In 1953, proprioceptive malfunction was proposed as a core feature of schizophrenia by Sandor Rado (Reference RadoRado, 1953). It was this hypothesis that spurred the development of a new method to investigate proprioceptive information processing through electroencephalographic event-related potentials (Reference Arnfred, Chen and EderArnfred et al, 2000).
A brisk change of load on a hand-held handle elicits a proprioceptive event-related potential consisting of an early contralateral component (P60/N70) followed by the more central orienting response components (N160-P200) and, when associated with an experimental task, also late cognitive components (P360) (Reference ArnfredArnfred, 2005).
Previous event-related potential research in schizophrenia has followed two directions. The early cortical processing of stimuli has been investigated using passive paradigms such as the auditory P50 gating paradigm, whereas later processing has been examined in active cognitive tasks. Similarly, we investigated proprioceptive event-related potentials in a passive paradigm of paired stimuli and an active task paradigm requiring the participants to discriminate between weight loads. Because deficient auditory P50 gating and attenuated auditory P300 amplitude are well documented in schizophrenia (Reference Braff and LightBraff & Light, 2004) we expected corresponding deficits in the proprioceptive event-related potentials, i.e. decreased gating of the early components and attenuated amplitude of the late target-related components.
METHOD
The study was approved by the local ethics committee and all participants gave written informed consent. We studied 12 male patients with schizophrenia and 24 healthy male controls. All participants were right-handed. The patients were diagnosed according to DSM–IV criteria (American Psychiatric Association, 1994), using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN version 2.1; Wing et al, Reference Wing, Babor and Brugha1990, Reference Wing, Sartorius and Ûstün1998). Their level of psychopathology was rated with the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS; Andreasen, Reference Andreasen1984a ,Reference Andreasen b ).
The age-range of the patients was 18–49 years (mean 33.8; s.d.=9.0) and of the controls was 19–54 years (mean 29.3; s.d.=7.9); the difference was not significant. The duration of illness ranged from 0.5 to 23 years (mean 9.5; s.d.=8.7; median 8.5) and the cumulative length of treatment periods with antipsychotic medication ranged from 1 to 28 months (mean 9.7; s.d.=9.8; median 6.5), apart from two patients who were drug naïve. Those previously treated had been without medication for at least 1 month, the range being 1–210 months (mean 54.4; s.d.=72.2; median 24). Mean positive symptom score was 4.3 (s.d.=2.3), mean negative symptoms score was 6.8 (s.d.=4.5), disorganised symptoms 2.9 (s.d.=2.6) and total sum of scores 14.0 (s.d.=4.0). Cognitive functions were assessed in both participant groups using the cognitive screening elements in the SCAN (the Mini-Mental State Examination, Oral Trail Making and Category Verbal Fluency), and through simple reaction-time and cognitive load reaction-time measures obtained by the California Computerized Assessment Battery (Reference MillerMiller, 1990). No significant differences were seen.
Proprioceptive evoked potentials
We recorded evoked potentials at the following electrode positions: FP1, FP2, Fz, Cz, Pz, C3′ and C4′, the last two placed 2 cm posterior to C3 and C4 above the somatosensory cortices. The proprioceptive stimuli were administered using a custom-built device, which applied 100 g instantaneous changes to a vertical static load (400g) of a paddle which the participants held freely in their hands with their wrists supported (Reference Arnfred, Chen and EderArnfred et al, 2000). To avoid fatigue and drowsiness, other experiments and tasks were interspersed between the runs with the proprioceptive stimulus. The passive paired data were recorded in a mixed-method paradigm where a loop of three stimulus pairs lasted 12 s. The reports of the other stimulus pairs (auditory clicks and right median nerve stimulation) are given elsewhere (Reference Arnfred, Chen and GlenthojArnfred et al, 2003; Reference Arnfred and ChenArnfred & Chen, 2004). The interstimulus interval between the paired load increments was 1 s. After artefact rejection and baseline correction, visual peak detection was performed on the event-related potential waveforms. The latencies and amplitudes of each of the components were analysed separately through repeated-measures analyses of variance.
RESULTS
Contrary to our expectations, we did not observe significant deficits in proprioceptive gating or attenuation of P300. Significant differences between the two groups were observed only in the passive paradigm and primarily at the first stimulus of the pair. The latency of contralateral parietal P60 was prolonged, i.e. left parietal electrode: controls 63 ms, patients 69 ms as seen in post-hoc t-tests (t 32: 2.52, P=0.02), Fig. 1. P200 amplitude was augmented at the central and contralateral electrodes in the patients, maximal difference at vertex: controls 4.45 μV, patients 6.72 μV (posthoc t 34: 2.40, P=0.02).
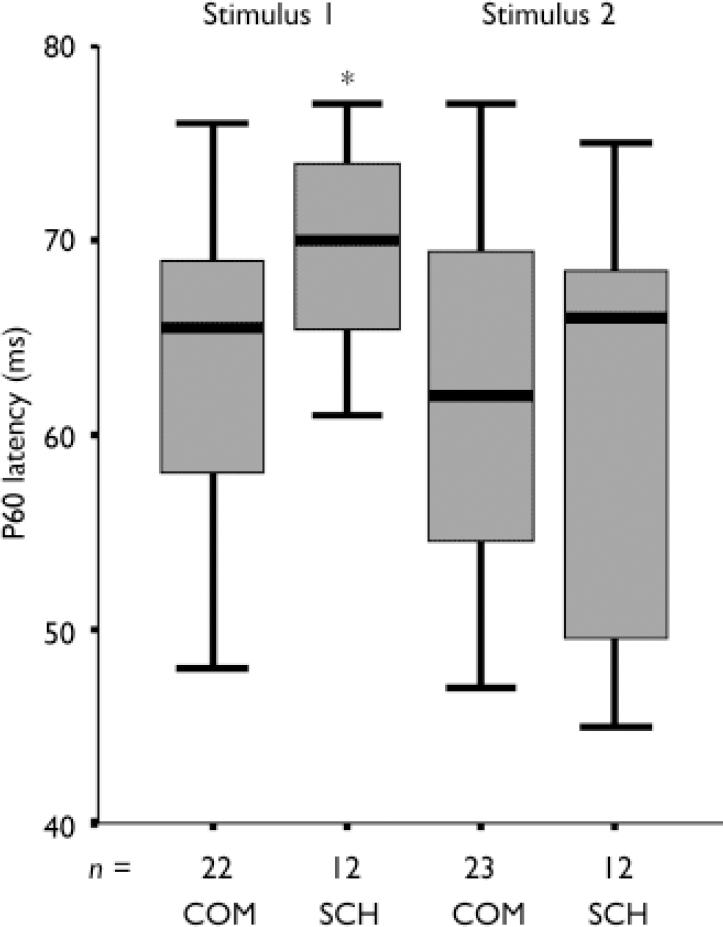
Fig. 1 Latency of contralateral parietal P60 component of the proprioceptive event-related potential. COM, healthy male controls. SCH, male patients with schizophrenia. Two stimuli of a 100g weight increment are presented 1 s apart.* The difference in latency is significant (P<0.02) only in the first stimulus of the pair.
In the second stimulus of the passive paradigm, the frontal N70 amplitude was greater in the patients: controls 2.49 μV(s.d.=0.98), patients 3.22 μV (s.d.=0.92); (post-hoc t 34: –2.14, P=0.04). Other components were comparable between the two groups.
DISCUSSION
The delayed latency of the initial activation of contralateral somatosensory cortex in the patient group was an unexpected and important finding, because the earlier stages of information processing are less influenced by top-down factors such as motivation and attention (Reference Stephan, Baldeweg and FristonStephan et al, 2006). The patients were stable without medication and lived alone, so the latency difference cannot be attributed to florid psychosis or to medication. Cognitive impairment was not significant, and no general mental slowing was observed.
We suggest that the delayed activation of contralateral somatosensory cortex at P60 is the result of imprecision in the cortical response based on a diminished or unstable maintenance of proprioceptive readiness. Increased amplitude of the early contralateral activity of the electrically induced somatosensory evoked potential has been observed when attention is directed at the hand of the stimulus, and this is assumed to be a manifestation of increased cortical readiness for the side in focus. (Reference Desmedt, Bourguet and Nguyen TranDesmedt et al, 1984). In a situation of voluntary isometric muscle contraction, the cortical readiness could be attenuated if the corollary discharge signal preparing the sensory cortices for feedback was weak or unstable. This interpretation is in agreement with the hypothesis of a deficiency of corollary discharge in schizophrenia (Reference Frith, Blakemore and WolpertFrith et al, 2000). As the P60 latency is normalised at the second stimulus, the difference is not owing to peripheral nerve transmission delay.
Although the augmented P200 amplitude at the first stimulus reflects an increased orienting response in the patients, in contrast to the commonly reported attenuation of amplitude, this effect lends support to our interpretation of diminished cortical readiness where a less-expected stimulus elicits a larger orienting response.
Our results support Rado's original hypothesis of a proprioceptive defect, whereas the lack of gating deficit is inconsistent with the ‘filtering’ theory. The proposed weakening of proprioceptive readiness could be understood as instability in feed-forward activations and, considering the influence of proprioception on the formation of self-awareness (Reference Aitken and TrevarthenAitken & Trevarthen, 1997), it could be basic to anomalies of self-experience, which are frequent in schizophrenia. It is, however, pertinent to verify this proposal in another study including psychiatric controls.
Acknowledgements
The stimulus-generating equipment was designed and built by S. Christoffersen, MSc., Department of Medical Physiology, University of Copenhagen.




eLetters
No eLetters have been published for this article.