1. Introduction
During radiation delivery to breast or chest wall a part of the dose is also received by heart. Mean doses of radiation to the heart from radiotherapy for breast cancer are typically about 1-2Gy for disease of the right breast. For disease of the left breast the doses are usually higher but vary widely and for some women including those in whom the distance of the heart to the thoracic wall is small and those who require internal mammary radiation, the mean doses may be around 10Gy (Darby et al., Reference Darby, Ewertz, McGale, Bennet, Blom-Goldman and Bronnum2013). Majority of studies reported in the literature are with conventional fraction (Aznar et al., Reference Aznar, Korreman, Pedersen, Josipovic and Specht2011; Correa et al., Reference Correa, Das, Litt, Ferrari, Hwang, Solin and Harris2008; Jin et al., Reference Jin, Chen, Deng, Liu, Huang and Huang2013; Krueger et al., Reference Krueger, Schipper, Koelling, Marsh, Butler and Pierce2004; Mulliez et al., Reference Mulliez, Speleers, Madani, Gersem, Veldeman and Neve2013; Muren et al., Reference Muren, Maurstad, Hafslund, Anker and Dahl2002; Nilsson et al., Reference Nilsson, Holmberg, Garmo, Duvernoy, Sjogren, Lagerqvist and Blomqvist2012; Remouchamps et al., Reference Remouchamps, Vicini, Sharpe, kestin, Martinez and Wong2003; Stewart et al., Reference Stewart, O’Farrell, Cormack, Hansen, Khan and Mutyala2008; Swanson et al., Reference Swanson, Grills, Ye, Teahan, Letts and Yan2013; Taylor et al., Reference Taylor, Povall, McGale, Nisbet, Dodwell, Smith and Darby2004). Hypofractionation is becoming a new standard in breast cancer irradiation. Hypofractionation is well established in WBI but it’s acceptance in PMRT is still very low. There is a concern that heart may receive more dose after mastectomy than BCS in patients with left breast cancer and it is one of the reasons for low acceptance of hypofractionation after mastectomy. The dose to the LAD artery is greater than the dose to the whole heart. Left sided breast cancer had a statistically significant increase in rate of stenosis in the coronary artery branches on the left anterior surface of the heart [mid, distal and distal diagonal branch of the LAD coronary artery].
In this prospective study we compared dose to the heart, LAD artery, and bilateral lungs and opposite breast between mastectomy and BCS in patients with left sided breast cancer with hypofractionation. The null hypothesis was that OARs receive higher dose after mastectomy in patients with left breast cancer.
2. Methods
A total of 30 patients of left side breast cancer after mastectomy or BCS, 15 each were included in the study. Inclusion criteria were the primary cancer of the left breast of any histological type, age 20–70 years, no metastasis and total mastectomy/BCS with resection margin free of tumour. Exclusion criteria were any history of prior malignancy, history of prior irradiation to the chest, pregnant or lactating women and collagen vascular disorders.
A CT based three dimensional planning using 100 ml of IV contrast was used to reconstruct radiotherapy target volume for treating breast/ chest and loco regional lymph nodes. The patients were positioned supine on a breast board with arm abducted above the head on arm rest in planning CT in radiotherapy department. CT axial cuts were taken from the level of larynx to upper abdomen, including both the lungs with a scan thickness and index of 3 mm. CT images were transferred to the Treatment Planning system (TPS). The chest wall or whole breast, heart, bilateral lungs and opposite breast were contoured using RTOG contouring guidelines. For LAD contouring, left coronary artery was identified and its course was followed. LAD was contoured from the place where it branches away from the main left coronary artery, and then runs towards the front and down towards the heart apex. It was divided in to proximal and distal parts. Distal LAD was the part which lies very near to the chest wall toward the apex of the heart. No additional margin was given to LAD.
The treatment parameters, patients and OARs outlines were exported to computerised TPS and plans were made using standard tangent fields (Figure 1a & b). Dose prescribed was 40Gy/16#/3 weeks both in BCS and mastectomy patients. Heart, bilateral lungs, LAD artery and opposite breast dose volume histogram were generated (Figure 2). From these, estimate of mean doses to heart, LAD, proximal LAD, distal LAD, bilateral lung and opposite breast, V5 of right lung, V5, V10 and V20 of left lung and V2 opposite breast were calculated and compared between BCS and mastectomy patients using student’s ‘t’ test.

Figure 1a. Axial CT showing planning of patient after mastectomy

Figure 1b. Axial CT showing radiotherapy planning of BCS patient
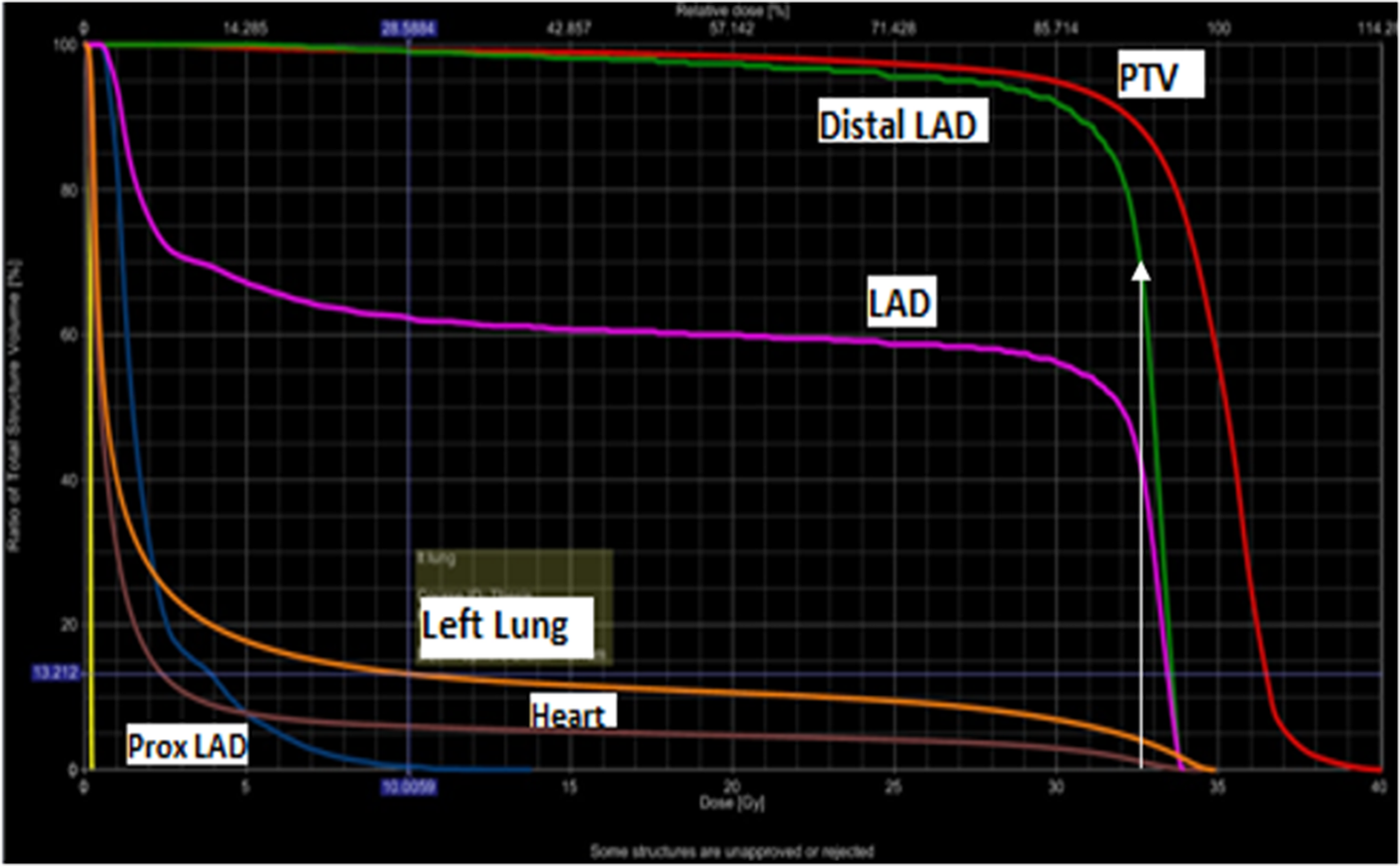
Figure 2. DVH showing dose received by different volume of OARs (70% of distal LAD is receiving 100% dose, white arrow)
3. Results
Mean age of the patients was 45 years(22–67 years). Total 15(50%) patients were premenopausal. Obesity was present in 5(16.6%) patients and 12(40%) patients were overweight. 15(50%) of patients had locally advanced disease and 17(57%) patients had positive nodes. Chemotherapy was given to 27(90%) patients, anthacyclins in 2(7%) and anthacyclins and taxanes in 25(93%) patients. Oestrogen receptor(ER) and progesterone receptors(PR) were positive in 12(40%), 7(23%) were only ER positive. 7(23%) were Her2-neu positive and only one received trastuzumab.
Mean doses to the heart, LAD, proximal LAD and distal LAD were 3.364Gy, 16.06Gy, 2.7Gy, 27.5Gy; and 4.219Gy, 14.653Gy, 4.306Gy, 24.6Gy, respectively for mastectomy and BCS patients (Table 1). There was no statistical significant difference in the doses to the heart between mastectomy and BCS patients.
Left lung mean dose, V5, V10 and V20 were 5.96Gy, 16%, 14%, 12.4%; and 7.69Gy, 21%, 18%, 16% in mastectomy and BCS patients, respectively. These were also not statistically different between the two techniques (Table 2).
Mean dose to the right lung was significantly less in mastectomy as compared to BCS patients, 0.29Gy vs. 0.51Gy, respectively(p = 0.007).
Mean dose to the opposite breast was significantly higher in patients with BCS than mastectomy (0.54Gy Vs 0.37Gy, p = 0.007) as shown in Table 2.
Similarly V2 to the right breast was also higher in BCS 1.43% as compared to 0.26% in mastectomy patients but it was not statistically significant[(p = 0.07)Table 2].
The dose to the distal LAD was significantly higher than proximal LAD both in BCS (24.6Gy Vs 4.3Gy, p = <0.0001) and mastectomy (27.5Gy Vs 2.7Gy, p = <0.0001) patients (Table 3). It can be seen in Figure 2 that 70% of distal LAD is receiving 100% dose (white arrow).
4. Discussion
In this dosimetric comparative study between BCS and mastectomy patients with left breast cancer there was no significant difference in dose to the heart, LAD, proximal LAD and distal LAD. Mean dose to the heart was 3.364Gy and 4.219Gy for mastectomy and BCS patients, respectively. In this study we used hypofractionated radiotherapy schedule of 40Gy/16#/3 weeks both in BCS and mastectomy patients.
Cardiac dose due to breast radiotherapy has been reported in many studies (Table 3) (Arenas et al., Reference Arenas, Hernandez, Farrus, Muller, Gascon and Pardo2014; Awad et al., Reference Awad, Zayed, Abotouk and Dawod2013; Borca et al., Reference Borca, Franco, Catuzzo, Migliaccio, Zenone and Aimonetto2012; Darby et al., Reference Darby, Ewertz, McGale, Bennet, Blom-Goldman and Bronnum2013; Gursel et al., Reference Gursel, Meydan, Ozbek and Ofluoglu2011; Pili et al., Reference Pili, Grimaldi, Fidanza, Florio, Petruzzelli and D’Errico2011; Schubert et al., Reference Schubert, Gondi, Sengbusch, Westerly, Soisson and Paliwal2011; Tan et al., Reference Tan, Wang, Qiu, Liu, Jia and Zeng2011; Yavas et al., Reference Yavas, Yavas and Acar2012). In majority of these studies conventional fractionation of 50Gy/25#/5 weeks with or without boost was used (Arenas et al., Reference Arenas, Hernandez, Farrus, Muller, Gascon and Pardo2014; Awad et al., Reference Awad, Zayed, Abotouk and Dawod2013; Borca et al., Reference Borca, Franco, Catuzzo, Migliaccio, Zenone and Aimonetto2012; Gursel et al., Reference Gursel, Meydan, Ozbek and Ofluoglu2011; Khullar et al., Reference Khullar, Datta, Vekadamanickam, Garg and Sinha2014; Schubert et al., Reference Schubert, Gondi, Sengbusch, Westerly, Soisson and Paliwal2011; Tan et al., Reference Tan, Wang, Qiu, Liu, Jia and Zeng2011; Yavas et al., Reference Yavas, Yavas and Acar2012) with few studies with hypofraction (Pili et al., Reference Pili, Grimaldi, Fidanza, Florio, Petruzzelli and D’Errico2011). We did not come across any study in the English literature where a comparison has been done for doses to the OARs between mastectomy and BCS in patients with left breast cancer. Mean dose to the heart was comparable between mastectomy and BCS. The reason for comparable dose in the present study could be the contouring guideline and different structures included for the whole breast irradiation(WBI) and chest wall irradiation(CWI) in these patients. For WBI the entire breast volume is contoured excluding 3-5 mm of the skin and pectoral muscles to constitute clinical target volume(CTV). For CWI skin, pectoral, chest wall muscles and ribs are included in the CTV. So heart and the left lung are very close to the CTV. For contouring whole breast, the breast borders are variable and depend on the extent of the breast. These variable borders invariably lead to a variable radiation volume to be treated in case of WBI and variable dose to the OARs. Whereas in PMRT, the chest wall borders or land marks are well defined and volume of irradiation is also well defined.
Cardiac RT dose reported in the literature vary with techniques, prescribed dose and the period in which patients were treated. Higher mean cardiac dose reported by Darby et al. was due to the inclusion of the patients from 1960–70s and retrospective analysis of dose on the basis of CT scans of a patient of typical anatomy (one size for all) which may not be representative of all population (Darby et al., Reference Darby, Ewertz, McGale, Bennet, Blom-Goldman and Bronnum2013). Higher mean cardiac doses of 7Gy in 1970s and 5Gy in 1980s were reported by Hooning et al. (Reference Hooning, Botma, Aleman, Baaijens, Bartelink and Klijn2007) and Taylor et al. (Reference Taylor, Nisbet, McGale and Darby2007) (10.5Gy in 1970s). These were due to the older technique of radiation from 1950–1980 and compulsory internal mammary irradiation at that time. Capezzali et al. reported lower cardiac dose (1.71Gy) in their study. It was due to lateral decubitous treatment position in which the breast falls away from the chest wall (Capezzali et al., Reference Capezzali, Kirova, Costa, Fournier-Bidoz, Aristei and Zygogianni2013). This treatment technique is not generally followed because of set up uncertainty. Higher doses to the OARs in other studies were due to the higher prescription dose to the PTV (50–62.4Gy) (Assaoui et al., Reference Assaoui, Toulba, Nouh, Lkhouyaali, Bensouda and Kebdani2012; Awad et al., Reference Awad, Zayed, Abotouk and Dawod2013; Guan et al., Reference Guan, Dong, Ding, Zhang, Huang and Liu2015; Ma et al., Reference Ma, Zhang, Lu, Wu, Wu and Huang2015; Pierce et al., Reference Pierce, Butler, Martel, Normolle, Koelling and Marsh2002; Tan et al., Reference Tan, Wang, Qiu, Liu, Jia and Zeng2011; Yavas et al., Reference Yavas, Yavas and Acar2012). We planned all our patients in supine position on breast board and used hypofractionation in all our patients. Some of the studies have also included patients with internal mammary irradiation which also contribute to higher doses to the OARs (Hjelstuen et al., Reference Hjelstuen, Mjaaland, Vikstrom and Dybvik2012; Khullar et al., Reference Khullar, Datta, Vekadamanickam, Garg and Sinha2014; Zhang et al., Reference Zhang, Mei, Chen, Hu, Hu and Zhang2015). We did not plan internal mammary radiation in this study. Since these older techniques and conventional schedules are not followed now so there was a need to know the doses to the OARs with hypofractionation which is becoming standard of care in adjuvant breast/chest wall irradiation.
Because of its anatomical location, LAD artery is likely to remain within the high dose volume from a tangential field arrangement even at low irradiated heart volume and is most prone for atherosclerosis(Figure 2). Few studies has reported dose received by LAD where it ranged from 0.1 to 46 Gy (Gursel et al., Reference Gursel, Meydan, Ozbek and Ofluoglu2011; Ma et al., Reference Ma, Zhang, Lu, Wu, Wu and Huang2015; Muren et al., Reference Muren, Maurstad, Hafslund, Anker and Dahl2002; Taylor et al., Reference Taylor, Povall, McGale, Nisbet, Dodwell, Smith and Darby2004; Reference Taylor, Nisbet, McGale and Darby2007; Yavas et al., Reference Yavas, Yavas and Acar2012). In our study dose to the LAD was 14.6Gy and 16Gy in patients with BCS and mastectomy, respectively. Very few studies have reported dose to the proximal LAD, where it ranged from 12–17.8Gy (Aznar et al., Reference Aznar, Korreman, Pedersen, Josipovic and Specht2011; Krueger et al., Reference Krueger, Schipper, Koelling, Marsh, Butler and Pierce2004). Only one study had reported dose to the distal LAD where it was 31.52Gy (Krueger et al., Reference Krueger, Schipper, Koelling, Marsh, Butler and Pierce2004). In the present study doses to proximal and distal LAD were lower as compared to other studies. It might be because of lower total dose used in our study with hypofractionation. With hypofractionation the total dose is reduced to compensate for high dose per fraction but it is biologically equivalent to the conventional fractionation. So with this equally effective dose, the dose to the OARs is reduced. A very low dose to the whole LAD artery seems to be associated with very low dose to the heart but the reverse statement is not true. That is why the whole LAD artery should be contoured as a risk organ along with the whole heart and should be used prospectively for plan optimism in left sided breast cancer. In our study dose to the distal LAD was significantly higher than the proximal LAD in both BCS and mastectomy patients (Fig 2). Distal LAD dose may be of paramount importance as it may contribute to the late effects on the heart. So it is important to report dose received by distal LAD artery.
In our study, there was no difference in mean dose, V5, V10 and V20 of left lung between BCS and mastectomy patients. It also confirms that hypofractionation can be practiced in patients with mastectomy for left breast cancer without concerns for late effects because these patients are likely to develop late toxicity comparable to those with WBI for which hypofractionation is well established. In a study by Remouchamps et al. (Reference Remouchamps, Vicini, Sharpe, kestin, Martinez and Wong2003) V20 to left lung was 17.1 to 22.3%, which is higher than our and the other studies reported in the literature where it mostly ranged from 8.2 to 14.8% (Borca et al., Reference Borca, Franco, Catuzzo, Migliaccio, Zenone and Aimonetto2012; Gursel et al., Reference Gursel, Meydan, Ozbek and Ofluoglu2011; Schubert et al., Reference Schubert, Gondi, Sengbusch, Westerly, Soisson and Paliwal2011; Ng et al., Reference Ng, Shuryak, Xu, Chao, Brenner and Burri2012).
In the present study no volume of right lung received 5Gy in both BCS and mastectomy patients because plans were made using standard tangent fields, so the chances of dose going right lung were very remote. Only two studies have reported mean dose to the right lung where it ranged from 0.1 to 0.8Gy (Schubert et al., Reference Schubert, Gondi, Sengbusch, Westerly, Soisson and Paliwal2011; Tan et al., Reference Tan, Wang, Qiu, Liu, Jia and Zeng2011). In the present study, mean dose to the right lung was 0.51Gy and 0.29Gy in patients with BCS and mastectomy, respectively. This is comparable to those reported in other studies.
Mean dose to the contralateral breast was higher in patients with BCS as compared to mastectomy in the present study. Rather et al. reported dose to the contra lateral breast of 5.34–6.40% of the total dose for tangential fields and it was 1.2–1.75% of the dose for supraclavicular fields in their study (Rather et al., Reference Rather, Haq, Khan, Khan and Sofi2014). Larger dose to contra lateral breast may be because of short perpendicular distance from the contra lateral breast surface to the geometric beam edge. Short perpendicular distance can be caused by shallow medial gantry angle or large pendulous breast. Higher mean dose to the right breast in our study can be explained by scattering from wedges used for compensation in BCS patients.
Limitation of the present study are small sample size for very variable patient group, bias inherent to the trial’s design, conclusions only apply to the specific technique used, variety anatomical background of the heart, and absence of clinical outcomes as this being only a dosimetric study. Boost in cases of BCS patients was not planned in the present study, which may also contribute doses to the OARs.
Based on the present study and other studies from the literature it can be concluded that the dose to the OARs varies from patient to patient depending upon the patient anatomy, indication (post mastectomy/BCS), contouring guidelines for various volumes and organ at risks (OARs), radiation technique, volume of treatment, dose and nature of study (prospective/retrospective). However, as hypofractionation is going to be the standard for breast radiotherapy, therefore if same dose fractionation is used in patients with BCS or after mastectomy doses to the OAR may not vary.
5. Conclusion
In the present study there was no difference between dose received by heart, LAD, proximal LAD and distal LAD; mean dose, V5, V10 and V20 of left lung between BCS and mastectomy patients. Mean dose to the contra lateral breast was higher in BCS patients. Therefore hypofractionation may be safely delivered in patients with left side breast cancer after mastectomy. It will contribute to increase the utilization of hypofractionation in PMRT setting.
Compliance with Ethical Standards
Funding
None.
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involved
human participants.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability
Data is available.
Authors’ contributions
Dr Budhi Singh Yadav: Concept design, Treatment planning, data collection and analysis, manuscript writing and approval.
Dr Deepak Kumar Das: Concept design, Treatment planning, data collection, analysis and approval.
Dr Narendra Kumar: Concept design, manuscript approval.
Dr Manphool Singhal: Concept design, manuscript approval.
Dr Ngangom Robert: Treatment planning, and approval.
Conflict of interest statements
None.
Role of funding source
None.
Ethics committee approval
Yes
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/exp.2021.8.






Comments
Comments to the Author: I recommend substantial revisions. While the conclusions seem logical, they are impossible to reproduce given the limited methods section. Therefore the reader is unable to adequately assess the scientific soundness and validity of the methods. Please elaborate on your methods: retrospective vs prospective inclusion/planning? Nodal irradiation is mentioned and is a major contributing factor to OAR dose. Which contouring guidelines are used for targets and OARs? Please elaborate on beam design. Describe patient characteristics.
The reported OAR parameters are reported without a measure of spread, please include one. There is no way for the reader to know that OAR doses were obtained with adequate target volume coverage, please clarify. Maybe include a whole group mean DVH for all ROIs.
When comparing to other papers, please clarify which use hypofractionation. Given the many factors influencing OAR doses the authors cite in the discussion, it is surprising they don’t clarify these factors for their current trial.
The article doesn’t cite limitations but obviously has some (e.g. small sample size for very variable patient group, bias inherent to the trial’s design, conclusions only apply to the specific technique used). Furthermore, the impact of a tumor bed boost is not discussed. It might well be that RT after BCS actually yields larger OAR doses than post mastectomy RT because of it.
The manuscript needs some language revisions but can be understood as is. Please explain abbreviations (e.g. TMAC). The title in the submission system (see proof p.1) doesn’t match the one from the abstract.