INTRODUCTION
Most newborns have passive immunity to measles due to transplacental transfer of maternal IgG antibodies, which occurs mainly in the last period of pregnancy [Reference Landor1]. After birth, the concentration of specific measles antibodies falls and the duration of passive immunity depends on initial antibody levels [Reference Black2, Reference Carson3].
The level of maternal measles antibodies varies according to how immunity was acquired (with mothers naturally infected by measles having a higher level than mothers acquiring immunity through vaccination) [Reference Pabst4–Reference Redd6], the time since immunization and possible reinforcement due to occasional contact with the wild virus or revaccination [Reference Leuridan and Van Damme7, Reference Cáceres, Strebel and Sutter8].
The risk of measles infection is very low while passive immunity persists in the first months of life but the probability of infection subsequently increases according to the level of circulation of the measles virus in the population. Without vaccination, most people are infected during early childhood and the percentage of susceptible adults is very low [Reference Nokes and Anderson9].
Vaccination is the most effective means of preventing measles, but its effectiveness is influenced by the duration of passive immunity, which interferes in the response to vaccination. The protective response induced by vaccination includes the humoral and cellular responses. Although the humoral response is poor after vaccination in children aged 6 months, it provokes a response by T cells that is independent of the effects of passive antibodies [Reference Gans10–Reference Gans12]. Gans et al. [Reference Gans11] reported that children aged 6 months have low antibody titres and low seroconversion rates after vaccination in the presence of passive antibodies and also found that only 27% of vaccinated children aged 6–12 months produced INF-γ after vaccination.
The correct age to initiate measles vaccination in a population should be established by balancing the risk of infection against the effectiveness of vaccination. When measles incidence is very high, the vaccination age should be advanced despite its lower effectiveness. In contrast, when the incidence is low, vaccination can be delayed, since infection in a period of a few months is improbable and vaccination effectiveness is higher at older ages [Reference Leuridan and Van Damme7, Reference Orenstein13, Reference Zhao14].
Measles vaccination was introduced in Catalonia in 1981 with administration of the measles-mumps-rubella (MMR) vaccine at age 12 months. A substantial reduction in disease incidence was soon observed but, in 1987, the age of administration was put back to 15 months to avoid primary failures and improve vaccination effectiveness. In 1988, a second dose of MMR was added at age 11 years, and was advanced to age 4 years in 1999 to increase the proportion of children aged <10 years who were immune to above the reported critical threshold of 95% [Reference Domínguez15–Reference Fine18] in order to achieve the objective of interrupting transmission. In 2000, the transmission of measles was interrupted in Catalonia and, since then, sporadic imported cases have occurred that have caused some infections in the indigenous population. During the period 2000–2005, the incidence of measles in Catalonia was very low, and outbreaks were related either to imported cases or affected families that had not received any dose of vaccine due to personal beliefs. No transmission chains involved the indigenous population [19]. However, in August 2006, a measles outbreak began that lasted until June 2007, with 381 confirmed cases [Reference Domínguez20]. The outbreak affected a large part of Catalonia and among those affected were highly susceptible unvaccinated children aged <15 months [Reference Domínguez20]. General interventions adopted to control the outbreak included administering one dose of MMR to infants aged 9 months [21]. The objective of this study was to determine the level of measles antibodies in a sample of infants vaccinated at 9 months and their mothers at the time of vaccination and the response to vaccination measured by antibody titres after vaccination.
MATERIAL AND METHODS
We conducted an observational study. Two blood samples obtained by capillary puncture before (S0) and 28 days after (S1) vaccination with the MMR vaccine were collected on filter paper [Reference Ibrahim22]. Mothers' samples (Sm) were also collected by capillary puncture. Samples were obtained between February and June 2007.
Subjects
Healthy children aged 9–14 months and their mothers from 11 health centres in Catalonia were included when parents gave written informed consent. Children in whom the MMR vaccine was contraindicated, previously vaccinated children, children with previous contact with a case of measles, those who had suffered measles and those who had received blood products or blood transfusions in the preceding 3 months, were excluded.
Variables
The date and place of birth, maternal breastfeeding, date of sampling and date of vaccination were recorded for all children included. The date and place of birth, the date of sampling and a history of measles infection or measles vaccination were recorded for all mothers. The maternal vaccination status was verified by reviewing the vaccination card.
The vaccines used in the study, Priorix® (GlaxoSmithKline, Belgium) and Triple MSD® (Sanofi Pasteur MSD, France), were administered subcutaneously.
The protocol was approved by the ethics committee of the Catalan Health Institute.
Laboratory tests
Specific measles IgG antibody titres were determined in the microbiology laboratory of the Hospital Clinic of Barcelona by enzyme-linked immunosorbent assay (Enzygnost® Anti-Measles Virus/IgG, Dade Behring, Germany) following a previously established protocol with some modifications [Reference Novello23]. Briefly, circles of filter paper measuring 0·6 cm in diameter were cut out and the blood reconstituted with 120 μl PBS buffer with 2% of bovine serum albumen (BSA), agitated for 30 min at room temperature to ensure thorough soaking of the discs, and incubated at 4°C overnight in microtitre plates. After overnight elution with buffer, the plates were agitated at room temperature for 30 min and centrifuged at 2200 g for 15 min. In a previous study, we observed no significant differences when comparing this elution protocol with elution with 0·3 m Tris buffer or with the Dade Behring Enzygnost sample supplied with the kit as recommended by Riddell et al. [Reference Riddell24]. The discs were estimated to contain about 15 μl of blood, equivalent to about 5–6 μl of serum. Supernatant of the blood samples in filter paper was treated as a dilution of 1:20 of serum. Subsequently, measles IgG antibodies at a final dilution of 1:231 were determined, according to the manufacturer's instructions. Samples with more than 0·2 optical density units were considered as positive. Measles antibody concentrations were measured in positive samples and expressed in mIU/ml on the basis of an internal reference serum (Anti-Measles Virus Reference Reagent P/N, provided by the manufacturer Dade Behring, Germany) processed together with the samples. The reference serum is calibrated on the First International Standard Preparation for Anti-Measles Serum of the WHO. The method has a limit of detection of 150 mIU/ml.
Seroconversion was defined as the detection of measles IgG antibodies after vaccination in children with no measles antibodies in the S0 sample.
Maternal antibody interference was defined as children who were positive before and seronegative after vaccination.
Statistical analysis
The χ2 test of association was used to compare proportions. The geometric mean titres (GMT) of antibody titres and their standard deviation were calculated (mean±s.d.).
The Mann–Whitney U test was used to compare quantitative variables that harmed the assumption of normality. An α-level of 0·05 was considered significant. Statistical analysis was performed using the SPSS statistical package (SPSS Inc., USA).
RESULTS
Of the 66 mothers, eight refused to participate and three were mothers of twins. Finally, 58 mothers, representing 61 children, participated.
S1 samples were obtained from 51 children. Maternal antibody titres (S0) were present in 37·7% (95% CI 25·5–49·8) of the recruited children aged 9–14 months, 45·1% (95% CI 26·1–64·3) of children aged 9 months, and 47·1% (95% CI 22·9–72·2) of children aged 10 months presented positive maternal antibodies. The overall percentage of unprotected children was 62·3% (95% CI 50·1–74·5).
The results for children with two samples are shown in Tables 1 and 2 and Figure 1.
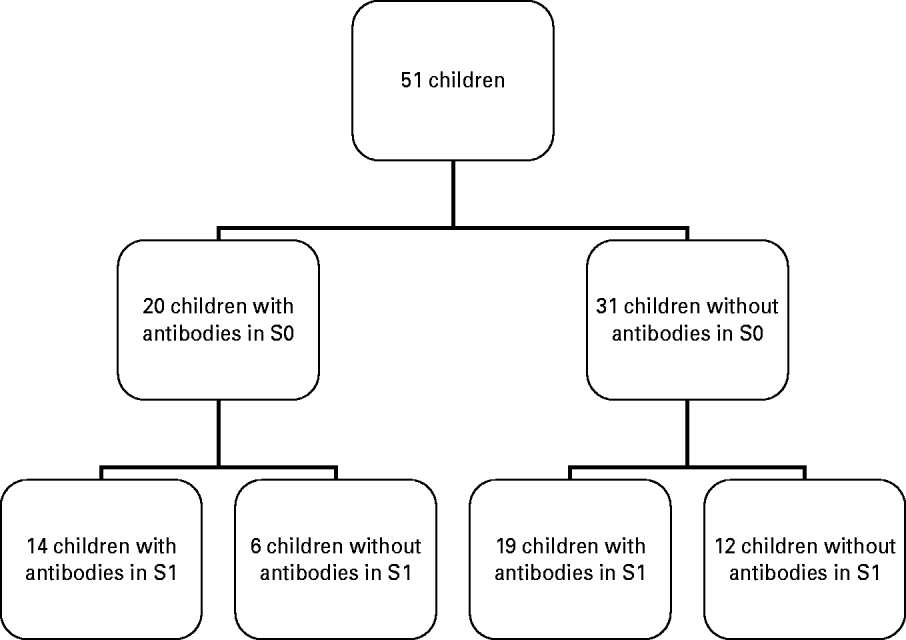
Fig. 1. Flowchart of antibody detection before (S0) and after (S1) vaccine administration.
Table 1. Distribution of children with two samples according to the presence or absence of measles antibodies and age (n=51)
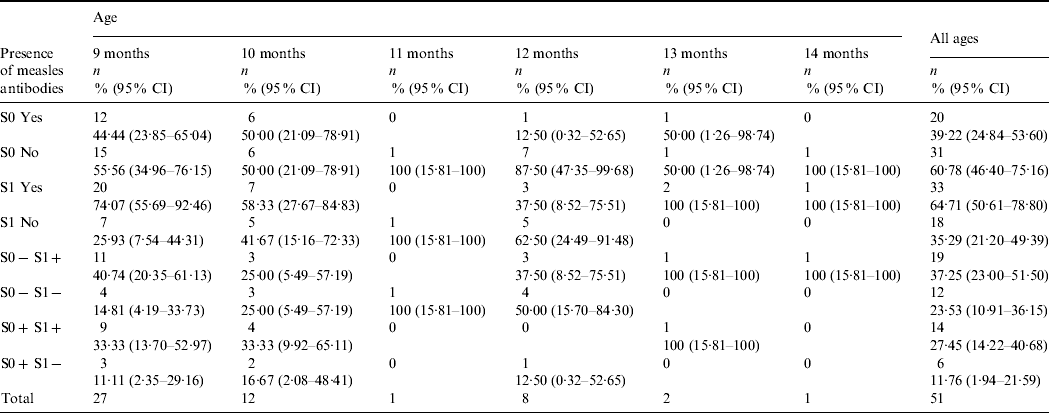
S0, Pre-vaccination blood sample; S1, post-vaccination blood sample.
Table 2. Distribution of antibody titres (GMT) according to the presence or absence of antibodies in S0 and S1
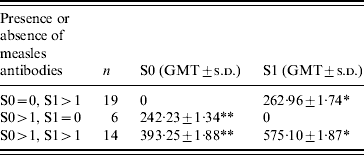
GMT, Geometric mean titres; s.d. standard deviation.
* P=0·002, ** P>0·05.
Global seroconversion in the 31 seronegative children was 61·3% (95% CI 42·5–80·0). By age, seroconversion was 73·3% (95% CI 44·9–92·2) in children aged 9 months, 50·0% (95% CI 11·8–88·2) in children aged 10 months, 42·8% (95% CI 9·9–81·6) in children aged 12 months and 100% in those aged 13 and 14 months (Table 1). Global seropositivity was 64·7% (95% CI 50·6–78·8).
The interference of maternal antibodies was 30·0% (95% CI 11·9–54·3) in all children and 33·3% (95% CI 7·5–70·1) in children aged 9 months.
Mean S0 titres in children in whom no antibodies were detected in S1 were lower (GMTS0: 242·23±1·34 mIU/ml) than in children in whom antibodies were detected in S0 and S1 (GMTS0: 393·25±1·88 mIU/ml; GMTS1: 575·10±1·87 mIU/ml; P=0·002). This latter level of S1 was also higher than in children who seroconverted (GMTS1: 262·96±1·75 mIU/ml; P>0·05).
A total of 84·33% of children were breast-fed, for a mean time of 6·54±3·64 months. There was no association between breastfeeding and antibody titres.
Of the 58 mothers studied, 15·5% (95% CI 5·3–25·7) reported having been vaccinated, 41·4% (95% CI 27·8–54·9) having had measles and 43·1% (95% CI 29·5–56·7) did not know their measles status.
A total of 67·2% (95% CI 54·3–80·2) of mothers had measles IgG antibodies, of whom 46·1% reported having had measles, 12·8% having been vaccinated and 41·0% did not know their measles status.
Antibody titres in mothers who reported having had measles were higher than in those reporting having been vaccinated (P=0·009) (Table 3). There were no significant differences in antibody titres between children of vaccinated mothers and those who had had measles. In seven mothers, the titre detected was >1000 mIU/ml and passive antibodies were not detected in two of their children.
Table 3. Measles immunity of mothers and their children according to the route of acquisition of immunity in mothers
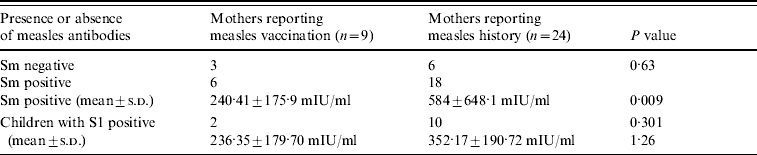
S0, Pre-vaccination blood sample; S1, post-vaccination blood sample; Sm, maternal blood sample; s.d. standard deviation.
Of the mothers presenting antibody titres, those aged >30 years had higher titres than those aged ⩽30 years (GMT: 786·06±3·43 mIU/ml; GMT: 329·99±2·18 mIU/ml, respectively; P<0·001) and the percentage that had measles was similar at 50% and 40%, respectively.
DISCUSSION
We determined levels of measles antibodies in a sample of Catalan children and their mothers in order to evaluate the best age to begin paediatric vaccination [Reference Leuridan and Van Damme7].
Delaying the administration of the first dose of measles vaccine increases the period of susceptibility to measles infection. We used an optimized method to measure measles-specific IgG levels in filter paper blood samples which showed a strong correlation with the results obtained from serum samples [Reference Novello23]. Assessment of measles antibody concentrations in dried blood spots has been widely used in previous studies and its usefulness in children is proven [Reference Ibrahim22, Reference Helfand25].
However, determination of virus-neutralizing antibodies is considered the best method of assessing protection against measles. In this study, an ELISA with a limit of detection of 150 mIU/ml for quantification of IgG levels was used as an alternative test. Therefore, in terms of protection, the results should be interpreted with caution, as there is not always close agreement between neutralization and ELISA analysis [Reference Van den Hofa26].
In children vaccinated at age 9 months, the response to vaccination was 74·0%. The fact that only 37·7% of children aged 9–14 months and 45·1% of children aged 9 months presented antibodies acquired passively from their mothers shows there is a wide period of susceptibility to measles infection, in agreement with the results of studies in the USA [Reference Maldonado5] and other countries [Reference Gans27]. However, other reports show that the concentration of maternal antibodies falls more rapidly in the first 6 months of life. In The Netherlands [Reference Van den Hof28], 45·5% of children aged 4 months were protected and other studies have found a level of protection of <10% in children aged >8 months [Reference Shaoyuan29–Reference Yerkovich32].
The rapid fall in antibodies may be due to lower passive transmission of maternal antibodies, which would result in a rapid fall in titres. This lower transmission might be explained by a lower antibody concentration in mothers, as observed in the mothers in our study, of whom 67·2% were seropositive.
Both the wild virus and vaccination produce lasting immunity. However, protection against measles is due not only to the action of antibodies, but also to cytotoxic cell immune mechanisms [Reference Gans11, Reference Gans12, Reference Gans27]. It has been suggested that, currently, immunity persists in adults and avoids infection despite new exposures to measles virus [Reference Domínguez20]. The outbreak in Catalonia corroborates this suggestion, since the number of cases in vaccinated subjects (including fertile women) was low, and only two cases occurred in mothers due to contact with infected offspring.
The lower concentration of measles antibodies found in vaccinated mothers compared to mothers infected by the virus in early childhood (P=0·009) has also been reported by other authors [Reference Leuridan and Van Damme7, Reference Zhao14, Reference Gans27, Reference Krugman33–Reference Gagneur35]. Leuridan et al. [Reference Leuridan34] estimated that the mean time of reduction of maternal antibodies was 3·78 months in children of mothers who had had measles and 0·97 months in children of vaccinated mothers. The lack of re-stimulation due to contact with the wild virus, which is typical when vaccination coverages are >95% and the circulation of measles is low or non-existent, might be another explanation.
Currently, in Catalonia, there is a transition in passive immunity in children: until recently, most mothers were immune due to infection in early childhood, whereas currently there is an increase in vaccinated mothers [Reference Domínguez36]. Maternal age is increasing in Catalonia, with 25% aged >35 years in 2006. Most mothers aged <30 years were vaccinated in early childhood (the cohort born in 1977 who were aged 11 years in 1988 and posterior cohorts). The elimination of indigenous measles in Catalonia after 2000 and the substantial reduction in incidence since at least 1990 have made contact with the wild virus improbable. Therefore, it seems logical that the concentration of antibodies in today's newborns and the duration of passive immunity against the disease are relatively low.
The seroconversion rate found in this study (61·3%) is lower than that reported by other studies [Reference Redd6, Reference Ibrahim22, Reference Khalil30, Reference Pabst, Boothe and Carson37], but comparison requires that the detection techniques used and the parameters of antibody levels are taken into account. Some authors define seroconversion as post-vaccination antibody titres fourfold greater than pre-vaccination levels [Reference Redd6, Reference Cáceres, Strebel and Sutter8, Reference Pabst, Boothe and Carson37]. Seroconversion, understood as the percentage of children who did not have protective antibody titres before vaccination but did after vaccination, was 61·29% in children aged 9–14 months and 64% in children aged 9 months. These results differ from those of Redd et al. [Reference Redd6], who found that 91·75% of children aged 9 and 12 months had seroconverted. The method used for sampling in our study may result in lower sensitivity than in phlebotomy samples. This could explain the differences observed in the seroconversion rate.
Although many published studies have also been conducted in small samples, the sample size is a limitation of our study, and limits the generalizability of the results.
Although it is reported that antibodies acquired passively from the mother interfere with seroconversion when children are vaccinated early [Reference Relly38, Reference Sato39], the present situation, i.e. passive transmission with low levels of passive acquisition and a rapid fall in levels, together with a rate of 33·3% of interference with seroconversion in children aged 9 months, suggests that advancing the age of administration of measles vaccine is a good strategy in the struggle to achieve and maintain elimination of measles. The response to a second dose of vaccine in children vaccinated before age 12 months has been good, as reported by other authors [Reference Hutchins40, Reference Stetler41].
In conclusion, our results show that the present situation in Catalonia of advancing the first dose of measles vaccination from 15 months to 12 months is the correct strategy given the increase in the time of susceptibility of infants to measles.
ACKNOWLEDGEMENTS
This study received financial support from the Directorate of Public Health, Department of Health, Generalitat of Catalonia, Barcelona, Spain and AGAUR (grant number 2009/SGR-42). The authors thank all participating parents and the physicians and nursing staff of paediatric wards for their collaboration.
APPENDIX. Members of the Working Group for the Study of Measles Immunity in Children
Agència de Salut Pública de Barcelona: Quique Muñoz and Glòria Rovira; Cap Ca N'Oriac: Soledad Casas; Cap Sagrera: Violeta Terrazas; Cap Hospitalet de l'Infant: Pediatrics Team; Cap Montcada: Irene Cercós, Angélica Diéguez, Susana Navarrete, Noemí Perez, Marta Serdà, Ester Toral, Lidia Troya, Amada Zamora; Cap Montnegre: Montse Gajans and Amparo Valles; Cap Valdoreix: Pilar Clemente and Olga Santaularia; Cap Sant Cugat: M Salut Cladellas, Laia Colomé, Lourdes Diaz, Gemma Jiménez; Dr Felipe Velasco Betes; Hospital de Nens de Barcelona: Orencio Urraca; Paido.Dex. Hospital Universitari Deixeus. Grup USP: Pediatrics Team.
DECLARATION OF INTEREST
None.






