampling units. First, in view of the proportion of urban to rural population, population density and fertility rate, twenty counties and ten districts were randomly selected from the entire province. Second, six townships from the sampled counties and three streets from the sampled districts were randomly selected. Third, six villages from the sampled townships and six communities from the sampled street were randomly selected. Subsequently, thirty and sixty women were randomly selected from the sampled village and community, respectively. With a response rate of 93 %, a total of 30 027 women consented to participate in the survey. All women were interviewed in person by trained study interviewers using a standardised and structured questionnaire. The questionnaire collected information on socio-demographic characteristics, reproductive history, family history of diseases, environmental/personal exposures, lifestyle factors, nutritional supplements use before conception and during pregnancy, and birth outcomes. Among all participants, 7750 women who had given birth to live children less than 12 months prior to recruitment were further interviewed to report dietary intakes during pregnancy. For this analysis, 443 participants were excluded because of multiple births (n 87), implausible energy intake (<4500 or >20 000 kJ/d)( Reference Meltzer, Brantsaeter and Ydersbond 17 ) (n 316), missing data on birth weight or gestational age (n 11), and gestational age <33 weeks or >42 weeks (n 29)( Reference Villar, Cheikh Ismail and Victora 18 ). Thus, the final population for analysis comprised 7307 women, and the median postpartum month when participants were interviewed was 3 (10th–90th percentiles: 0–7). The flow diagram of recruitment in the present study is provided in the online Supplementary Fig. S1.
The survey was conducted according to the guidelines laid down in the Declaration of Helsinki. All study procedures were approved by the ethics review committee of the Xi’an Jiaotong University Health Science Center, and written informed consent was obtained from each participant before enrolment.
Assessment of nutrient intake
All participants were asked to report the brand/type and duration of all nutritional supplements they consumed during the following four stages: before conception (12 weeks before pregnancy), first trimester (1–12 weeks), second trimester (13–27 weeks) and third trimester (≥28 weeks). For folic acid supplements, users were defined as those who used folic acid supplements alone or folic acid-containing multivitamins before or during pregnancy, while non-users were defined as those who never took folic acid supplements alone or folic acid-containing multivitamins before and during pregnancy. The definitions of users and non-users mentioned above also applied to Ca and Fe supplements.
Women who had infants less than 12 months of age (median, 3 months; 10th–90th percentile, 0–7 months) were also asked to report their diets during pregnancy via a semi-quantitative FFQ. Considering that dietary patterns and intakes did not change substantially during pregnancy( Reference Rifas-Shiman, Rich-Edwards and Willett 19 – Reference Crozier, Robinson and Godfrey 21 ), and that it is burdensome and costly to collect dietary data at more than one period during pregnancy, we assessed average dietary intakes throughout pregnancy at one time in this investigation. The FFQ throughout pregnancy was adapted from the previously validated FFQ, which was designed for pregnant women during the third trimester in Northwest China( Reference Cheng, Yan and Dibley 22 ). Pearson’s correlation coefficients between the FFQ and the 24-h recalls were 0·66 for folate and 0·53–0·70 for other nutrients( Reference Cheng, Yan and Dibley 22 ). The questionnaire used in this survey has a food list of 107 items, including grains and potatoes, vegetables, fruit, meat and meat products, eggs, soya bean products and nuts, dairy and dairy products, fish, shrimp and shellfish, fungi and algae, alcoholic and non-alcoholic beverages, sugar, snacks and fast foods, and edible oil and condiments. The frequency scale of five food items (vegetable oil, animal oil, salt, sauce and sugar) was open-ended and recorded as kg per month and the number of family members who regularly consumed them (children under 12 years old were counted as one half of a person). The other 102 food items had a choice of eight frequency categories ranging from ‘never or almost not’ to ‘two or more times per d’. With the assistance of food photographs( Reference Cheng, Zhou and Zhang 23 ), estimates of portion sizes for the 102 food items were recorded as large, medium or small.
The daily supplemental folic acid intake was calculated by multiplying the folic acid content of each reported supplement by the number of supplement tablets taken per d. The daily intakes of total energy and folate were estimated by multiplying the consumption of each food item by the nutrient composition per gram of the corresponding food as obtained from the China Food Composition Table( 24 , 25 ). Dietary folate intake was adjusted for total energy intake using the residual method( Reference Willett 26 ). The average supplemental folic acid intake during the first, second and third trimesters was converted into the dietary folate equivalent by multiplying with 1·7 and added to the dietary folate intake throughout pregnancy to calculate total folate intake during pregnancy.
Ascertainment of birth outcomes
Birth outcomes, including sex, birth date, gestational age, birth weight and birth length, were abstracted from the Medical Certificate of Birth. Birth weight was measured to the nearest 10 g. Gestational age was calculated in weeks based on the first day of the last menstrual period. Preterm birth was defined as a delivery before 37 completed weeks. The sex- and gestational age-adjusted birth weight Z score was calculated according to international standards developed by the International Fetal and Newborn Growth Consortium for the 21st Century( Reference Villar, Cheikh Ismail and Victora 18 ). SGA was defined as birth weight Z score below the 10th percentile.
Potential covariates
Potential covariates mainly consisted of two parts: socio-demographic characteristics, including geographic area (northern, southern or central), maternal age at delivery (continuous), maternal education (primary school or below, junior school, or senior high school or above), maternal occupation (farmer or others), household wealth index (continuous), and parity (primiparous or multiparous); and health-related behaviours during pregnancy, including passive smoking (yes or no), alcohol consumption (yes or no), pregnancy complications (yes or no), medication use (yes or no), Fe supplementation (yes or no), and Ca supplementation (yes or no). The household wealth index was constructed from five variables reflecting family economic level (housing conditions, type of vehicle, income resources, and type and number of household appliances) through principal component analysis( Reference Pei, Kang and Zhao 27 ). Passive smoking was defined as being exposed to tobacco smoke from others for at least 15 min/d. Self-reported pregnancy complications included a series of diseases, such as anaemia, gestational hypertension, gestational diabetes, and intrahepatic cholestasis of pregnancy.
Statistical analysis
Comparisons of proportions or means of baseline characteristics between SGA and non-SGA infants were performed using χ2 tests or t tests. As the proportion of women taking folic acid supplements after the 12th week of pregnancy was small, we combined the second trimester users and the third trimester users into one group of women who took folic acid supplements during the second or third trimester. The duration of folic acid supplementation during each pregnancy stage was interpreted as change per 10-d increase and classified into two groups by the median value. Both dietary folate intake and total folate intake were log-transformed to improve normality before analyses and categorised into tertiles.
As the survey data obtained from the multistage sampling design were hierarchically structured, multilevel models were performed to estimate the association of folate (dietary folate, supplemental folic acid and total folate) intake with the risk of SGA births. First, a four-level model where levels 1, 2, 3, and 4 represent the individual, village (street), township (community) and county (district), respectively, was established. After running the empty model, we only observed significant within-group homogeneity (intra-class correlation = 0·04, P < 0·05) at the county (district) level. Thus, the simplified two-level logistic regression model with a random intercept at the county (district) level was applied to estimate the OR and 95 % CI for SGA births associated with folate intake before and after controlling for confounders.
To avoid incomplete adjustment or over-adjustment, we identified a minimum set of confounders from the potential covariates by using a directed acyclic graph with the DAGitty programme (version 2.3; Johannes Textor)( Reference Textor, Hardt and Knuppel 28 ). The selected potential confounders included geographic area, maternal age at delivery (continuous), maternal education, maternal occupation, household wealth index (continuous) and parity (online Supplementary Fig. S2). Tests for linear trend were performed by entering the median value of each categorical level into regression models as a continuous variable. In addition, a two-level linear regression analysis was conducted to estimate the associations between birth weight Z score and folate intake following the same strategy as that for SGA.
To rule out the potential synergistic effect between folic acid and other nutrients, we conducted sensitivity analyses by excluding women who reported folic acid-containing multivitamins use during any stage of pregnancy (n 645). All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc.). Results were considered significant with a two-sided P < 0·05.
Results
Population characteristics
Among the 7307 participants, the prevalence of SGA births was 10·6 %. Distributions of baseline characteristics of participants by SGA status are presented in Table 1. Compared with women who delivered non-SGA infants, those who gave birth to SGA infants were more likely to have a lower education level, to be farmers, to be poorer, and were less likely to take Ca supplements. The distributions of geographic area, maternal age at delivery, parity, passive smoking, alcohol consumption, pregnancy complications, medication use and Fe supplementation were similar between the non-SGA and SGA groups. In addition, SGA infants had longer gestational age than non-SGA infants. SGA births were negatively associated with preterm births.
Table 1. Comparison of baseline characteristics of participants by status of small-for-gestational-age (SGA) births in Shaanxi Province, Northwest China
(Numbers and percentages; mean values and standard deviations)
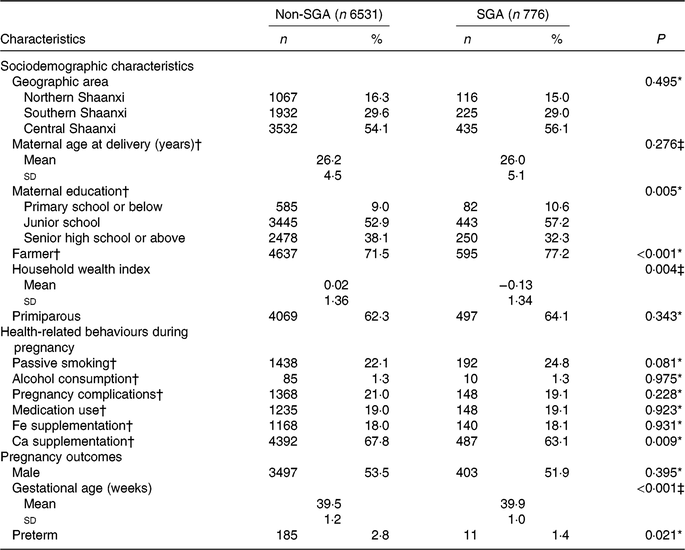
* Comparison by χ2 test.
† Information was missing for sixty-one participants on maternal age at delivery, twenty-four participants on maternal education, fifty-one participants on maternal occupation, nineteen participants on passive smoking, twelve participants on alcohol consumption, three participants on pregnancy complications, sixteen participants on medication use, thirty participants on Fe supplementation, and fifty-four participants on Ca supplementation.
‡ Comparison by t test.
Dietary folate intake throughout pregnancy and small-for-gestational-age
The median intake of dietary folate throughout pregnancy was 299·3 (interquartile range (IQR) 145·6) μg/d among all participants and was 203·3 (IQR 76·0), 299·3 (IQR 44·3), and 421·3 (IQR 125·4) μg/d in the first, second, and third tertiles, respectively. The association between dietary folate intake and the risk of SGA births was not significant whether dietary folate intake was analysed as a continuous variable or categorical variable. Adjustment for selected potential confounders did not meaningfully change the results (Table 2).
Table 2. Association between dietary folate intake throughout pregnancy and risk of small-for-gestational-age births in Shaanxi Province, Northwest China*
(Odds ratios and 95 % confidence intervals)
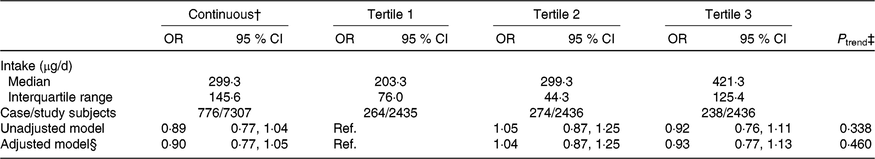
Ref., reference.
* Two-level logistic regression models were used to estimate OR and 95 % CI.
† Per one-unit increase in the log-transformed value of dietary folate intake.
‡ P trend was obtained by using the median value of each folate intake tertile as a continuous variable in the regression models.
§ Adjusted for geographic area, maternal age at delivery (continuous), maternal education, maternal occupation, household wealth index (continuous) and parity.
Folic acid supplementation during each pregnancy stage and small-for-gestational-age
Overall, 76·0 % of women (n 5524) used folic acid supplements before conception or during pregnancy. The prevalence of taking folic acid supplements alone before conception, and during the first, second and third trimesters was 20·0 % (n 1463), 65·3 % (n 4771), 9·2 % (n 674), and 4·0 % (n 290), respectively. The prevalence of taking folic acid-containing multivitamins before conception, and during the first, second and third trimesters was 0·3 % (n 21), 0·9 % (n 68), 4·8 % (n 352), and 3·0 % (n 222), respectively. The dose of folic acid in most multivitamins was 400 μg/d. The associations between folic acid supplementation during each pregnancy stage and SGA risk are presented in Fig. 1. Women who took folic acid supplements before conception or during pregnancy had a 23 % lower risk of delivering an SGA infant (OR 0·77; 95 % CI 0·64, 0·91). When taking the timing into account, folic acid supplementation during the first trimester was associated with a 22 % reduction in the risk of SGA births (OR 0·78; 95 % CI 0·67, 0·92), whereas no significant association was observed when folic acid supplements were taken before conception or during the second or third trimester. When investigating the duration, a longer duration of folic acid supplementation before conception or during pregnancy (≤90 d v. non-use: OR 0·78; 95 % CI 0·65 0·94; >90 d v. non-use: OR 0·71; 95 % CI 0·56, 0·91; P trend = 0·011; per 10-d increase: OR 0·98; 95 % CI 0·97, 0·99) or during the first trimester (≤60 d v. non-use: OR 0·80; 95 % CI 0·66, 0·96; >60 d v. non-use: OR 0·78; 95 % CI 0·65, 0·94; P trend = 0·010; per 10-d increase: OR 0·97; 95 % CI 0·95, 0·99) was negatively associated with SGA risk (Table 3).
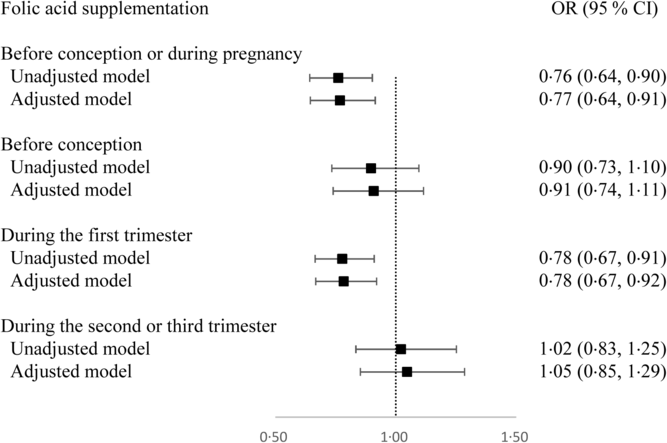
Fig. 1. Odds ratios of small-for-gestational-age births associated with folic acid supplementation during each pregnancy stage in Shaanxi Province, Northwest China. Two-level logistic regression models were used to estimate odds ratios and 95 % confidence intervals. The adjusted model was adjusted for geographic area, maternal age at delivery (continuous), maternal education, maternal occupation, household wealth index (continuous) and parity.
Table 3. Association between the duration of folic acid supplementation during each pregnancy stage and risk of small-for-gestational-age births in Shaanxi Province, Northwest China*
(Odds ratios and 95 % confidence intervals)
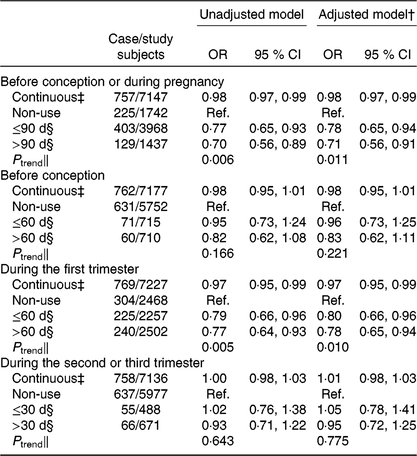
Ref., reference.
* Two-level logistic regression models were used to estimate OR and 95 % CI.
† Adjusted for geographic area, maternal age at delivery (continuous), maternal education, maternal occupation, household wealth index (continuous) and parity.
‡ Per 10-d increase in the duration of folic acid supplementation.
§ The duration of folic acid supplementation during each pregnancy stage was classified into two groups by the median value.
‖ P trend was obtained by using the median duration of folic acid supplementation per group as a continuous variable in the regression models.
Total folate intake during pregnancy and small-for-gestational-age
The median intake of total folate during pregnancy was 426·0 (IQR 239·7) μg/d among all participants and was 277·5 (IQR 99·4), 426·0 (IQR 77·2), and 625·8 (IQR 195·7) μg/d in the first, second, and third tertiles, respectively. After adjustment for selected potential confounders, a higher total folate intake was associated with a lower risk of SGA births (highest tertile v. lowest tertile: OR 0·77; 95 % CI 0·64, 0·94; P trend = 0·010; per one-unit increase in the log-transformed value: OR 0·81; 95 % CI 0·69, 0·95) (Table 4).
Table 4. Association between total folate intake during pregnancy and risk of small-for-gestational-age births in Shaanxi Province, Northwest China*
(Odds ratios and 95 % confidence intervals)
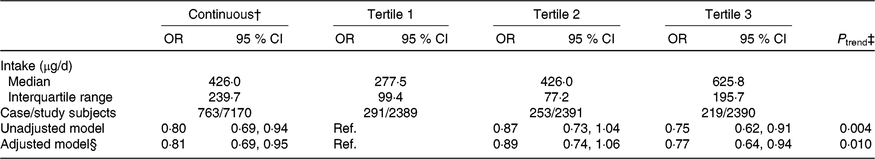
Ref., reference.
* Two-level logistic regression models were used to estimate OR and 95 % CI.
† Per one-unit increase in the log-transformed value of total folate intake.
‡ P trend was obtained by using the median value of each total folate intake tertile as continuous variable in regression models.
§ Adjusted for geographic area, maternal age at delivery (continuous), maternal education, maternal occupation, household wealth index (continuous) and parity.
Folate (dietary folate, supplemental folic acid and total folate) intake and birth weight Z score
When controlling for selected potential confounders, dietary folate intake throughout pregnancy was positively associated with birth weight Z score (per one-unit increase in the log-transformed value: β-coefficient 0·058; 95 % CI 0·007, 0·108). For every 10-d increase in the duration of folic acid supplementation before conception or during pregnancy, the birth weight Z score increased by 0·006 (95 % CI 0·001, 0·010). For every 10-d increase in the duration of folic acid supplementation during the first trimester, the birth weight Z score increased by 0·008 (95 % CI 0·001, 0·014). A higher total folate intake during pregnancy was associated with a higher birth weight Z score (per one-unit increase in the log-transformed value: β-coefficient 0·104; 95 % CI 0·053, 0·156) (online Supplementary Table S1).
Sensitivity analysis
Additional sensitivity analysis excluding women who reported folic acid-containing multivitamins use before conception or during pregnancy (n 645) did not change these results (data not shown).
Discussion
In this population-based cross-sectional study, we found that both a longer duration of folic acid supplementation during the first trimester and a higher total folate intake during pregnancy were associated with a reduced risk of SGA births. The dietary folate intake throughout pregnancy, the duration of folic acid supplementation during the first trimester and the total folate intake during pregnancy were positively associated with the birth weight Z score.
The requirement of folate increases during pregnancy due to the increase in maternal blood volume, the growth of fetus and placenta, and the enlargement of the uterus( Reference Bailey 29 ). In light of the importance of folate in preventing neural tube defects, women of childbearing age worldwide are recommended to take folic acid supplements from 3 months before conception to the first trimester( Reference Gomes, Lopes and Pinto 2 ). In the past two decades, it has been reported that folic acid supplementation during the periconceptional period may protect pregnant women against the risk of a wide spectrum of adverse outcomes, such as pre-eclampsia( Reference Wang, Zhao and Qiu 30 ), preterm delivery( Reference Liu, Lv and Zhang 31 ), fetal growth restriction( Reference Timmermans, Jaddoe and Hofman 6 – Reference Lamers, MacFarlane and O’Connor 9 ) and congenital heart defects( Reference Liu, Joseph and Luo 32 ). However, except for neural tube defects, the potential preventative effect of folate in relation to adverse pregnancy outcomes such as SGA remains controversial. The Generation R study found that women who started folic acid supplements preconceptionally have a reduced risk of SGA births( Reference Timmermans, Jaddoe and Hofman 6 ), and further demonstrated that low folate concentration during early pregnancy was associated with lower placental weight and birth weight and increased risks of SGA births( Reference Bergen, Jaddoe and Timmermans 33 ). Similarly, a large prospective cohort study in Southern China showed that taking folic acid supplements in the first trimester or in the periconceptional period can reduce the risk of SGA births( Reference Li, Li and Ye 7 ). In contrast, the Danish National Birth cohort study did not observe any associations between preconceptional and postconceptional folic acid alone use and SGA risk( Reference Catov, Bodnar and Olsen 34 ). Possible reasons for the inconsistency between studies include differences in baseline nutritional status, genetic background, sample size, controlling for confounders, or the prevalence and dose of folic acid supplementation. In the present study, we found that a higher supplemental folic acid intake during early pregnancy was negatively associated with SGA risk and positively associated with the birth weight Z score. Currently, the underlying molecular mechanisms whereby folic acid can prevent adverse pregnancy outcomes are poorly understood. It is speculated that epigenetic modifications, especially DNA methylation, may be involved in these processes( Reference McGee, Bainbridge and Fontaine-Bisson 14 ). Folate is an essential component in the one-carbon metabolism pathway as it can provide methyl groups for a series of biochemical reactions, including DNA methylation. Abnormal methylation patterns, which are caused by maternal folate malnutrition, have a critical effect on fetal gene expression and short-term and long-term health( Reference Anderson, Sant and Dolinoy 35 ).
Compared with the periconceptional period, the benefits of folate in middle and late pregnancy were less studied. One study conducted in Norwegian pregnant women found no evidence that plasma folate concentrations, food folate intake, or folic acid supplementation during the second trimester was associated with the risk of SGA births( Reference Nilsen, Vollset and Monsen 10 ). Due to the small proportion of pregnant women who took folic acid supplements after early pregnancy, the second trimester users and the third trimester users were combined into one group in the present study. We also did not observe any significant association between folic acid supplementation during the second or third trimester and the risk of SGA births and the birth weight Z score. Our results suggested that fetal growth is most affected by maternal folate deficiencies during the early stage of pregnancy. A plausible explanation for this finding is that DNA methylation patterns required for normal tissue development were established during the early stage of embryonic development( Reference Nafee, Farrell and Carroll 36 ).
Our study showed that a higher dietary folate intake throughout pregnancy was associated with a higher birth weight Z score and that total folate intake during pregnancy was negatively associated with SGA risks and positively associated with the birth weight Z score. As dietary folate is less stable and has a substantially lower bioavailability than synthetic folic acid( Reference McGee, Bainbridge and Fontaine-Bisson 14 ), the association of total folate intake during pregnancy with SGA risk in the present study was largely attributed to folic acid supplements. Large-scale cohort studies with more comprehensive information on folate status (dietary folate, folic acid supplements, serum folate, and erythrocyte folate) at multiple time points (before conception, in early pregnancy, in middle pregnancy, and in late pregnancy) are needed to further demonstrate the association of folate intake with intra-uterine growth restriction.
Despite the unequivocal effect of folic acid intervention in preventing neural tube defects, concerns have been raised regarding the potential risk of maternal folate intake. A study in Spain showed that periconceptional use of more than 1 mg/d of folic acid supplements was associated with decreased birth height( Reference Pastor-Valero, Navarrete-Munoz and Rebagliato 11 ). In India, to prevent neural tube defects, pregnant women are often prescribed high doses of folic acid (5 mg or more per d), even though they are already in the second trimester. The Pune Maternal Nutrition Study found that higher maternal erythrocyte folate concentrations at 28 weeks predicted higher insulin resistance and greater adiposity in offspring( Reference Yajnik, Deshpande and Jackson 37 ). In addition, in a study of 490 children in Australia, maternal folic acid supplementation (median, 300·0 μg/d; range, 27·4–895·4 μg/d) in late pregnancy was associated with an increased risk of childhood asthma at 3·5 years( Reference Whitrow, Moore and Rumbold 38 ). The aforementioned epidemiological evidence suggested that both the dosage and timing of folic acid supplementation were vital for the short-term and long-term health of offspring. Unlike many countries that choose fortification of staple foods with folic acid as the primary strategy to prevent neural tube defects, the Chinese government began distributing folic acid supplements free of charge to women of childbearing age in rural areas in 2009( 4 ). One study conducted in China showed that although plasma folate concentrations among pregnant women increased dramatically after the folic acid supplementation programme, gaps remained between areas with high and low prevalence of neural tube defects( Reference Liu, Gao and Zhang 39 ). While 7·6 % of women in the high-prevalence area were folate deficient, few women (0·3 %) in the low-prevalence area had plasma folate concentrations below the cut-off values for folate deficiency( Reference Liu, Gao and Zhang 39 ). The proportion of folate deficiency in the area with low prevalence of neural tube defects is close to that of Japan (0·5 %)( Reference Yila, Araki and Sasaki 40 ). To our knowledge, reports on the undesirable consequences of folic acid supplements in China are scarce. More longitudinal studies are warranted to provide a scientific basis for the development of policies and recommendations for folic acid supplementation.
The main strength of the present study is that our findings can be extended to the entire Shaanxi Province, which is attributed to the adoption of a stratified multistage random sampling method. Moreover, the assessment of folate from both diet and supplements allowed us to comprehensively examine the effect of nutrient intake on birth outcomes. However, our findings should be interpreted cautiously in light of some methodological limitations. First, due to the cross-sectional design of this survey, the observed association between folic acid supplements and the risk of SGA births could not be characterised as causal. Second, in the present study, women were asked to retrospectively report nutrient intakes and behavioural factors during pregnancy at 0–12 months (median, 3 months; 10th–90th percentile, 0–7 months) after delivery. Despite previous studies indicating that dietary intakes and supplements use during pregnancy can be recalled accurately after many years( Reference Bunin, Gyllstrom and Brown 41 , Reference Bosco, Tseng and Spector 42 ), we cannot rule out exposure misclassification due to recall bias. However, we believe that the exposure misclassification could be non-differential as the protective effect of folate on adverse pregnancy outcomes other than neural tube defects was not known during this investigation. Third, we used a FFQ for the dietary intakes assessment, which is not precise in estimating absolute intakes. However, compared with other methods, such as food records and 24-h recalls, the FFQ can reflect intakes over a longer periods of time and can be used to rank intakes( Reference Beaton, Milner and Corey 43 ). Fourth, we assessed the average dietary intakes during pregnancy at one time due to the convenience and low cost in large epidemiological studies. Although some studies suggested that dietary patterns and intakes did not change appreciably from early pregnancy to third trimester( Reference Rifas-Shiman, Rich-Edwards and Willett 19 – Reference Crozier, Robinson and Godfrey 21 ), the fluctuation of dietary intakes during pregnancy in the Chinese population remains to be demonstrated. Fifth, although we control for several confounders in our models, residual confounding may still exist. For example, we did not collect pre-pregnancy BMI, which was reported to be negatively associated with the risk of SGA births( Reference Li, Liu and Guo 44 ). However, as previous studies in China suggested that maternal BMI was negatively associated with folic acid supplementation( Reference Li, Li and Ye 7 , Reference Zheng, Guan and Zhao 8 ), the association of supplemental folic acid intake with SGA risk in the present study could be underestimated( Reference Mehio-Sibai, Feinleib and Sibai 45 ). Sixth, since a number of statistical tests were conducted in the present study, the probability of the type I error increased. Last, because of the lack of maternal or neonatal biomarkers, such as erythrocyte folate and serum folate, the relationship between folate intake and the risk of SGA births cannot be further confirmed.
In conclusion, in the cross-sectional study in Northwest China, we found that folic acid supplementation during the first trimester and a higher total folate intake during pregnancy were associated with a reduced risk of SGA births. More studies are needed to elucidate the effects of the timing of folic acid supplementation on fetal growth and development and the mechanisms involved.
Acknowledgements
The authors are grateful to all participants and investigators in the present study.
The present study was founded by the National Key Research and Development Program of China (grant no. 2017YFC0907200, grant no. 2017YFC0907201), the National Natural Science Foundation of China (grant no. 81230016), the Project of birth defect control and prevention in Shaanxi (grant no. Sxwsjswzfcght2016-013) and the China Postdoctoral Science Foundation (grant no. 2015M582678). The funders had no role in study design, data collection and analysis, and decision to submit the manuscript for publication.
S. D. and H. Y. conceived and designed the study; R. Z. and Z. Z. conducted the research; S. L., F. L., C. L., M. X., L. G., B. Z., and M. L. performed the statistical analysis; S. l., D. L., X. L., Y. C., R. Z., Z. Z., G. S., and Y. L. wrote the paper; All authors reviewed and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001272








