Introduction
A priority in suicide risk assessment is the identification not only of individuals who are at risk of dying by suicide, but also the periods when their risk is elevated (for review, Glenn & Nock, Reference Glenn and Nock2014). Because intensive clinical and supportive interventions are difficult to apply for long periods of time, a means to identify individuals at near-term risk of suicide can significantly aid suicide prevention efforts by informing which among a population of at-risk individuals can benefit from intensive efforts during an upcoming window of time. While many epidemiological risk factors have been identified that associate with lifetime risk of suicide attempt, these may be distinguished from warning signs or near-term factors that indicate more proximal risk within an upcoming window of days to weeks (Franklin et al., Reference Franklin, Ribeiro, Fox, Bentley, Kleiman, Huang and Nock2016). Illustrating how little is known about proximal risk, a meta-analysis of 50 years of suicide risk factor research reported a mean follow-up period across studies identifying risk factors of 10 years, with only 5% of studies examining a follow-up period of ⩽6 months (Franklin et al., Reference Franklin, Ribeiro, Fox, Bentley, Kleiman, Huang and Nock2016). This is likely due to an inherent methodological challenge: using shorter follow-up periods reduces the likelihood of observing a rare event such as a suicide attempt (Glenn & Nock, Reference Glenn and Nock2014). Despite these challenges, detection of warning signs to indicate proximal risk would be of immense clinical value (Gordon, Avenevoli, & Pearson, Reference Gordon, Avenevoli and Pearson2020) and new research in this area is emerging (e.g. Bagge et al., Reference Bagge, Littlefield, Wiegand, Hawkins, Trim, Schumacher and Conner2022).
Suicidal behavior has been linked to identifiable behavior patterns on a range of neurocognitive tasks (Richard-Devantoy, Berlim, & Jollant, Reference Richard-Devantoy, Berlim and Jollant2014). Such tasks can provide objective markers, and also potentially provide a window into cognitive mechanisms involved with suicidal behavior. For example, reduced ability to inhibit prepotent motor responses has been observed in individuals with a history of suicidal behavior (Mann et al., Reference Mann, Arango, Avenevoli, Brent, Champagne, Clayton and Wenzel2009). One such task, the Go/No-go (GNG) task, requires rapidly responding (‘Go’) to target stimuli but occasionally withholding responses (‘No-go’) to infrequent foil stimuli. Several retrospective cross-sectional studies report increased false alarm rates (i.e., failures to inhibit) in participants with a history of suicide attempt (e.g. Richard-Devantoy et al., Reference Richard-Devantoy, Jollant, Kefi, Turecki, Olié, Annweiler and Le Gall2012; Westheide et al., Reference Westheide, Quednow, Kuhn, Hoppe, Cooper-Mahkorn, Hawellek and Wagner2008). In more fine-grained analysis, false alarm rates were higher among patients with a suicide attempt in the prior week compared to those with suicide attempt in the prior year, suggesting reduced response inhibition may be sensitive for near-term suicide attempt (Interian et al., Reference Interian, Myers, Chesin, Kline, Hill, King and Keilp2020). An important open question is therefore whether cognitive processes may be associated with, and even predict, short-term risk.
As a first step toward this goal, the current study builds on existing research by examining whether GNG performance is prospectively associated with suicide attempt. The study considers a sample of participants at high-risk for suicide enrolled in a randomized clinical trial (RCT). The trial, which showed reductions in suicidal behavior (Interian et al., Reference Interian, Chesin, Stanley, Latorre, St. Hill, Miller and Kline2021), observed suicide-related events with enough frequency to meaningfully examine shorter observation windows. Many of these participants also completed GNG at baseline and at several points during a follow-up year. Our primary hypothesis was that GNG performance was predictive of near-term (90-days) suicide attempt, above and beyond standard variables used in suicide risk assessment (i.e., number of previous suicide attempts, level of suicidal ideation).
We also applied a computational model to determine whether observed differences in GNG performance could be understood in terms of altered latent cognitive processes among those with an upcoming suicide attempt. Evidence accumulation models such as the linear ballistic accumulator (LBA) assume that, during speeded decision-making, evidence is gradually accumulated for possible responses, until a winning response is triggered. These models attempt to fit the entire distribution of reaction times (RTs) for correct and incorrect responses, and estimate individual-level latent cognitive parameters, such as response bias, response caution, and decisional efficiency, that may help explain observable behavior. Here, we applied a Bayesian version of the LBA (Brown & Heathcote, Reference Brown and Heathcote2008; Donkin, Brown, Heathcote, & Wagenmakers, Reference Donkin, Brown, Heathcote and Wagenmakers2011) to the GNG data. Our exploratory hypothesis was that one or more LBA variables would be predictive of near-term suicide attempt, above and beyond the standard suicide risk variables, potentially suggesting specific cognitive processes altered in at-risk individuals entering a period of high risk for suicide attempt.
Methods
Participants
This is a secondary analysis of data from 136 Veterans enrolled in a 12-month RCT of Mindfulness-Based Cognitive Behavioral Therapy for Suicide Prevention (MBCT-S) (Interian et al., Reference Interian, Chesin, Stanley, Latorre, St. Hill, Miller and Kline2021). Veterans were recruited at VA New Jersey Health Care System (VANJHCS) following an index suicide-related episode, ranging from suicide attempt (SA)to suicidal ideation resulting in acute hospitalization and/or engagement with Veterans Health Administration (VHA) suicide prevention services (Katz, Reference Katz2012; Stanley & Brown, Reference Stanley and Brown2012). These services included suicide safety planning, clinical monitoring and attempts to engage in regular mental health care.
Inclusion criteria for the RCT were both (1) severe suicidal ideation in the prior 30 days and (2) past-year actual, aborted, or interrupted suicide attempt (Posner, Brodsky, Yershova, Buchanan, & Mann, Reference Posner, Brodsky, Yershova, Buchanan, Mann and Nock2014) or placement on the VHA high-risk for suicide list. Full inclusion and exclusion criteria for the RCT are provided in the Supplementary material. As part of the RCT, all participants had access to a full range of standard mental health treatments; the treatment condition also received MBCT-S.
Overview of procedures
At baseline (T1), participants completed a clinical interview and several questionnaires (Interian et al., Reference Interian, Chesin, Stanley, Latorre, St. Hill, Miller and Kline2021; Kline et al., Reference Kline, Chesin, Latorre, Miller, St. Hill, Shcherbakov and Interian2016). Suicide behavior counts and worst-point suicidal ideation severity were determined using the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., Reference Posner, Brown, Stanley, Brent, Yershova, Oquendo and Mann2011), using previously-published case classification criteria (Interian et al., Reference Interian, Chesin, Kline, Miller, St. Hill, Latorre and Stanley2018). Suicidal ideation severity during the prior week was assessed with the Beck Scale for Suicidal Ideation (SSI) (Beck, Kovacs, & Weissman, Reference Beck, Kovacs and Weissman1979).
Participants were followed for one year, with follow-up testing (T2, T3) approximately 3 and 6 months post-T1. These follow-up sessions included updated C-SSRS (covering the period since last session) and SSI (covering prior week).
At each session, participants completed several computer-based tests of neurocognitive processes, including GNG and a color-word Stroop interference task (MacLeod, Reference MacLeod1991; Stroop, Reference Stroop1935). In most cases, neurocognitive testing occurred immediately after collection of clinical and self-report data.
Go-no-go (GNG) task
The GNG task was previously described (Interian et al., Reference Interian, Myers, Chesin, Kline, Hill, King and Keilp2020; Keilp et al., Reference Keilp, Beers, Burke, Melhem, Oquendo, Brent and Mann2014a; Moore et al., Reference Moore, Gur, Thomas, Brown, Nock, Savitt and Stein2019) and is adapted from the original bimodal matching GNG task described in Keilp, Sackeim, and Mann (Reference Keilp, Sackeim and Mann2005). On each trial, an X or Y appeared on the screen (Fig. 1a). Participants were instructed to press a key (‘Go response’) when X appeared in one of three locations in the top half of the screen area (target), but to withhold keypresses (‘No-go response’) when Y appeared in one of the upper locations (identity foil), or when either X or Y appeared in one of three locations in the bottom half of the screen (location foil). Stimuli appeared for 300 ms followed by 1200 ms blank screen (total trial length 1.5 s). The task included 225 trials, including 144 targets and 81 foils (36 identity foils; 45 location foils including 36 X and 9 Y); in one instance, only the first 85 trials were recorded due to computer failure.
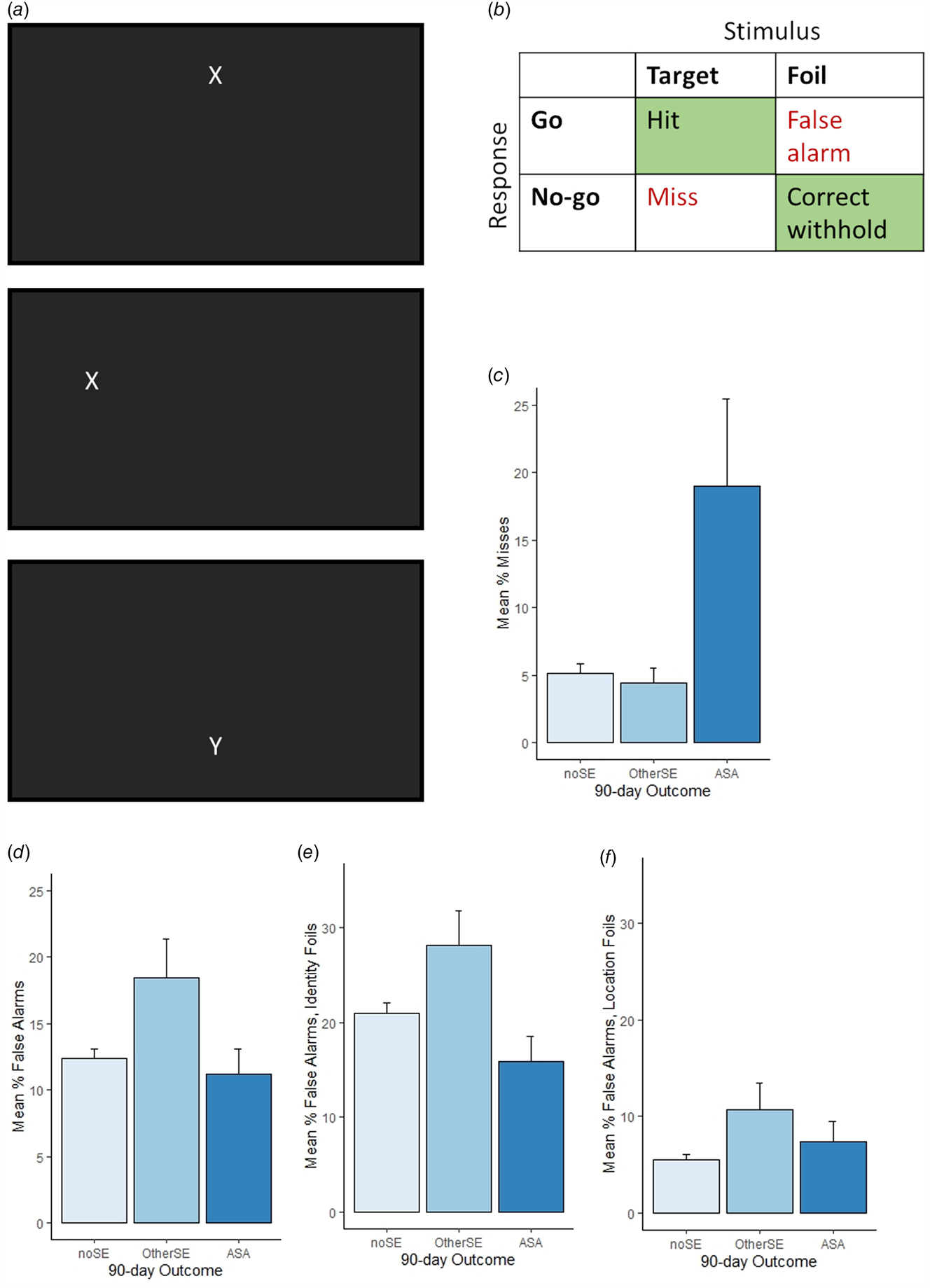
Fig. 1. Go/No-go task and results, across all n = 284 GNG datafiles; note participants who completed more than one testing session are represented more than once. (a) Participants were instructed to respond (Go) to rapidly-presented target stimuli (X in upper half of screen) but withhold response (No-go) to infrequent identity foils (Y in upper half of screen) and location foils (X or Y in lower half of screen). Top and middle show example screenshots on target trials; bottom shows example location foil trial. (b) Go responses to targets were scored as Hits and No-go responses as misses (aka omission errors). No-go responses to foils were scored as correct withholds, and Go responses as false alarms (aka commission errors). (c) Misses were highest when 90-day follow-up included an actual suicide attempt (ASA group, n = 18), compared to outcomes including other suicide-related event excluding ASA (OtherSE group, n = 29) or no suicide-related event within follow-up window (noSE, n = 237). (d) In contrast, false alarms were higher in the OtherSE group compared to the ASA or noSE groups. (e) False alarm rates to identity foils (Y in correct location) and (f) false alarm rates to location foils (X or Y in incorrect location). Error bars show SEM.
Following prior studies with GNG tasks (e.g. Gomez, Ratcliff, & Perea, Reference Gomez, Ratcliff and Perea2007; Huang-Pollock et al., Reference Huang-Pollock, Ratcliff, McKoon, Roule, Warner, Feldman and Wise2020; Ratcliff, Huang-Pollock, & McKoon, Reference Ratcliff, Huang-Pollock and McKoon2018; Weigard & Huang-Pollock, Reference Weigard and Huang-Pollock2017), we discarded responses with RT < 200 ms as anticipatory responses, and discarded responses with RT > 1s as likely reflecting inattention or other interfering cognitive processes (Lerche & Voss, Reference Lerche and Voss2019; Ratcliff, Reference Ratcliff1993). A mean of 0.96 short-RT trials (s.d. 4.3) and 0.80 long-RT trials (s.d. 1.76) per GNG datafile were dropped.
Key behavioral variables (Fig. 1b) were percent misses (aka omission errors) and percent false alarms (aka commission errors).
Stroop task
The color-word Stroop task was previously described (Interian et al., Reference Interian, Myers, Chesin, Kline, Hill, King and Keilp2020; Keilp et al., Reference Keilp, Wyatt, Gorlyn, Oquendo, Burke and Mann2014a; Moore et al., Reference Moore, Gur, Thomas, Brown, Nock, Savitt and Stein2019). On each trial, participants saw a word (RED, GREEN, or BLUE) and pressed a key to indicate the font color (red, blue, or green). The task included 52 congruent trials (e.g., word RED printed in red font) and 58 incongruent trials (e.g., word RED in green font). Words remained present on screen until the correct response was made, with intertrial intervals of 50 ms.
A d-score for interference was calculated as the difference between mean RT for incongruent v. congruent trials, divided by mean RT for congruent trials; higher (positive) d-scores indicated greater interference and thus poorer attentional control.
LBA modeling
A Bayesian version of the LBA adapted for the GNG task (Fig. 2a) was fit to each GNG datafile, using the Dynamic Models of Choice (DMC) package v. 190819 (Heathcote et al., Reference Heathcote, Lin, Reynolds, Strickland, Gretton and Matzke2019) and base R functions (R Core Team, 2017) to estimate posterior distributions for eight latent cognitive variables: non-decision time (t0), starting point variability (A), boundary offsets (BNo−go and BGo for No-go and Go accumulators, respectively), and mean slope parameters (v) for each combination of stimulus type (foil v. target) and accumulator (Go v. No-go). Full details of LBA model building and testing appear in the Supplementary material.

Fig. 2. The linear ballistic accumulator model (LBA) adapted to apply to Go/No-go task. (a) Schematic of the LBA model, showing one evidence accumulator for each response (here, No-go and Go); at the start of each trial, a starting point for each accumulator is drawn from the uniform distribution U [0…A]; evidence accumulation in each accumulator then follows a trajectory (red lines) with slope drawn from a normal distribution with mean v (where v may be different in each accumulator and for each stimulus type). The first accumulator to reach a threshold A + B (dashed line) ‘wins’ and the corresponding response is triggered. In the example shown here, boundary offset BNo−go > BGo, creating a relative bias in favor of Go responses (less distance to travel to reach threshold in the Go accumulator); however, the mean slope v on foil trials is greater in the No-go than Go accumulator, meaning that evidence accumulation proceeds more swiftly in the No-go accumulator, favoring the correct (No-go) response. Mean slope v on target trials (not shown) is typically steeper in the Go than the No-go accumulator, favoring the correct (Go) response. Total reaction time (RT) on this trial is the time for the winning accumulator to reach threshold plus non-decision time (t0) representing time to encode the stimulus and execute the response. Variability in RT and response across trials is provided by trial-to-trial variability in starting point and in slope. Values of eight free parameters (t0, A, BNo-go, BGo, and v for each combination of stimulus and response) are imputed for each datafile such that the resulting LBA model best predicts the observed RT distributions. (b) Response bias for Go responses, defined as 100*(BNo–go – BGo) is greatest in the OtherSE group, consistent with this group's high rate of false alarms. (c, d) Decisional efficiency for targets and foils are defined as the difference in v between correct and incorrect responses to that type of stimulus, where larger (positive) values indicate more efficiency in deciding to execute the correct response; here, the ASA group has lowest decisional efficiency for targets, consistent with this group's relatively high miss rate, and the highest decisional efficiency for foils. Error bars show SEM.
The medians of posterior distributions for each datafile were used as point estimates (Zhang et al., Reference Zhang, Rittman, Nombela, Fois, Coyle-Gilchrist, Barker and Rowe2016). Following Karalunas, Weigard, and Alperin (Reference Karalunas, Weigard and Alperin2020), after estimating posteriors for each GNG datafile, a measure of response bias for Go responses was calculated as 100*(BNo −go-BGo), where values > 0 indicate greater boundary offset for the No-go than Go accumulator (easier to reach threshold for Go responses). Decisional efficiency for targets and foils was calculated as vtarget-Go–vtarget-No-go and vfoil-No-go–vfoil-Go, respectively, where larger (positive) values indicate more efficiency in deciding to execute the correct response for that trial type. These three metrics (response bias for Go responses, decisional efficiency for targets, and decisional efficiency for foils) constituted our primary results from the LBA analysis.
Prospective (90-day) outcome evaluation
For each GNG datafile, we categorized the outcome into one of three mutually exclusive categories: (1) ‘ASA’ if the participant had 1+ actual suicide attempt during the 90 days subsequent to GNG testing; (2) ‘OtherSE’ if the participant had no ASA during this window but at least one other suicide-related event (SE), including interrupted/aborted suicide attempt, preparatory behavior (e.g., writing a note, assembling a method) or suicide-related hospital admission (e.g., emergency department visit or acute psychiatry admission related to suicidal ideation); or (3) ‘noSE’ if the participant had neither ASA nor other SE within the 90-day follow-up window.
ASA and other suicide behaviors were determined from clinician-administered C-SSRS at each available timepoint; medical chart review was used to capture SI-related hospital admissions. In one case, chart review also identified an ASA that occurred after a patient had been lost to contact. In two cases, participants had two GNG sessions occurring within <90 days, and the same ASA fell within the follow-up window for both sessions.
Of the 310 available GNG datafiles, four were dropped because they could not be associated with an outcome due to censoring (death from natural causes, study withdrawal, or study end within <90 days) and an additional 22 (~7%) were dropped due to apparent noncompliance or failure to understand task instructions (participant never made any ‘Go’ responses, and may have been pressing the wrong keyboard key, n = 10; participant made 90–100% errors to (only) one type of foil and likely misunderstood the requirement to inhibit responding to both types of foil, n = 12). There were no obvious differences in demographics, clinical profile or outcome distribution among these dropped files compared to the remaining 284 GNG files (results not shown).
Statistical analysis
The dependent variable for all analyses was outcome category: ASA, OtherSE, or noSE (reference category). Generalized estimating equations (GEEs) were used to test the effects of predictors on this multinomial dependent variable. Given that the same patient could contribute multiple data points (maximum of 3 observations per subject), we accounted for within-subject clustering. We also controlled for the effects of the testing session (T1, T2, T3) by modeling it as a categorical factor. Results were reported as odds ratios (OR) with 95% confidence interval (CI); threshold for significance was set at 0.05.
GEE models were estimated using SAS Enterprise (version 7.18, SAS Institute Inc, Cary, NC). First, in a simple model of GNG behavior across outcome groups, we used separate GEEs to model the dependent variable against GNG percent misses (failure to respond Go to target) and GNG percent false alarms (failure to withhold response to foil), adjusting only for testing session number. Second, to test our hypothesis regarding predictive value of behavioral measures, we used a GEE to evaluate the incremental utility of GNG behavioral scores in predicting the response variable, over standard suicide risk variables (number of lifetime ASAs, SSI at time of testing), as well as other pertinent covariates, such as testing session number, age, gender, lifetime history of traumatic brain injury (TBI), receipt of study treatment during the RCT to account for treatment effects on suicide outcomes, and an index of executive attention (Stroop d-score).
To explore the LBA variables, the same methods were used, except using LBA estimates of response bias and decisional efficiency as predictors.
Results
Sample characteristics
Table 1 summarizes demographic and clinical information for the 136 Veterans. Thirteen (9.6%) participants had 1+ actual suicide attempt (ASA) during the one-year follow-up period, while 25 (18.4%) had no ASA but 1+ OtherSE. After data cleansing, n = 284 GNG datafiles obtained from 130 unique participants were analyzed.
Table 1. Demographic and clinical information at baseline testing (T1)

GNG datafiles were classified into outcome groups based on the 90-day window following GNG testing, resulting in 18 datafiles classed as ASA (2 from female participants, 11.1%), including one case with two ASAs in the 90-day window. Another 29 were classed as OtherSE (5 from female participants, 17.2%); of these, 9 cases involved SI-related hospital admissions without suicide-related behavior, 7 involved aborted/interrupted attempts, and the remaining 13 cases involved preparatory behavior. The remaining 237 were classed noSE (29 from female participants, 12.2%). Detailed GNG results for each outcome group are summarized in the online Supplementary Table.
GNG task performance and prediction of near-term suicide outcomes
Compared to the noSE reference group, misses were higher in the ASA group [Fig. 1c; OR 1.05, (1.02–1.08), p < 0.001], but not in the OtherSE group [OR 0.99, (0.96–1.03), p = 0.713]. Conversely, false alarm rates were higher in the OtherSE group [Fig. 1d; OR 1.04, (1.01–1.07), p = 0.022], but not in the ASA group [OR 0.99, (0.94–1.04), p = 0.632]. Figure 1e and f show false alarms for the two subtypes of foil separately, illustrating that the OtherSE group made more false alarms on both the relatively easy location foils and the more difficult identity foils.
To relate ORs to observed GNG differences, we exponentiated raw beta coefficients multiplied by the observed group mean differences. Thus, while every unit increase in misses corresponded to a 5% increase in odds of ASA, the observed mean difference in misses (14%) corresponded to OR 1.92, or a 92% increase in odds of ASA within the next 90 days. Similarly, while every unit increase in false alarm rate corresponded to a 4% increase in odds of OtherSE, the observed mean difference in false alarms (6%) corresponded to OR 1.25, or a 25% increase in odds of OtherSE (excluding ASA) within the next 90 days.
Our primary hypothesis was that GNG variables could predict upcoming ASA, above and beyond the contributions of other standard suicide risk variables. Table 2 summarizes results of the GEE using GNG variables as predictors, adjusting for suicide risk variables and other pertinent covariates. As expected, suicidal ideation at the time of testing increased the odds of an upcoming ASA while receipt of study treatment in the RCT decreased the odds of ASA. Consistent with our hypothesis, increased rate of GNG misses increased the odds of ASA, independently of the other covariates. Increased rate of GNG false alarms was associated with increased odds of upcoming OtherSE (excluding ASA), independently of the other covariates.
Table 2. Predicting suicide-related behavior (actual suicide attempt or other suicidal event excluding ASA) within 90 days based on GNG behavioral variables: results from GEE, with session as repeated-measure, adjusted by key suicide-related covariates
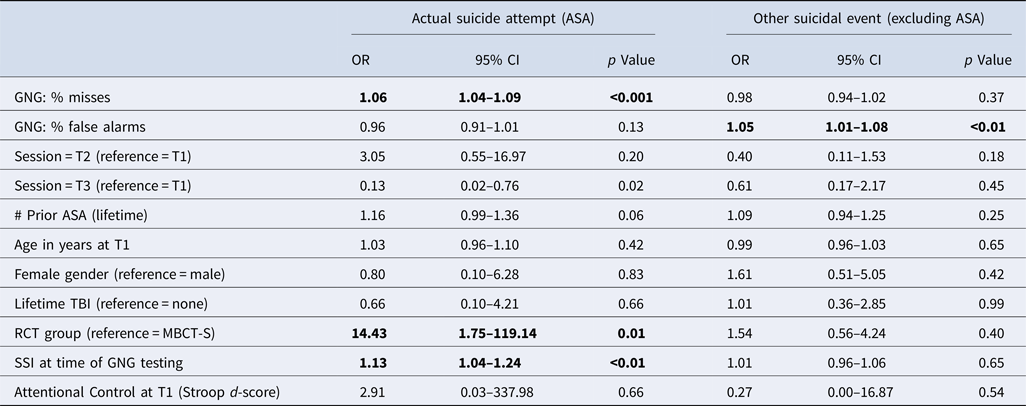
GNG, Go/No-go task; RCT, randomized clinical trial for Mindfulness-Based Cognitive Therapy for Suicide (MBCT-S); SSI, Scale for Suicidal Ideation score.
Note. Bold indicates predictors for which p < 0.05 for either the Actual Suicide Attempt (ASA) or Other Suicidal Event outcome.
Latent parameters from evidence accumulation model
Of the 284 GNG datafiles analyzed above, the LBA could not be applied to 22 datafiles containing <2 false alarms (so RT variance could not be calculated). The LBA model was run on the remaining 262 datafiles (17 ASA, 28 OtherSE, 217 noSE).
Posterior parameter estimates are shown along with other detailed results in the online Supplementary Table. As shown in Fig. 2b, the OtherSE group had a stronger response bias for Go responding than the noSE reference group [OR 1.02 (1.01–1.04), p = 0.009], while the ASA group did not [OR 1.01 (0.99–1.03), p = 0.20]. The pattern of stronger response bias for Go in the OtherSE group is consistent with their high rate of false alarms on the GNG task.
The ASA group had lower decisional efficiency for targets [Fig. 2c; OR 0.63 (0.46–0.87), p = 0.004], while the OtherSE group did not [OR 0.80 (0.57–1.11), p = 0.18]. This relative difficulty of the ASA group in accumulating evidence towards a correct response to targets may explain their high rates of misses on the GNG task. Neither the OtherSE nor ASA group differed from the noSE group in decisional efficiency for foils [Fig. 2d; OtherSE OR 1.13 (0.80–1.59), p = 0.48; ASA OR 1.40 (0.81–2.43), p = 0.23].
To relate ORs to observed parameter differences, the observed differences between ASA and noSE on decisional efficiency for targets (−0.79) increased the odds of ASA by 43%. The observed 14-point difference between OtherSE and noSE on response bias increased the odds of OtherSE by 34%.
To test our exploratory hypothesis, Table 3 summarizes results of the GEE using LBA parameters as predictors, adjusting for standard suicide risk variables and other covariates. Response bias (favoring Go responding) was strongly related to upcoming OtherSE, adjusting for the covariates; however, both decisional efficiency for targets and decisional efficiency for foils were significantly related to ASA within 90 days, after adjusting for the covariates: Every unit increase in decisional efficiency for foils more than doubled the odds of an ASA; while every unit decrease in decisional efficiency for targets increased the odds of an ASA.
Table 3. Predicting suicide-related behavior within 90 days based on LBA variables: results from GEE, with session as repeated-measure, adjusted by key suicide-related covariates

LBA, Linear ballistic accumulator model; Response bias for Go values >0 indicate bias for Go over No-go responses; Decisional efficiency for targets/foils >0 indicate faster evidence accumulation for correct than incorrect responses to targets and foils, respectively; RCT, randomized clinical trial for Mindfulness-Based Cognitive Therapy for Suicide (MBCT-S); SSI, Scale for Suicidal Ideation score.
Note. Bold indicates predictors for which p < 0.05 for either the Actual Suicide Attempt (ASA) or Other Suicidal Event outcome.
Discussion
A priority for suicide prevention is identifying which among a set of individuals considered at high-risk for suicide are most likely to attempt suicide within a short-term window, so that clinical resources can be appropriately targeted. Several prior studies have associated GNG performance with prior suicide attempt (Interian et al., Reference Interian, Myers, Chesin, Kline, Hill, King and Keilp2020; Richard-Devantoy et al., Reference Richard-Devantoy, Jollant, Kefi, Turecki, Olié, Annweiler and Le Gall2012; Westheide et al., Reference Westheide, Quednow, Kuhn, Hoppe, Cooper-Mahkorn, Hawellek and Wagner2008); the current study investigated whether GNG performance could be used to prospectively predict future ASAs.
In our sample of high-risk Veterans, increased false alarms were predictive of an upcoming SE excluding ASA. This is consistent with our own prior analysis showing a relationship between recent (prior) ASA and false alarms at baseline in this dataset (Interian et al., Reference Interian, Myers, Chesin, Kline, Hill, King and Keilp2020) and other studies associating prior SE with decreased response inhibition (Richard-Devantoy et al., Reference Richard-Devantoy, Jollant, Kefi, Turecki, Olié, Annweiler and Le Gall2012; Westheide et al., Reference Westheide, Quednow, Kuhn, Hoppe, Cooper-Mahkorn, Hawellek and Wagner2008). LBA modeling suggested that high false alarm rate in the OtherSE group might reflect a general response bias favoring Go over No-go responses.
However, it was GNG misses, i.e., failures to respond to rapidly-presented targets, that strongly and selectively associated with upcoming ASAs, and that distinguished the ASA group from both the noSE and OtherSE groups. Other prior studies have similarly reported increased miss rates as an important indicator of suicidality (Harfmann, Rhyner, & Ingram, Reference Harfmann, Rhyner and Ingram2019; Westheide et al., Reference Westheide, Quednow, Kuhn, Hoppe, Cooper-Mahkorn, Hawellek and Wagner2008; Wright, Lipszyc, Dupuis, Thayapararajah, & Schachar, Reference Wright, Lipszyc, Dupuis, Thayapararajah and Schachar2014). The significant association of misses with ASA, after accounting for other variables, also held in a supplemental analysis where the GEE was run with binary outcome coding ASA v. all other outcomes (results not shown).
Increased miss rates in the ASA group could reflect several cognitive processes. First, it could reflect a general impairment in attention; however, this group did not show evidence of general attention/concentration deficits as indexed by Stroop task d-scores. Increased miss rate could also reflect a general psychomotor slowing which would reduce the likelihood of completing a Go response before the end of the 1.5-s trial period; however, the ASA group showed no slowing of behavioral RT, compared to the other groups (online Supplementary Table).
To explore specific cognitive processes that could underlie an increased miss rate in the ASA group, we turned to computational modeling, which provides a way to examine and quantify latent processes that could govern individual- and group-level differences in performance, but that are not directly evident from analysis of behavioral indices alone. The model results suggested no evidence of general slowing of stimulus encoding and motor response time (t0) nor of a general response bias favoring No-go in the ASA group. Rather, the behavior of those with upcoming ASA was consistent with reduced decisional efficiency for targets (Fig. 2d). Here, the reduced decisional efficiency for targets in the ASA (but not OtherSE or noSE) group suggests qualitative, not merely quantitative, differences in cognitive processes in a subset of at-risk individuals who will soon attempt suicide.
A key strength of computational modeling is to propose formal, mechanistic and biologically plausible mechanisms underlying observable behaviors (Millner, Robinaugh, & Nock, Reference Millner, Robinaugh and Nock2020). In electrophysiology studies, decisional efficiency has been linked with the build-up rate of the P3b component of the P300 event-related potential (Kelly & O'Connell, Reference Kelly and Connell2013), and appears to originate from temporal-parietal activity associated with stimulus detection and attention (Polich, Reference Polich2007). Interestingly, reduced decisional efficiency is emerging as a neurocognitive risk factor in a range of psychopathologies (for review, see Weigard & Sripada, Reference Weigard and Sripada2021). A few studies have suggested that decisional efficiency can be increased by catecholamine agonists that modulate brain signal-to-noise ratios (Peters, Vega, Weinstein, Mitchell, & Kayser, Reference Peters, Vega, Weinstein, Mitchell and Kayser2020; Weigard, Heathcote, & Sripada, Reference Weigard, Heathcote and Sripada2019), which may be particularly relevant given that brain serotonergic systems have been associated with suicidality (Oquendo et al., Reference Oquendo, Sullivan, Sudol, Baca-Garcia, Stanley, Sublette and Mann2014; van Heeringen & Mann, Reference van Heeringen and Mann2014). This raises the intriguing possibility of therapeutic intervention to modify brain substrates underlying reduced decisional efficiency, which might in turn help to remediate dysfunctional cognitive and executive processes underlying suicidality. This also illustrates a key utility of computational modeling, which is to identify cognitive phenotypes that can help bridge between brain circuits and clinical behavior.
Further studies are obviously indicated to further explore these ideas, including replication of the current results, before any clinical recommendations can be made. Nevertheless, the strength and specificity of the relationship between misses and ASAs in the current study suggest that GNG misses may be a proximal warning sign of suicide risk, as well as an indicator providing some insight into cognitive processes that may change as high-risk individuals enter a period of acute risk for suicide. If the current results were appropriately replicated/validated, the GNG might represent a fairly short, computer-based screening tool that could be administered in an acute care setting or delivered via Internet or mobile device for more routine follow-up; positive screens (e.g., an increase in an individual's GNG miss rate) could trigger specific interventions including increased clinical encounters, more intensive safety planning, or increased monitoring efforts during periods of elevated risk (Stanley et al., Reference Stanley, Brown, Brenner, Galfalvy, Currier, Knox and Green2018).
The fact that decreased response inhibition (increased false alarm rate) was associated in our study with OtherSE, but not with upcoming ASA, may at first appear at odds with the idea that decreased inhibition might mediate the transition from suicidal thoughts to suicide attempts (i.e., from ideation to action). One possible interpretation is that different neurocognitive deficits may be more prominent during different phases of the transition from suicidal ideation to suicide attempt; for example, decision-making difficulties may lead to a downward spiral of mood and function, while impulsivity may be associated when individuals take action to escape the pain. Another possibility is that there may be different deficits at play with different subpopulations who exhibit different types of attempts. This would be consistent with recent suggestions that cognitive impulsivity (e.g., reduced ability to delay gratification) is decreased in individuals with high-lethality suicide attempts (Anestis, Soberay, Gutierrez, Hernández, & Joiner, Reference Anestis, Soberay, Gutierrez, Hernández and Joiner2014; Dombrovski et al., Reference Dombrovski, Szanto, Siegle, Wallace, Forman, Sahakian and Clark2011; Keilp et al., Reference Keilp, Wyatt, Gorlyn, Oquendo, Burke and Mann2014b). These, together with the current results, suggest that some attempts may be characterized by decisional difficulties and less reactive responding, while other attempts may be characterized by greater reactivity and impulsivity. The emerging field of ecological momentary assessment may shed light on these questions, by allowing short cognitive tasks to be administered in a more naturalistic setting, potentially allowing the detection of more rapid fluctuations in cognitive processes closer to the time of an upcoming ASA (e.g. Le et al., Reference Le, Moscardini, Cowan, Elvevåg, Holmlund, Foltz and Cohen2021).
It is also useful to compare rates of GNG errors in our high-risk group against a sample of putatively healthy control participants. For example, Hoffman et al. (Reference Hoffman, Taylor, Campbell-Sills, Thomas, Sun, Naifeh and Stein2022) administered this same GNG task to a sample of over 38 000 Army Soldiers (17% females; mean age 20.97 years), of whom the vast majority (98%) reported no lifetime history of suicide attempt. This study reported a mean of ~10% false alarms, quite similar to the overall rate in the current study (Fig. 1c and online Supplementary Table); a follow-up cohort (including 3.4% with ‘new-onset’ suicide attempt during the 3–7 year follow-up period), again reported ~12% false alarms. Hoffman et al. reported that GNG false alarms was a significant predictor of retrospective history of lifetime suicide attempts, but not a significant predictor in a prospective model predicting emergence of new-onset ASA at follow-up. This seems to lend credence to the finding in the current study that reduced inhibition, as indexed by GNG false alarms, is not a significant prospective predictor, at least when measurements are made weeks or months before the attempt. Again, assessments made more frequently, and/or closer to the time of an upcoming ASA, might detect more rapid fluctuations in these cognitive processes, and thus be more successful prospective predictors.
A key limitation of our study is the low incidence of outcome events (particularly ASAs), even within our high-risk sample. Indeed, the low frequency of attempts, even among high-risk individuals, has been a prediction challenge clinically, but also for research studies. Other limitations of the current study include underrepresentation of females among our Veteran participants which may have masked gender differences, and lack of information on psychotropic medication that could have influenced behavior. Also, about 7% of GNG datafiles were excluded due to apparent subject non-compliance or failure to understand task instructions, and a further ~10% of GNG datafiles could not be subjected to LBA modeling due to too few false alarms, suggesting some participants may have been trading speed for accuracy. It would be interesting to see if the current pattern of results were replicated with a simpler GNG task, putatively requiring less cognitive load than the current task which involved multiple types of foil. It is also important that all participants were enrolled in a treatment trial, which may have modified their behavior across time. Indeed, fewer participants in the MBCT-S treatment group had ASAs during follow-up than those in the control group (Interian et al., Reference Interian, Chesin, Stanley, Latorre, St. Hill, Miller and Kline2021). Nevertheless, current results remained significant after adjusting for treatment group effects in the multivariate models. In fact, any study enrolling at-risk participants is likely to be complicated by the ethical necessity for interventions related to suicide prevention.
Despite these limitations, our finding of increased miss rates associated with upcoming suicide attempt suggests there may overt behavioral profiles that can be detected in advance of an upcoming ASA, and also points to the potential utility of neurocognitive deficits to provide objective measures to complement existing clinically-assessed warning signs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722001003
Financial support
This work was supported by the U.S. Department of Veterans Affairs (VA) through Health Services Research and Development Service #IIR 12-134 (IA), Clinical Sciences Research and Development Service (CSR&D) Merit Awards #I01 CX001826 (CEM), #I01 CX002093 (MSG and EAH), and #I01 CX002094 (ABN), and VA Biomedical Sciences Research and Development Service Merit Award #I01 BX004561 (KDB). Dr Hazlett was supported by a VA CSR&D Research Career Scientist Award (1 IK6 CX001738). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of interest
Dr Stanley receives royalties from the Research Foundation for Mental Hygiene, Inc. for the Columbia Suicide Severity Rating Scale. The remaining authors have no actual or potential conflicts to disclose.








