INTRODUCTION
Reduced environments are found throughout the world's ocean seafloor and sediments and typically arise from a lack of dissolved oxygen or excessive organic inputs to the seabed, such as particulate organic matter (marine snow) or large animal carcasses (Tunnicliffe et al., Reference Tunnicliffe, Juniper, Sibuet and Tyler2003). They also arise from geological conditions, for example, cold seeps that are typically located along continental margins and associated with sub-seabed reservoirs of hydrocarbons (often methane) and sulphide-rich fluid expelled to the seafloor by gravitational and tectonic forces (Levin, Reference Levin2005). Methane seeps, mud volcanoes, pock marks, carbonate slabs and brine pools are all examples of cold-seep chemosynthetic habitats (Cordes et al., Reference Cordes, Cunha, Galeron, Mora, Olu-Le Roy, Sibuet, Van Gaever, Vanreusal and Levin2010). At cold seeps, microbial consortia anaerobically oxidize methane, reduce sulphate to sulphide and chemically acquire the necessary energy for metabolism, growth and replication (Boetius et al., Reference Boetius, Ravenschlag, Schubert, Rickert, Widdel, Gieseke, Amann, Barker Jùrgensen, Witte and Pfannkuche2000; Orphan et al., Reference Orphan, House, Hinrichs, McKeegan and DeLong2002; Joye et al., Reference Joye, Boetius, Orcutt, Montoya, Schulz, Erickson and Lugo2004; Dubilier et al., Reference Dubilier, Bergin and Lott2008; Boetius & Wenzhöfer, Reference Boetius and Wenzhöfer2013). The biochemical reactions result in the precipitation of authigenic carbonate which can lead to the formation of solid crusts surrounding seeps and vents that in turn provide substrate for other fauna (Bayon et al., Reference Bayon, Dupre, Ponzevera, Etoubleau, Cheron, Mascle, Boetius and de Lange2013).
The bacteria occur either as chemosynthetic symbionts of other organisms (Dubilier et al., Reference Dubilier, Bergin and Lott2008) or as free-living bacteria. The free-living bacteria often form extensive filamentous mats across the seafloor (Boetius & Wenzhöfer, Reference Boetius and Wenzhöfer2013). Only a few higher marine taxa have evolved the specialized adaptations to tolerate high concentrations of sulphide and live symbiotically with the bacteria (Dubilier et al., Reference Dubilier, Bergin and Lott2008). These include, among others: mussels of the subfamily Bathymodiolinae; bivalve clams of the family Vesicomyidae, Thyasiridae, Lucinidae and Solemyidae; annelid worms of the family Siboglinidae; and sponges of the family Cladorhizidae. The species and communities of cold-seep sediments are often dominated by endemic fauna (Olu et al., Reference Olu, Lance, Sibuet, Henry, Fiala-Médioni and Dinet1997; Levin, Reference Levin2005; Vanreusel et al., Reference Vanreusel, Andersen, Boetius, Connelly, Cunha, Decker, Hilario, Kormas, Maignien, Olu and Pachiadaki2009). Cold-seep habitats are highly heterogeneous (Cordes et al., Reference Cordes, Cunha, Galeron, Mora, Olu-Le Roy, Sibuet, Van Gaever, Vanreusal and Levin2010) and contrast starkly with adjacent deep-sea habitats (Bowles et al., Reference Bowles, Hunter, Samarkin and Joye2016). The biomass of cold-seep communities can be orders of magnitude greater than the surrounding deep-sea habitat (Tunnicliffe, Reference Tunnicliffe1992).
Although worldwide fewer than 100 active seeps have been described (German et al., Reference German, Ramırez-Llodra, Baker and Tyler2011), they are thought to be much more widespread along continental margins than currently documented. This is likely to be the case in the deep north-east Atlantic, where as far as we know, they are few and far between. Only three confirmed sites have so far been described: at ~72°N lies the Haakon Mosby mud volcano in the Barents Sea (Niemann et al., Reference Niemann, Lösekann, de Beer, Elvert, Nadalig, Knittel, Sauter, Schluter, Klages, Foucher and Boetius2006; Jerosch et al., Reference Jerosch, Schluter, Foucher, Allais, Klages and Edy2007); at around 64°N, offshore from Norway, is found the Nyegga pock-mark region (Krylova et al., Reference Krylova, Gebruk, Portnova, Todt and Haflidason2011); and south of Spain, at 36°N, are found the mud volcanoes of the Gulf of Cadiz (Rodrigues et al., Reference Rodrigues, Webster, Cunha, Duperron and Weightman2010; Cunha et al., Reference Cunha, Rodrigues, Genio, Hilario, Ravara and Pfannkuche2013). This apparent rarity of cold seeps in the North-east Atlantic is unlikely to be real and highlights a major gap in our knowledge of deep-water benthic ecosystems. Most of the sedimentary basins of the North Atlantic margin contain potential hydrocarbon source rocks and this includes the Hatton–Rockall basin, a large expanse of submerged continental crust that lies ~500 km west of the British Isles (Hitchen, Reference Hitchen2004). The underlying sediments of the Hatton–Rockall Basin are characterized by an extensive polygonal fault system which has been suggested to be a currently active process resulting in fluid expulsion (Berndt et al., Reference Berndt, Jacobs, Evans, Gay, Elliott, Long and Hitchen2012). As seabed fluid expulsion is often associated with chemosynthetic ecosystems (Sibuet & Olu, Reference Sibuet and Olu1998), the Hatton–Rockall Basin is a candidate area for harbouring cold-seep ecosystems.
In 2012 a benthic sampling net deployed at the deepest point (1200 m) of the western margin of Rockall Bank recovered two new species of chemosymbiotic bivalve of the families Vesicomyidae and Thyasiridae (Oliver & Drewery, Reference Oliver and Drewery2014). These specimens suggested a cold-seep ecosystem (hereafter referred to as the Scotia Seep) within the UK continental shelf claim. However, the occurrence of these ‘indicator’ species is not itself proof of a cold-seep, as they may be associated with reduced sediments arising from biotic inputs. In order to assess the seabed habitats in the vicinity of where these species were found, a visual survey of the area was made using a towed camera to produce the first account of the site and map the seabed habitats present.
MATERIALS AND METHODS
Study site
The Hatton–Rockall Basin is a large, sedimentary deep-water habitat (1000–1500 m water depth). Bounded by the Rockall Bank to the east and the Hatton Bank to the west and north, it is located around 500 km west of the UK in international waters, but within the extended continental shelf claim of the UK (Figure 1, inset map). The study site (provisionally named the Scotia Seep after MRV ‘Scotia’) was located at ~57°57′N 15°33′W at the bottom of the western slope of the Rockall Bank at depths of between 1100 and 1216 m.

Fig. 1. Map of the study site and the habitats found along the towed camera transects. Baited camera deployments are also indicated (circles) as are the sites of active vents. Bathymetric contours are plotted every 10 m from 1100 m to 1210 m. The 1190 m isobath is shown in black to indicate the area in which the majority of cold-seep habitats were observed. Inset map gives location of the putative cold seep (red dot).
Visual survey
Eight towed camera transects were made aboard MRV ‘Scotia’ totalling ~12.5 h and 40 km of video footage (Figure 1, main map). Transects spanned a depth range of 1040–1216 m. Seabed imagery was obtained using a towed body, consisting of an aluminium frame fitted with a Kongsberg Maritime colour HD video camera and six high-intensity undersea lamps and 30 cm spaced lasers for scale (McIntyre et al., Reference McIntyre, Drewery, Eerks-Medrano and Neat2016). The camera also had the option to take strobe-lit stills which was done to obtain images for close inspection. In addition, the frame housed sensors for pressure (depth), bearing, altitude from seabed, pitch and heave, all recording at 1 s intervals throughout. The towed body was attached to the research vessel with a 4000 m ‘Netsonde’ co-axial cable and towed at a speed of between 1.5–2.5 knots at an elevation of between 2 and 5 m above the seafloor. An ultra-short baseline beacon (USBL) provided positional information on the towed camera, calculating the position of the body relative to the vessel. Transmission from the USBL was, however, intermittent and for many video sequences the position of the camera had to be estimated from the length of wire out and the seabed depth using standard spherical geometry (assuming the body to lie directly behind the vessel). Comparison of estimated positions with known positions from the USBL indicated estimated positions were on average within 164 m (±22 m) of the known position. In addition to the towed video five point observations were made from a free-fall baited lander system with time-lapse cameras that took horizontal digital stills of the seabed (Linley, Reference Linley2016). These were used to supplement the towed camera data.
Habitat classification
The identification of species from digital stills images and HD video is frequently problematic in the absence of physical samples; however visible species were identified to the lowest possible taxonomic level. Following detailed analysis of the HD video that involved species identification when possible and an assessment of the seabed type, six broad habitat types were evident that appeared to be generally exclusive of one another. These were: bacterial mats covering flat seabed and raised areas; sponges in bioturbated sediment; coral gardens; sand-rich sediment; sediment with tube-dwelling worms; zero visibility (when the seabed was obscured by dense flocculent matter). Segments of video were classified to one of these habitat types and using time referenced positions were then mapped in ArcGIS.
Bathymetry mapping
The ship-board Olex seabed mapping system (http://www.olex.no) generated a bathymetric map of the area based on echo-sounder readings. This was used to generate 10 m depth contours from 1100 to 1210 m water depth in ArcGIS onto which results are presented.
RESULTS
Location and topography
The putative cold-seep habitat was revealed within a distinct narrow depression (Figure 1) beneath a steep escarpment to the east (Rockall Bank) and a more gradual ascent to the west (the Hatton–Rockall Basin). At its deepest point the depression reached 1216 m. There were two distinct basins along the north-south axis of the basin where water depth exceeded 1200 m, separated by a ridge that rose to around 1180 m water depth. To the south and north of the trench, the seabed gradually shallowed before levelling out at around 1100 m water depth.
Habitats and distribution
BACTERIAL MATS
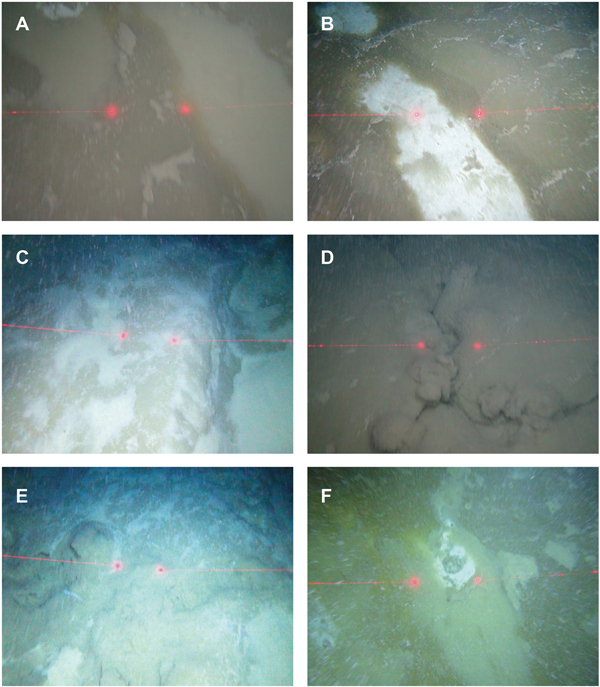
Large expanses of the seafloor appeared to be covered in dense bacterial mats. The bacterial mats appeared as patches on the seabed (Figure 2A and Supplementary video) between areas of fine sediment (Figure 2B) and areas where flocculent matter had settled to form a fluffy lighter brown layer (Figure 2A, B). The bacterial mats were mainly devoid of any emergent epifauna with the exception of sporadic, small unidentified anemones (Supplementary video). The bacterial mats were encountered mainly at depths of 1190 m and deeper (Table 1). At times the bacterial mats covered distinct three-dimensional features referred to as ‘green mounds’ in Figure 2C and Table 1. These mounds rose an estimated 1 m above the surrounding seafloor, forming elongate ridges. At least four separate sediment-rich fluid plumes emanating from the seafloor were observed (Figure 2D, E and Supplementary video). Small concentric features of the seabed were sometimes evident (Figure 2F) that resembled holes or burrows. Visibility was variable along transects, a result of often dense flocculent matter in the water column. Fish (e.g. Corephaeneoides rupestris, Chimaera opalesence, Synaphobranchus kaupi) were seen swimming above the bacterial mats.
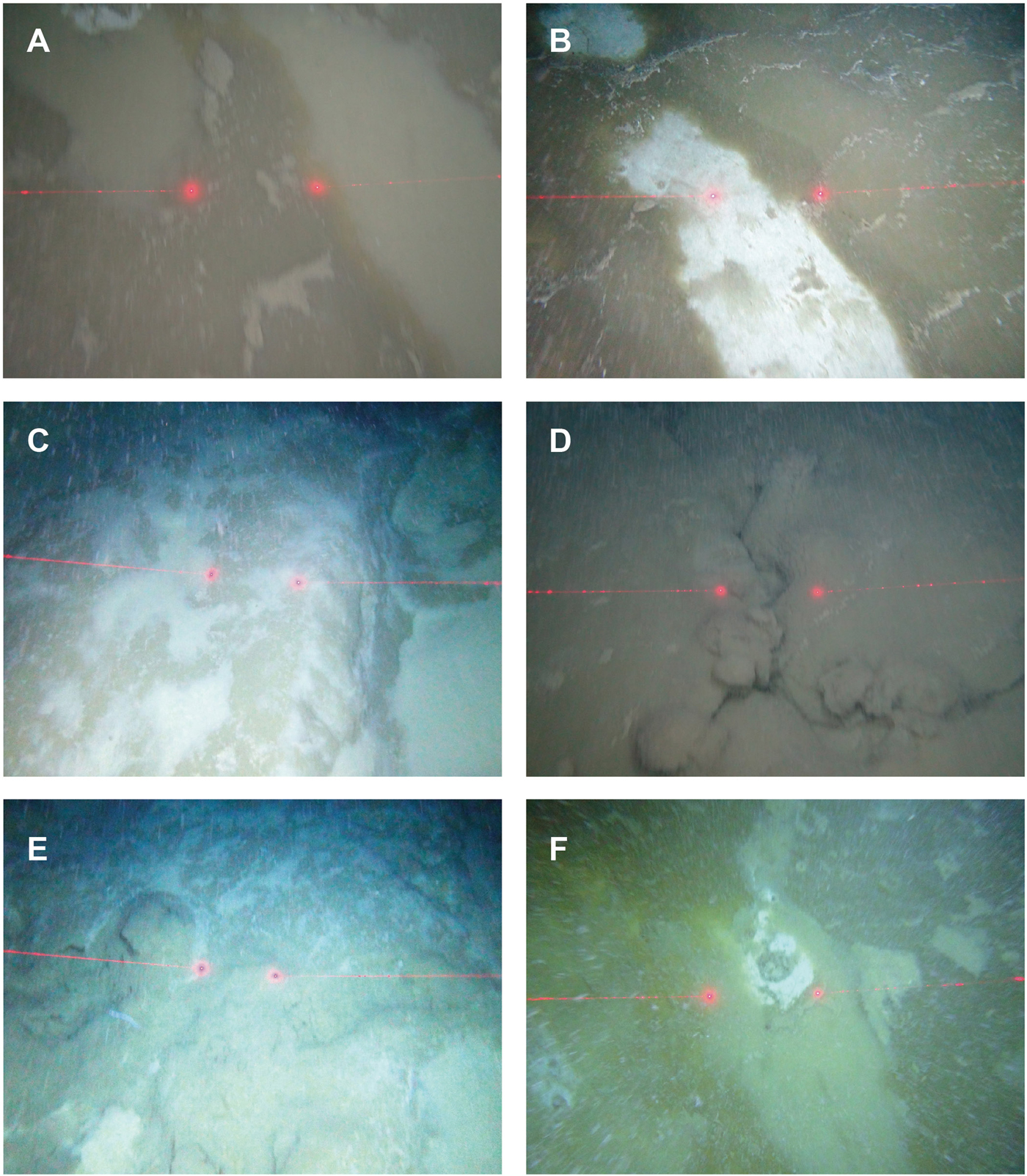
Fig. 2. Seabed imagery of cold-seep habitats. (A) and (B) The bacterial mats shrouding the seabed. The paler areas are flocculent matter that has settled from the water column onto the mats. (C) An example of the raised elongate mounds on the seabed covered with bacterial mats. (D) and (E) Active fluid seepage in association with the raised mounds. (F) An example of what appears to be a hole in the seabed between the bacterial mats. Red laser points are 30 cm apart.
Table 1. Depth ranges (derived from pressure sensor on towed camera) for each of the habitat types observed in the study.
SPONGES IN BIOTURBATED SEDIMENT
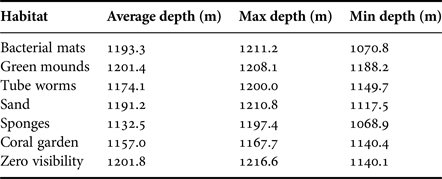
This habitat was characterized by soft, bioturbated sediment (Figure 3A). Megafauna included sponges, of which the hexactinellid Pheronema carpenteri, the encrusting yellow sponge Hexadella sp. and a stalked sponge of the genus Hyalonema were identified. What appeared to be Xenophyophores were evident, although at times it was difficult to distinguish these from species of sponge. The squat lobster Munida sp. was seen frequently, sometimes partially burrowed in the sediment. The biotope was further characterized by cerianthid anemones, of which Pachycerianthus sp. was identified. Other commonly observed taxa included holothurians of the genus Benthogone and various large echinoids including Calveriosoma sp. This habitat was characteristic of the shallower western slopes (1130–1180 m) of the study area (Table 1). Fish were observed including Lepidon eques. This habitat rapidly disappeared at depths of around 1180 m and greater, transitioning into either the bacterial mat or tubeworm-dominated habitats.
Fig. 3. Seabed imagery of habitats on slopes adjacent to the cold seep. (A) Bioturbated sediments with sponges/and or xenophyophores. (B) Coral gardens with gorgonian corals, stony corals and anemones. (C) Tube-dwelling worm dominated sediments. (D) Close up of the tube-dwelling worm dominated sediments (image acquired from a baited lander deployment).
CORAL GARDENS
This habitat (Figure 3B) was typified by the presence of gorgonian corals (sea fans) of the genus Placogorgia, stony coral (Madrepora oculata), alcyonaceans (soft corals) and the anemone Bolocera sp. The slope was steep and the seabed composed of outcrops of rock and areas of gravel with intermittent patches of fine-grained sediments. Fish were observed including Lepidon eques. This habitat was only observed in the eastern reaches of the study area, coincident with the steep flank of Rockall Bank along a narrow depth range of between 1140 and 1167 m (Table 1).
SAND-RICH SEDIMENT
In this habitat the sediment comprised muddy sand with occasional gravel-sized clasts. It was predominantly not bioturbated and lacked sponges. Occasional boulders were colonized by an unidentified species of barnacle. Other benthic fauna included small cerianthid anemones and holothurians including Benthogone sp. This habitat was observed on the ridge between the two basins, but also extended into the basins in places and was characteristic of the southern limit of the basin as the depth decreased.
TUBE-DWELLING POLYCHAETE WORM SEDIMENT
This habitat was characterized by the presence of high densities of small unidentified tube-dwelling polychaete worms (Figure 3C, D). At times there appeared also to be a light covering of bacterial mat. There was no evidence of bioturbation and no sponges or other benthic megafauna. This habitat was typically seen adjacent to and at marginally shallower water depths than the bacterial mats habitat (Table 1).
ZERO-VISIBILITY
This category was reserved for sections of transects where the seabed was completely obscured by dense flocculent matter in the water column (see supplementary video). The location of this cloud of flocculent matter was predominantly encountered in the deepest sections of the study area (Table 1), although in two transects areas of zero-visibility were encountered at shallower water depths and in one instance it was encountered where the seabed had been visible on another transect.
DISCUSSION
Extensive areas of what appeared to be bacterial mats smothering the seafloor were observed in this study. The localized nature of the mats and their absence from adjacent shallower areas suggest a focal source of reduced sediments, hydrogen sulphide production and/or methane. The bacterial mats could be indicative of a chemosynthetically active cold-seep ecosystem as has been reported from numerous sites across the world's oceans (Boetius & Wenzhöfer, Reference Boetius and Wenzhöfer2013). Alternatively they could be sustained through excessive accumulation of photosynthetically derived particulate organic matter (Gooday & Lambshead, Reference Gooday and Lambshead1989). This would fit with the bacterial mats being found predominantly in the deepest part of the study area (below 1190 m) and also with the survey being undertaken in mid-summer following the spring phytoplankton bloom in the NE Atlantic. The presence of bacterial mats together with the initial discoveries of chemosynthetic clams (Oliver & Drewery, Reference Oliver and Drewery2014) provides strong evidence for the presence of a reduced sediment condition and possibly the first cold-seep ecosystem to have been found within the continental shelf limits of the UK. In places the seafloor was obscured due to dense flocculent matter in the water column, but again it is not clear at this stage if this is bacterially derived flocculants from the seabed or is pelagic derived phytodetritus. Bacterial mats and dense particulate matter in the water column have been previously reported from cold seeps from the Barents Sea (Grunke et al., Reference Grunke, Lichtschlag, de Beer, Felden, Salman, Ramette, Schulz-Vogt and Boetius2012), the Gulf of Mexico (Sassen et al., Reference Sassen, Roberts, Aharon, Larkin, Chinn and Carney1993), the Californian slope (Levin, Reference Levin2003) and the New Zealand margin (Baco et al., Reference Baco, Rowden, Levin, Smith and Bowden2009). The near-bottom water throughout the area was clearly not toxic to fish as numerous species were observed swimming directly above the seabed.
Assuming that most of the seabed below 1190 m water depth is covered in bacterial mats this would equate to an area of at least 10 km2, similar in scale to cold seeps found on the slope of the Gulf of Mexico (Sibuet & Olu, Reference Sibuet and Olu1998). While there was no evidence for gas bubbling or ‘shimmering water’ from the seabed, as is often reported from cold seeps and mud volcanoes (Skarke et al., Reference Skarke, Ruppel, Kodis, Brothers and Lobecker2014), sediment-rich fluid plumes were observed that might suggest an active geological process. Water currents, fish motion and pressure waves from the towed camera could have caused sediment disturbance, but the plumes were sporadic and very localized, occurring both in absence and presence of fish (see Supplementary video). The fluid plumes were seen in association with unusual elongate three-dimensional features on the seabed. Directed sampling of the sediments, mounds and plumes is needed to confirm their origin and nature.
The video footage did not reveal any obvious aggregations of bivalves in the bacterial mats as has been reported from other cold seep sites (Barry et al., Reference Barry, Greene, Orange, Baxter, Robison, Kochevar, Nybakken and McHugh1996; Sibuet & Olu, Reference Sibuet and Olu1998; Baco et al., Reference Baco, Rowden, Levin, Smith and Bowden2009), but this may simply reflect the small size and burrowing habits of the Vesicomyidae and Thyasiridae clams described by Oliver & Drewery (Reference Oliver and Drewery2014). Overall there was a scarcity of benthic epifauna associated with the bacterial mats, with the exception of small, dark red anemones that appeared sporadically. These remain to be identified and it is not known if they belong to the several genera of cerianthid anemones that are known to associate with reduced deep-sea habitats (Rodríguez & Daly, Reference Rodríguez and Daly2010). Dense fields of what appeared to be small (2–3 cm in height) tube-dwelling polychaete worms were observed in areas adjacent to the bacterial mats. They clearly were not large enough and did not have the characteristic bright red tentacles to suggest they were species of the family Siboglinidae (that are well known from cold seeps) and it is not clear whether they are chemosynthetic or not. It is interesting however that they appeared in a transition zone between the bacterial mats and the sponges and bioturbated sediment habitat observed at shallower depths.
In the area beyond the reduced sediments, on the shallower flanks of the depression, numerous sessile and more typical deep-sea species were observed. These included hexactinellid sponges Hyalonema sp. (stalked sponge) and Pheronema carpenteri, the ophiuroid Ophiocten gracilis and possibly Xenophyophores. This habitat occurred primarily on the shallower western slope of the study area and was characterized by bioturbation of the sediment and the presence of the squat lobster Munida sp. and the holothurian, Benthogone sp. These species and similar biotopes have been reported from numerous sites nearby in the Rockall Trough and Hatton–Rockall Basin (Hughes & Gage, Reference Hughes and Gage2004; Narayanaswamy et al., Reference Narayanaswamy, Hughes, Howell, Davies and Jacobs2013; McIntyre et al., Reference McIntyre, Drewery, Eerks-Medrano and Neat2016). The coral garden habitat occurred on the steep eastern slope of the study area and again contrasted sharply with the seabed in the depression. Various gorgonians, the anemone Phelliactis sp. and soft corals (alcyonaceans) were seen in this area. Coral gardens are frequently associated with escarpment features with steep gradients, boulders and exposed bedrock (Davies et al., Reference Davies, Stewart, Narayanaswamy, Jacobs, Spicer, Golding and Howell2015).
The habitats observed at the Scotia Seep contrasted starkly from the surrounding deep seafloor and slopes. A concentric pattern of habitats was apparent with a central area located in the deepest part of the depression covered with dense bacterial mats, elongate raised features and sediment-rich fluid plumes. Away from the bacterial mats there appears to be transition into sediments with tube-dwelling worms, and in turn to more typical deep-sea bioturbated sediments and communities. Concentric patterns of chemosynthetic habitat radiating out from active seep areas have been previously described (Olu et al., Reference Olu, Lance, Sibuet, Henry, Fiala-Médioni and Dinet1997; Levin, Reference Levin2003, Reference Levin2005; Levin et al., Reference Levin, Baco, Bowden, Colaco, Cordes, Cunha, Demopoulos, Gobin, Grupe, Le, Metaxas, Netburn, Rouse, Thurber, Tunnicliffe, Van Dover, Vanreusel and Watling2016).
Chemosynthetic ecosystems are currently understood to be rare in the NE Atlantic with only the three sites previously mentioned confirmed to date. Given that these are thousands of kilometres away from the current study site, it is perhaps not surprising that species from the Scotia Seep have turned out to be new to science (Oliver & Drewery, Reference Oliver and Drewery2014). Interestingly, west of the Scotia Seep there is a network of kilometre-scale polygonal faults that extend across the Hatton–Rockall Basin (Berndt et al., Reference Berndt, Jacobs, Evans, Gay, Elliott, Long and Hitchen2012). It is possible the Scotia Seep is connected in some way to this geological fault system. The observation of flocculent matter above the seabed suggests it could have a significant effect on the overlying water column. Methane plumes from cold seeps in the Gulf of Mexico, at depths similar to this study are thought to support planktonic microbial communities from the seabed into the surface water (Rakowski et al., Reference Rakowski, Magen, Bosman, Gillies, Rogers and Chanton2015).
In conclusion while the habitats described are clearly atypical of the seabed sediments in the north Atlantic for these depths, it is not clear whether it is a geological driven cold seep ecosystem or a biologically driven reduced environment. It might even arise from both if geological conditions (emissions of hydrogen sulphide rich fluids) give rise to toxic seafloor conditions for detritivores, the absence of which allows phytodetritus to build up. Geochemical analyses of sediment samples and genetic bar-coding of the microbial and invertebrate communities are currently underway and should better define the biogeochemical nature of the Scotia Seep.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418000115
ACKNOWLEDGEMENTS
The MRV ‘Scotia’ 0915S expedition was awarded to the Marine Alliance for Science and Technology Scotland (MASTS) Deep-Sea Forum through an open call for ship time by Marine Scotland Science (Scottish Government). We are grateful for help from the other scientists aboard survey 0915S and the officers and crew of MRV ‘Scotia’ for their professional assistance at sea. H.A.S. publishes with permission of the Executive Director of the British Geological Survey (Natural Environment Research Council).
FINANCIAL SUPPORT
The MASTS Deep-Sea Forum received support from Scottish Funding Council (grant reference HR09011).