The disorder now termed ‘hyperphagic short stature’ (HSS; Reference Skuse, Albanese and StanhopeSkuse et al, 1996) was first identified over 30 years ago (Powell et al, Reference Powell, Brasel and Raiti1967a ,Reference Powell, Brasel and Raiti b ), and has since been described by a variety of synonyms, including ‘psychosocial short dwarfism’ (e.g. Reference Mouridsen and NielsenMouridsen & Nielsen, 1990). More recently, diagnostic criteria have been defined and shown to have predictive validity, and evidence has been reported of a genetic predisposition to the disorder (Reference Skuse, Albanese and StanhopeSkuseet al, 1996). There is a high rate of familial aggregation in full sibships of HSS-affected families (Reference Skuse, Albanese and StanhopeSkuse et al, 1996). In a series of HSS probands, ascertained using the single incomplete ascertainment method, 40% had an affected full sibling (further details available from the author upon request). One half-sibling of a proband was affected; she shared the same biological mother as the proband, and their fathers were brothers. No unrelated children living in the HSS proband's household or comparison group children were affected. We propose that this pattern of familial aggregation indicates that HSS is due to a gene of major effect, which is inherited in a Mendelian pattern.
Hyperphagic short stature and Prader—Willi syndrome compared
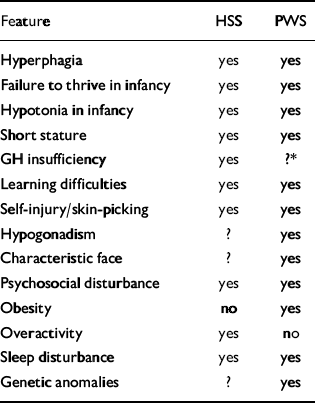
Many signs of HSS are remarkably similar to Prader—Willi syndrome (PWS), although to date no study has directly compared the conditions. Children with HSS show hyperphagia, including gorging, pica, food hoarding (Reference Bowden and HopwoodBowden & Hopwood, 1982; Reference Mouridsen and NielsenMouridsen & Nielsen, 1990) and polydipsia (Reference Skuse, Albanese and StanhopeSkuse et al, 1996). Hyperphagia is also a cardinal symptom of PWS (Reference Cassidy and CassidyCassidy, 1992). Sleep cycle disruption is described in both HSS (Reference Gilmour and SkuseGilmour & Skuse, 1999) and PWS (Reference Helbing-Zwanenburg, Kamphulsen and MourtazevHelbing-Zwanenburg et al, 1993). Short stature is a common feature of PWS (Reference Cassidy and CassidyCassidy, 1992), although there is debate about whether individuals have primary growth hormone deficiency (e.g. Reference Angulo, Castro-Magana, Uy and CassidyAngulo et al, 1992; Reference Ritzen, Bolme, Hall and CassidyRitzen et al, 1992). Growth hormone insufficiency is invariable in HSS (Reference Skuse, Albanese and StanhopeSkuse et al, 1996), but it is exquisitely responsive to environmental circumstances. We hypothesise that the dysregulation of appetite, growth and sleep characteristic of HSS might constitute an inherited predisposition to hypothalamic dysfunction, as has been described in PWS (Reference Cassidy and CassidyCassidy, 1992).
The developmental histories of each condition closely correspond. Infants with PWS have poor muscle tone, feeding problems and delayed motor milestones (Reference ButlerButler, 1990; Reference Cassidy and CassidyCassidy, 1992). Failure to thrive and feeding problems commonly occur in HSS (Reference Bowden and HopwoodBowden & Hopwood, 1982), together with delayed motor milestones (Reference Hopwood and BeckerHopwood & Becker, 1979; Reference Ferholt, Rotnem and GenelFerholt et al, 1985) and hypotonia (Reference Gilmour and SkuseGilmour & Skuse, 1999). The onset of hyperphagia is delayed until early childhood in PWS (Reference Holm, Butler, Hanchett and CassidyHolm Et al, 1993). In HSS, hyperphagia is not evident until children are at least 3 years old. Both conditions are associated with global learning disability (Reference Bowden and HopwoodBowden & Hopwood, 1982; Reference Money, Annecillo and KellyMoney et al, 1983; Reference Ferholt, Rotnem and GenelFerholt et al, 1985; Reference ButlerButler, 1990; Reference Skuse, Albanese and StanhopeSkuse et al, 1996), although the degree of learning disability is less marked in HSS than in PWS. Greater degrees of learning disability may be associated with increased risk of behavioural problems in children with PWS (Reference Dykens, Hodapp and WalshDykens et al, 1992), although this is not a consistent finding (e.g. Reference Dykens and CassidyDykens & Cassidy, 1995).
Skin-picking is characteristic of the PWS phenotype (Reference GreenswagGreenswag, 1987). Children with HSS also show self-injury, including skinpicking (Reference Money, Annecillo and HutchinsonMoney et al, 1985). Oppositional behaviour (Reference Blizzard and BulatovicBlizzard & Bulatovic, 1992), deliberate enuresis and encopresis (e.g. Reference Money, Annecillo and HutchinsonMoney et al, 1985; Reference Blizzard and BulatovicBlizzard & Bulatovic, 1992; Reference Skuse, Albanese and StanhopeSkuse et al, 1996) and depression (Reference Ferholt, Rotnem and GenelFerholt et al, 1985) are associated with HSS. Many children with PWS show marked internalising and externalising disorders (Reference Dykens, Hodapp and WalshDykens et al, 1992; Reference Dykens and CassidyDykens & Cassidy, 1995; Reference van Lieshout, De Meyer and Curfsvan Lieshout et al, 1998a ). These may be provoked by food restriction (Reference ButlerButler, 1990), although this is unlikely to be the only causative mechanism. The disturbance is more severe in individuals with a chromosomal deletion compared with those with uniparental disomy, though the quality is the same (Reference Dykens, Cassidy and KingDykens et al, 1999).
Differentiating features
While there are striking parallels between the two conditions, some features differentiate them. Children with PWS are characteristically obese and lethargic (Reference ButlerButler, 1990; Reference Cassidy and CassidyCassidy, 1992), while those with HSS have a body mass index (BMI) in the normal range (Reference Bowden and HopwoodBowden & Hopwood, 1982; Reference Mouridsen and NielsenMouridsen & Nielsen, 1990; Reference Skuse, Albanese and StanhopeSkuse et al, 1996) and are usually overactive (Reference Skuse, Albanese and StanhopeSkuse et al, 1996). Their overactivity may account for the lack of weight gain despite their hyperphagia, but the possibility of a hypermetabolic disorder has not yet been investigated. Symptoms of psychosis (Reference ClarkeClarke, 1998) and obsessive-compulsive features (e.g. Reference Dykens, Leckman and CassidyDykenset al, 1996) have been described in individuals with PWS. Such symptomatology has not been described in the HSS population, but nor has it been investigated directly. Clinical features of the two conditions are compared and contrasted in Table 1.
Table 1 The clinical features of hyperphagic short stature (HSS) and Prader—Willi syndrome (PWS)
| Feature | HSS | PWS |
|---|---|---|
| Hyperphagia | yes | yes |
| Failure to thrive in infancy | yes | yes |
| Hypotonia in infancy | yes | yes |
| Short stature | yes | yes |
| GH insufficiency | yes | ?* |
| Learning difficulties | yes | yes |
| Self-injury/skin-picking | yes | yes |
| Hypogonadism | ? | yes |
| Characteristic face | ? | yes |
| Psychosocial disturbance | yes | yes |
| Obesity | no | yes |
| Overactivity | yes | no |
| Sleep disturbance | yes | yes |
| Genetic anomalies | ? | yes |
While a high incidence of familial aggregation is common in HSS (Reference Bowden and HopwoodBowden & Hopwood, 1982; Reference Money, Annecillo and KellyMoney et al, 1983; Reference Skuse, Albanese and StanhopeSkuse et al, 1996), it is rare in PWS, with most cases being sporadic genetic anomalies (Reference Cassidy and CassidyCassidy, 1992). Prader-Willi syndrome is a congenital condition with an established anomaly at chromosome 15q11-13 (see Nicolls et al, 1999). This is commonly due to a deletion at the critical region (Reference Led better, Riccardi and AirhartLedbetteret al, 1981) or uniparental disomy in which the affected individual has two maternally derived copies of the critical region on chromosome 15 and no paternally derived copy (Nicolls et al, 1999). This observation and rare ‘imprintor mutations’ associated with PWS indicate that it is an imprinted condition (Nicolls et al, 1999). ‘Imprinting’ means that a gene is silent or expressed in children depending on whether it is inherited from the mother or father. A mutation in a normally imprinted gene means that the gender of the transmitting parent determines whether the child manifests the disorder or not. The phenotype will not be expressed if the mutated allele is silenced.
Intrafamilial stress
In the case of HSS, we propose that a gene-environment interaction is necessary for the key clinical features to become apparent. Children who meet diagnostic criteria for HSS invariably live in conditions of stress. The nature of that stress is often intra-familial abuse (Reference Bowden and HopwoodBowden & Hopwood, 1982; Reference Skuse, Albanese and StanhopeSkuse et al, 1996), but not necessarily so. For example, one child in our series lived in extremely poor housing conditions and was not the subject of parental abuse; another developed symptoms during a civil war. However, abuse of a child by a parent is a potent form of stress, and so is especially likely to precipitate the clinical features. Removing the child from the stressful environment coincides with the rapid recovery of growth hormone levels from insufficient to high normal levels (Reference Skuse, Albanese and StanhopeSkuse et al, 1996); hyperphagia and associated sleep disruption also resolve within a matter of days (Reference Skuse, Albanese and StanhopeSkuseet al, 1996). Over a substantial period of time separated from the stressor, catch-up growth occurs (e.g. Powell et al, Reference Powell, Brasel and Raiti1967a ,Reference Powell, Brasel and Raiti b ). In contrast, the non-specific emotional and behavioural disturbances (Reference Gilmour and SkuseGilmour & Skuse, 1999) associated with living in psychosocial adversity, which are common to affected and unaffected children, do not show such a rapid response to environmental change. Re-introduction to the original environment is associated with a return of the key clinical features and a limitation or cessation of linear growth (Reference Taitz and KingTaitz & King, 1988).
While stress does not provoke the PWS symptoms, high levels of intrafamilial stress are reported in association with the disorder. Families of a child with PWS describe more negative family and child characteristics and greater pessimism about the future than comparison families who have a child with learning disability (Reference Hodapp, Dykens and MasinoHodappet al, 1997). They regard themselves as angry, controlling, inconsistent and subject to marital conflict (Reference van Lieshout, De Meyer and Curfsvan Lieshout et al, 1998b ). The PWS behavioural phenotype is reported to be particularly demanding on a family's coping resources (Reference Hodapp, Dykens and MasinoHodapp et al, 1997; Reference van Lieshout, De Meyer and Curfsvan Lieshout et al, 1998b ). In other words, it is generally accepted that managing the PWS child induces intrafamilial stress. No previous study has considered that managing children with a genetic predisposition to HSS may induce stress in their families too.
Aims
We aimed to assess, using standardised measures, the degree of similarity between the clinical features of HSS and PWS. We also wished to test the hypothesis that the major locus predisposing to HSS co-inherits with the PWS locus at chromosome 15q11-13 or that there is an abnormal pattern of methylation at this locus.
We predicted that equivalently high levels of intrafamilial stress and arousal would be identified in the families of PWS and HSS probands, using a measure of expressed emotion (EE; Reference Vaughn and LeffVaughn & Leff, 1976). In general, parents rated as ‘high EE’ report higher levels of family conflict and mental health problems (Reference Hibbs, Hamburger and KruesiHibbs et al, 1993) and their children show higher levels of physiological arousal (Reference Hibbs, Zahn and HamburgerHibbs et al, 1992). Children living in conditions of persistent stress show attenuated hypothalamic-pituitary-adrenal (HPA) axis reactivity following an acutely stressful stimulus (Reference Kaufman, Birmaher and PerelKaufman et al, 1997). This characteristic pattern is due to a physiological adaptation to their chronically stressful environment (e.g. Reference Kirschbaum and HellhammerKirschbaum & Hellhammer, 1989). We also predicted that HSS, and possibly also PWS, subjects would show a blunted HPA response to an exogenous stressful stimulus.
METHOD
Inclusion criteria
Children were diagnosed as HSS (n=25) if their behaviour met the criteria in the HSS diagnostic algorithm (Reference Skuse, Albanese and StanhopeSkuse et al, 1996). Children were identified as PWS (n=30) on the basis of a clinical or genetic diagnosis. In the recruitment literature supplied to professionals, we stipulated that children with a convincing clinical PWS diagnosis but a negative cytogenetic investigation could be included in the study, because of the poor sensitivity of cytogenetic testing (Reference ButlerButler, 1990). The more recently introduced methylation test (Reference Dittrich, Robinson and KnoblauchDittrich et al, 1992; Reference Buiting, Dittrich and RobinsonBuiting et al, 1994) has excellent sensitivity (Reference Gillessen-Kaesbach, Robinson and LohmannGillessen-Kaesbach et al, 1995). Any PWS group child with a negative result following a methylation test was excluded. Specific data on the numbers of children with a clinical rather than a genetic identification were not available to the authors.
Recruitment
Children with HSS were recruited largely from specialist growth clinics (n=24). We also advertised in women's magazines, asking for families with a hyperphagic child to contact us. One additional case of HSS was identified this way. Fifty per cent of the PWS sample (n=15) were recruited through the parent support group, the Prader-Willi Association (UK). Seventeen per cent (n=5) were recruited from the advertisements. Paediatricians and psychiatrists referred others. Owing to the methods of recruitment that we were required to employ, we are unable to report the true refusal rate.
Measures
Direct assessment
Anthropometric measures were obtained using standard clinic equipment by trained personnel. We used the Castlemead Growth Programme (Reference Boyce and ColeBoyce & Cole, 1993) to convert heights and weights into standard deviation scores, corrected for age. A standard deviation score of −1.88 corresponds approximately to the third centile in height. The short form of the Wechsler Intelligence Scale for Children (3rd UK edition) (WISC-III-UK) and the Wechsler Pre-school and Primary Scale of Intelligence (revised) (WPPSI-R) were used to assess cognitive ability (Wechsler, Reference Wechsler1990, Reference Wechsler1992). Using four sub-scales, full-scale, performance and verbal IQ scores were pro-rated.
Parent and teacher report
The Hyperphagic Short Stature Diagnostic Interview (HSSDI) was designed specifically for the study of HSS children. No existing published measure was suitable. The interview is semi-structured, requiring parents to provide specific examples of their child's behaviour. Ratings (0-3) were justified on the basis of reported behaviour, rather than parental opinion or generalisations. The behaviours described were rated against specified behavioural anchors. This method is more consistent than asking parents to make their own judgements about the relative severity or abnormality of behaviour. Frequency, severity and the context in which behaviour occurred were noted. Previous studies have confirmed the predictive validity of this instrument for HSS (Reference Skuse, Albanese and StanhopeSkuse et al, 1996).
Teachers also completed the HSS Teacher Questionnaire (Reference Skuse, Albanese and StanhopeSkuse et al, 1996). Copies of this questionnaire and the HSSDI are available from the author upon request. Parents and teachers completed the Child Behaviour Checklist (CBCL) and the Teacher's Report Form (TRF) respectively (Achenbach, Reference Achenbach1991a ,Reference Achenbach b ).
Molecular genetics
Two investigative methods were used: a methylation status test and sibling pair linkage analyses. The critical region of PWS is imprinted, which means that the parental origin of a critical region plays a crucial role in normal development. Normally only the paternal allele is expressed at 15q11-13, while those with PWS have only the maternal allele or maternal disomy (two maternal alleles) at this region, and therefore no paternal allele. The methylation test is sensitive to allelic expression. A normal methylation pattern in the current investigation indicates that PWS is highly improbable.
Sibling-pair linkage analysis is a more stringent test of the PWS co-inheritance theory as it can, for example, exclude rare point mutations. For details of the probes used, see Buiting et al (Reference Buiting, Dittrich and Robinson1994). Sibling-pair linkage analysis is based on the premise that loci close to one another on the chromosome will stay together during meiosis, but that loci further apart have a higher probability of recombining. If the PWS locus is not associated with HSS then affected siblings would share the DNA marker alleles according to standard Mendelian ratios. In other words, there would be evidence of allelic discordance between affected siblings across the PWS region. The markers that we used in the current study are tightly linked to the critical region. Four CA repeat markers covering different parts of the critical PWS region were used: IR4, D15S210, GABRB3 and MS14. Protocols are available from the author upon request.
Intrafamilial stress measure
The Camberwell Family Interview (Reference Vaughn and LeffVaughn & Leff, 1976) provided ratings of expressed emotion on the following scales: Critical comments, Positive remarks, Emotional overinvolvement (EOI), Hostility, and Warmth. Caregivers who score over 3 on EOI or are rated 1 and above on hostility, or make six or more critical comments, are rated ‘high EE’. The ratings of EE reported in this study were completed by a trained rater, unaware of case status, but aware of the child's IQ and age. In the rating of EOI a correction was made for children with moderate to severe learning disabilities (an IQ less than 50), who were judged to require protection beyond that expected in normal children of equivalent age.
Physiological stress reactivity
Each child's HPA reactivity was measured by inducing elevated levels of cortisol through a brief period of intensive exercise. Cortisol levels were measured serially using salivary cortisol assays, which provide an accurate measure of plasma free cortisol (Reference Vining, McGinley and MaksvytisVininget al, 1983). Physical stress such as exercise increases salivary cortisol levels in normal children (Reference Kirschbaum and HellhammerKirschbaum & Hellhammer, 1989). We used the Harvard Step Test (Reference Kirkendal, Mohanty and GonasunKirkendal et al, 1987) and children took 60 steps per minute, which increased their heart rate to about 150 beats per minute, a level that is normally associated with a significant increase in salivary cortisol levels (R. Lane, personal communication, 1996). The full protocol is available from the author upon request. In children who were subject to chronically high levels of day-to-day stress, we expected to see an attenuation of the exercise-induced cortisol response.
Comparison group (salivary cortisol only)
Twenty unselected children from a mainstream primary school, aged 8-9 years (of whom 12 were boys), provided normative salivary cortisol samples. Parents were informed by letter of our study. The exercise was carried out in the early afternoon, with all children participating simultaneously.
RESULTS
Participants
Demographic variables are closely associated with parenting quality and family stress (Reference McLoydMcLoyd, 1998). There were no differences in mothers' or fathers' educational attainment. Socio-economic status levels were equivalent for the parents. Of the mothers, 32% of HSS and 22% of PWS mothers had never worked, were unemployed or students; 56% and 59% respectively were in manual occupations, and 12% and 18% respectively were in non-manual occupations. For fathers, the proportions were as follows for the HSS and PWS groups respectively: 6% and 0%, 60% and 65%, and 33% and 34%. Maternal ages were similar in the PWS (34.2 years, s.d. 6.0) and HSS groups (34.6 years, s.d. 7.9), as was paternal age (38.8 years, s.d. 7.5 and 39.4 years, s.d. 8.8, respectively). Of the HSS group 36% were living with their biological mother compared with 86% in the PWS group (χ2=3.9 (1), P<0.05). Twenty-eight per cent of HSS families and 30% of PWS families were single parents. The children's age ranges were 3.1-16.9 years in the HSS group and 5.6-15.3 years in the PWS group, with means of 9.1 (s.d. 3.8) years and 8.8 (s.d. 2.8) years. Twenty-eight per cent of the HSS and 33% of the PWS groups were girls. Mainstream school was attended by 57% of PWS children, compared with 72% of the HSS group (χ2=6.8 (1), P<0.01).
Child characteristics
In the HSS group IQ scores ranged from 50 to 118; the range was 40 to 91 in the PWS group. Because 40 is the lowest IQ score attainable on the WISC (41 on the WPPSI-R) it may be that a floor effect has biased the estimate of mean IQ in the PWS sample; in this sample 23% of children (n=7) scored at the floor of the test. Lower IQ scores are associated with greater emotional and behavioural disturbance in general (e.g. Reference Goodman, Simonoff and StevensonGoodman et al, 1995). Accordingly, we considered using IQ as a covariate in all the following analyses, but did not do so on the grounds that our aim was to report and compare absolute levels of behavioural disturbance in the two samples.
The HSS children were significantly shorter than the PWS children, but the PWS group had a significantly higher BMI (Table 2). Six of the children in the PWS group had been treated with growth hormone. There was no significant difference in height or in BMI between those who had been so treated and those who had not.
Table 2 Cognitive ability and anthropometric data: group means and analyses

| Variable | HSS (n=25) | PWS (n=30) | ||
|---|---|---|---|---|
| Mean | (s.d.) | Mean | (s.d.) | |
| Full-scale IQ | 77.7 | (17.8) | 54.0 | (13.7)** |
| Performance IQ | 83.4 | (18.7) | 56.0 | (11.8)** |
| Verbal IQ | 76.3 | (18.2) | 59.8 | (16.6)** |
| Standardised height1 | -2.6 | (1.1) | -1.5 | (1.5)* |
| Standardised body mass index | -0.1 | (1.2) | 2.4 | (2.0)** |
Parent and teacher reports
Standardised population (T) scores (mean 50, s.d. 10) on the Child Behaviour Checklist and the Teacher Report Form (Achenbach, Reference Achenbach1991a ,Reference Achenbach b ) are reported in Table 3. Standardised T scores of 65-69 fall into the ‘borderline clinical’ range, while scores of 70 and above can be regarded as clinically significant (Achenbach, Reference Achenbach1991a ,Reference Achenbach b ). On the basis of teacher reports both groups show broadly similar group profiles. Parents of PWS children reported more thought problems and social problems than the HSS caregivers, who reported significantly more anxious/depressed symptomatology. The total-problems scores were similar in both groups.
Table 3 Population Child Behaviour Checklist (CBCL) and Teacher's Report Form (TRF) scores: group means and analyses

| Problem | CBCL score (parent) | TRF score (teacher) | ||||||
|---|---|---|---|---|---|---|---|---|
| HSS (n=17) | PWS (n=28) | HSS (n=24) | PWS (n=25) | |||||
| Mean | (s.d.) | Mean | (s.d.) | Mean | (s.d.) | Mean | (s.d.) | |
| Total problems | 65.3 | (11.3) | 67.0 | (7.2) | 60.9 | (9.6) | 64.7 | (9.3) |
| Internalising | 60.3 | (12.3) | 57.8 | (7.0) | 56.2 | (11.3) | 59.6 | (9.1) |
| Externalising | 61.0 | (12.9) | 61.6 | (10.0) | 59.8 | (9.9) | 63.2 | (9.4) |
| Withdrawn | 62.5 | (11.4) | 61.3 | (6.9) | 58.6 | (10.5) | 58.8 | (6.9) |
| Somatic complaints | 60.1 | (8.7) | 60.7 | (8.3) | 56.1 | (9.1) | 62.1 | (8.0)* |
| Anxious/depressed | 59.5 | (11.4) | 52.9 | (4.1)* | 57.8 | (7.1) | 57.2 | (8.7) |
| Social problems | 64.2 | (9.2) | 71.8 | (9.2)* | 62.2 | (5.7) | 62.6 | (7.4) |
| Thought problems | 60.9 | (7.8) | 69.2 | (7.3)** | 57.9 | (9.4) | 61.5 | (10.2) |
| Attention problems | 70.0 | (11.7) | 65.4 | (9.3) | 61.5 | (9.8) | 61.2 | (7.9) |
| Delinquent behaviour | 62.8 | (10.5) | 60.0 | (8.8) | 59.3 | (7.1) | 60.0 | (8.5) |
| Aggressive behaviour | 62.7 | (11.3) | 63.0 | (10.1) | 60.8 | (9.5) | 64.7 | (10.7) |
| Sex problems | 54.2 | (7.6) | 55.1 | (8.1) | ||||
Hyperphagia
Thirteen variables relating to appetite disturbance, from the HSSDI and the Teacher Questionnaire (school data were unavailable for one child in the PWS group), were entered into a principal components analysis (PCA) with varimax rotation. The data were generated from a larger data-set, including the children from this study and a consecutive series (n=25) of children living in psychosocial adversity. Three factors were identified. ‘General hyperphagia’ explained 33.2% of the variance, ‘school-specific hyperphagia’ explained a further 15.7%, and ‘pica/polydipsia’ explained 9.7% (Table 4). Scores were compiled for the three variables derived from this analysis by summing together the untransformed values for all relevant questions.
Table 4 Factor analysis coefficient loadings on hyperphagia factors

| Variable | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| General hyperphagia | School-specific hyperphagia | Pica/polydipsia | |
| Behaviour at home | |||
| Stealing food: kitchen cupboards1 | 0.91 | 0.13 | 0.04 |
| Stealing food: fridge1 | 0.92 | 0.11 | 0.09 |
| Stealing food: plates at mealtimes1 | 0.63 | 0.14 | 0.34 |
| Hoarding food1 | 0.55 | -0.04 | -0.15 |
| Overeating requiring restraint1 | 0.69 | 0.13 | 0.01 |
| Gorging and vomiting1 | 0.28 | 0.31 | 0.11 |
| Polydipsia1 | 0.11 | -0.03 | 0.78 |
| Pica1 | 0.55 | -0.04 | 0.44 |
| Chewing non-food items1 | -0.05 | -0.11 | 0.21 |
| Behaviour at school | |||
| Foraging in rubbish bins2 | -0.01 | 0.50 | 0.72 |
| Pica2 | -0.03 | 0.70 | 0.54 |
| Stealing food at school1 | 0.39 | 0.55 | -0.12 |
| Stealing food at school2 | 0.14 | 0.88 | 0.02 |
Full-scale IQ did not correlate significantly with hyperphagia at school, general hyperphagia or pica/polydipsia in either group. There were no significant differences between the groups, with α set at 0.05, on any of the factor summary scores for acute hyperphagia, general hyperphagia or pica/polydipsia. Figure 1 shows that the quality of the appetite disturbance was similar in both groups.

Fig. 1 Item profile of appetite disturbance by group: hyperphagic short stature (▪) and Prader—Willi syndrome (□).
Parents' reports of their management techniques for hyperphagia were considered in mutually exclusive categories. There are no significant group differences. Three (12%) of the HSS caregivers reported that they did not try to prevent their child from taking food at all, despite an excessive appetite, and three (10%) of the PWS group did not use preventive measures. Six (32%) of the HSS caregivers used verbal warnings not to take food, compared with 11 (37%) in the PWS group. Nine (36%) of the HSS families used locks on food cupboards, compared with 13 (43%) in the PWS group. Excluding the child from the kitchen or other areas of the house was reported in four (16%) of the HSS cases compared with two (7%) of the PWS cases. There was a trend in the PWS group for more behaviour problems (measured as a sum of parent— and teacher-reported total-problems scores) to be associated with more restrictive techniques of denying children's access to food. Mean behaviour problem scores in the PWS group were 126 (s.d. 15) for children whose parents used verbal warnings, 136 (s.d. 14) for those who used locks on food cupboards, and 140 (s.d. 6) for children who were excluded altogether from certain areas of the house. In the HSS group equivalent scores were 121 (s.d. 22), 123 (s.d. 28) and 131 (s.d. 9) respectively for each management tactic.
Sleep
In the HSSDI, parents were asked to describe their children's sleep patterns. Sixty-three per cent of the PWS group and 44% of the HSS group had frequent and persistent sleep disturbance, including night roaming, to a degree that was developmentally inappropriate: a non-significant group difference.
Molecular genetic studies
Fourteen children in the HSS group were tested with the methylation status test (Reference Dittrich, Robinson and KnoblauchDittrich et al, 1992; Reference Buiting, Dittrich and RobinsonBuiting et al, 1994). All showed a normal methylation pattern at 15q11-13, which indicates that all children with HSS had one paternally derived allele and one maternally derived allele at the critical region of chromosome 15. For the sibling pair linkage analysis, two family pedigrees were tested using four marker probes (Reference Mutirangura, Greenberg and Butler MerlinMutirangura et al, 1993) across the PWS region. In family studies these closely linked markers can be used to establish the array of alleles (haplotype) for individual chromosomes. The pattern of haplotype inheritance is shown in Fig. 2. In family C, DNA was available from both biological parents and all three affected full siblings, but was not available from a further unaffected half-sibling. While the index case (JC) and his affected sister (CC) have inherited the same two haplotypes, GC, who is also affected, has no haplotype in common. This is evidence against a locus for HSS being located in the PWS region, regardless of the Mendelian pattern of inheritance of HSS.

Fig. 2 Family C: sibling pair linkage analysis.
In family M, maternal but not paternal DNA was available (family M are part of a retrospective series, not the current sample). Samples of DNA were gathered from two affected and three unaffected full siblings (Fig. 3). The paternal haplotypes have been inferred on the basis of his children's allele pattern. The children LM and LaM were both affected, and although they almost certainly inherited the same maternal and paternal alleles at D15S210, MS14 and possibly at GABRB3, so did an unaffected sibling (LiM). These data from family M are also evidence against a locus for HSS being located in the PWS region.

Fig. 3 Family M: sibling pair linkage analysis.
Intrafamilial stress
Each sub-scale of the Camberwell Family Interview (Reference Vaughn and LeffVaughn & Leff, 1976) was analysed separately in order to develop a more detailed profile for each index child. Critical comments were classified according to whether they were food-related or not (Table 5). Examples of food-related comments include: ‘Nine times out of ten he can't be bloody hungry. Why he needs it or why he wants it is a complete mystery to me.’ The total number of food-related comments was significantly related to the degree of hyperphagia in the PWS group (r=0.4, P<0.05). Although the trend was similar in HSS, it was not significant. In the PWS group caregivers made proportionately more food-related critical comments relative to all critical comments (52%) than the HSS group caregivers (32%), but this difference did not reach significance. Twelve (50%) of the HSS caregivers were rated as high EE compared with seven (23%) in the PWS group (χ2=4.2 (1),P<0.05).
Table 5 Family functioning: group means and analyses

| Expressed emotion | HSS (n=24) | PWS (n=30) | ||
|---|---|---|---|---|
| Mean | (s.d.) | Mean | (s.d.) | |
| Critical comments1 | 5.0 | (3.9) | 3.0 | (3.0)* |
| Food-related critical comments1 | 2.4 | (2.5) | 1.4 | (2.1) |
| Hostility2 | 0.5 | (1.0) | 0.2 | (0.6) |
| Emotional overinvolvement2 | 1.0 | (1.3) | 0.7 | (0.9) |
| Positive remarks1 | 1.7 | (1.5) | 2.0 | (1.4) |
| Warmth2 | 2.1 | (1.4) | 3.4 | (1.1)** |
Hostility was rated on a scale of 0 to 3. Vostanis & Nicholls (Reference Vostanis and Nicholls1992) found none of the parents in a non-referred group scored above 0. One (3%) of the PWS caregivers expressed hostility (a score of 1 to 3), compared with five (21%) of the HSS group caregivers (χ2=2.4 (2), not significant). There were no significant group differences in EOI, positive remarks or food-related critical comments.
Child's physiological reactivity
We aimed to discover the patterns of HPA reaction and recovery following an acute stressor (an exercise step test) using salivary cortisol assays. Obesity, a common feature of PWS, is associated with elevated cortisol levels in plasma and saliva. Therefore we recruited, in addition to our case samples, a normal comparison group. The test was administered in early afternoon in all cases. There was no significant relationship with baseline cortisol level and BMI in either the HSS or PWS samples. These results are consistent with previous reports of baseline cortisol levels in the normal range for children with PWS (Reference Tu, Hartridge and IzawaTu et al, 1992). Mean pulse rates at baseline were 95.9 beats per minute (s.d. 15.7) and 92.9 beats/min (s.d. 15.1) for the HSS and PWS groups respectively. Heart rates were 161.3 beats/min (s.d. 21.3) in the HSS group and 161.1 beats/min (s.d. 17.9) in the PWS group after the step exercise. A repeated measures analysis of variance (ANOVA) showed a significant increase in pulse rate pre— and post-exercise for the whole sample (F (45, 1)=463, P<0.001), but no group × pulse rate change interaction (F (45, 1)=0.05, not significant). Pulse rate data were not available for the community comparison children. Table 6 shows the cortisol levels for the groups pre-test and at 10, 20, 30 and 60 minutes after the exercise stress. Peak response is predicted approximately 30 minutes after the exercise stress (Reference Kirschbaum and HellhammerKirschbaum & Hellhammer, 1989). There was wide variation within the groups, with significantly greater variability among PWS than HSS children, especially at 10 minutes (F=5.5, P<0.01), 20 minutes (F=5.9, P<0.05) and 30 minutes (F=11.1,P<0.01) after exercise. Such variability reduces the power to detect significant mean differences between groups (Reference Kiess, Meidert and DressendorferKiess et al, 1995). No group differences were detected overall, nor for any specific sample, and there were no group × time of assay effects, using polynomial contrasts on the assay.
Table 6 Salivary cortisol measures (nM/I): group means and analyses

| Saliva sample assay | HSS (n=24) | PWS (n=26) | Comparison (n=20) | |||
|---|---|---|---|---|---|---|
| Mean | (s.d.) | Mean | (s.d.) | Mean | (s.d.) | |
| Pre-test | 8.3 | (8.9) | 10.5 | (10.8) | 6.7 | (4.2) |
| Sample 2 (+ 10 min) | 8.8 | (7.8) | 15.3 | (20.4) | 6.4 | (5.2) |
| Sample 3 (+20 min) | 9.4 | (7.6) | 15.9 | (20.7) | 7.3 | (6.7) |
| Sample 4 (+30 min) | 7.7 | (7.1) | 15.0 | (20.2) | 7.9 | (6.7) |
| Sample 5 (+60 min) | 8.8 | (6.0) | 12.5 | (12.8) | 6.6 | (6.4) |
DISCUSSION
This study presents empirical data describing the marked similarities between HSS and PWS. It is striking that the degree and quality of hyperphagia, according to parent and teacher report, is indistinguishable between the groups. There is comparable frequency of sleep disturbance according to parent report, and children in both groups are of short stature. The severity of psychosocial disturbance associated with the disorder was similar in both groups. There were significant differences in developmental ability, with the HSS children having less severe learning disabilities than those with PWS, overall.
Sample size and bias
The specificity and sensitivity of both molecular genetic techniques provide strong evidence of the exclusion of the HSS locus from the PWS region. The sample sizes for the psychological assessments were small, limiting the power to detect group differences. Accordingly, comparisons of means between PWS and HSS samples were liable to be associated with type II errors. On the other hand, statistically significant differences would have been associated with large effect sizes (Reference CohenCohen, 1992), equivalent to identifying phenomena of clinical significance.
Both samples were largely recruited through medical services. It is likely that the children included had more severe clinical problems than one would observe in an epidemiological survey. Given that our aim was to compare the conditions, it is appropriate that both groups were recruited through similar routes, so any bias will apply equally to both conditions. It does not make the comparison invalid.
Future genetic investigations
Molecular genetic analyses were conducted to test for co-inheritance of the PWS locus in HSS sibling pairs. Neither methylation patterns nor sibling pair linkage analysis indicates that HSS subjects have an anomaly at the PWS critical region. If children with HSS do indeed have a genetic predisposition to the condition, alternative candidate genes need to be evaluated, including those involved in appetite and growth regulation. A chromosomal anomaly may be associated with HSS, but cytogenetic studies have not yet been conducted.
Physiological reactivity
The salivary cortisol results were difficult to interpret. Neither the HSS nor the PWS group profiles differed significantly from the community comparison group. Lack of mean group differences could be attributable in part to the wide within-group variability, which was especially marked in children with PWS. The level of ‘stress’ experienced during the exercise may not have increased cortisol levels to an adequate degree. Kirschbaum & Hellhammer (Reference Kirschbaum and Hellhammer1989) obtained a peak response that was 250% greater than baseline salivary cortisol levels. We induced increases of only 10%, 53% and 12% in the HSS, PWS and community comparison groups respectively. A more challenging stressor, other than exercise, might have produced significant results, but raises ethical considerations.
Hyperphagia may precipitate stress
Given the broad similarities between HSS and PWS we might assume that having a child with either condition would have a similar impact on the family. A re-evaluation of the direction of effects that lead to intra-familial stress in HSS has important clinical implications. It is often assumed that HSS is entirely caused by psychosocial adversity. Children are often subjected to care proceedings on the grounds that their symptoms usually resolve in an alternative family, and catch-up growth ensues. However, our experience over the past decade has shown that, in many instances, after a ‘honeymoon’ period, the child's unregulated behaviour recommences in foster care, leading eventually to stress and rejection. Growth in stature then reduces or ceases altogether. In support of our hypothesis that hyperphagia induces similar reactions from families with HSS and PWS, there were no group differences in the number of food-related critical comments. The PWS group caregivers were all aware that hyperphagia is part of their child's condition, information that was not available to families in the HSS group at the time of the EE rating. The number of critical comments was related to the degree of hyperphagia in both samples. As shown in Fig. 4, the clinical features of HSS, which are certainly exacerbated by stress, may also contribute to intrafamilial stress.

Fig. 4 Hypothesised stress interactions in hyperphagic short stature (HSS).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Hyperphagic short stature (HSS) is invariably associated with stress. In some cases affected children are at risk of abuse and neglect. Children presenting with the features of HSS would benefit from a thorough assessment of their circumstances.
-
• Families who have a child with HSS or Prader—Willi syndrome will need support specifically to manage the hyperphagia.
-
• Expressed emotion could be a valuable outcome variable for intervention aimed at hyperphagia management.
LIMITATIONS
-
• The sample sizes are small, although of sufficient size to detect large, clinically significant effect sizes.
-
• The study describes a clinically referred sample. Replication with a community sample is important.
-
• As is often the case in developmental psychology, profiles were generated by parental report. It would be useful to describe appetite and sleep profiles using direct, objective measures.
Acknowledgements
We thank Helen Middleton Price, Jenny Jones, Joanne Newbolt, Richard Stanhope, the Prader—Willi Association (UK) and the families who took part in the study. We are grateful to Jennifer Smith for her administrative support. The Wellcome Trust funded this project.













eLetters
No eLetters have been published for this article.