Introduction
Biodiversity encompasses the diversity and variability of organisms along with the ecological complexity of habitats (Webb et al. Reference Webb, Ackerly, McPeek and Donoghue2002). It serves as the foundation for human survival and development and plays a pivotal role in maintaining ecosystem stability. However, in recent years, the rapid pace of industrialization and burgeoning human economic demands, climate change, deforestation, environmental pollution, and invasive species have precipitated a swift decline in biodiversity (Zhai et al. Reference Zhai, Cannon, Slik, Zhang and Dai2012; Zou et al. Reference Zou, Wang, Zuo, Zhang and He2023). Plant diversity, a significant component of biological diversity, includes an estimated 300,000 to 350,000 plant species worldwide, driving global material cycling and energy flow processes. The species diversity within a plant community reflects the composition, structure, and function of each component, illustrating the community’s appearance, structural characteristics, and dynamic changes (Hu et al. Reference Hu, Wang, Jiang, Yang, Yang, Li and Yang2023). Currently, the study of plant diversity has emerged as a focal point in forest ecology research, with increasing attention from scientific researchers toward understanding how environmental factors influence plant composition and species diversity (Weigel et al. Reference Weigel, Gilles, Klisz, Manthey and Kreyling2019; Zhang et al. Reference Zhang, Yang, Wang, Dan, Yin, Li, Liu and Qi2023).
The primary goal of forest restoration is to enhance ecosystem services and other ecological functions, making natural forest restoration the best choice. However, the approach to restoring artificial forests must be tailored to local conditions, considering factors such as the extent of land degradation, hydrothermal conditions, and proximity to the native habitats of animals and plants. Methods may include natural regeneration, planting of a diverse array of native trees, shrubs, and grasses, as well as artificial interventions to facilitate natural regeneration etc. Hua et al. (Reference Hua, Bruijnzeel, Meli, Martin, Zhang, Nakagawa, Miao, Wang, Mcevoy, Pena-Arancibia, Brancalion, Smith, Edwards and Balmford2022). Despite this, considerable expertise has been gained in the establishment and management of artificial forests, which may continue to play a significant role in current forest restoration practices, contributing to the swift achievement of global ecosystem restoration goals. The high-productivity timber supply of artificial forests can reduce or even avoid deforestation of natural forests by humans, thereby protecting forests with richer ecological functions and improving both ecological efficiency and benefits (Wang et al. Reference Wang, Li, Yang, Ceng, Xia, Wang, Qi and Hao2022).
Natural rubber (Hevea brasiliensis) is an important industrial raw material. Rubber plantations are primarily found in China, yet the unscientific and unreasonable cultivation of rubber poses threats to biodiversity and animal habitats (Ahrends et al. Reference Ahrends, Hollingsworth, Ziegler, Fox, Chen, Su and Xu2015; Leepromrath et al. Reference Leepromrath, Zhu, Zhou, Zhou, Li and Zhou2021). Current research on the plant diversity of rubber plantations is predominantly focused on exploring the development and utilization of understory resources. Long-term cultivation of rubber trees changed the functional diversity of soil microbial communities (Zhou et al. Reference Zhou, Wang, Li, Friedman and Wang2017). Hainan Province has been identified as a suitable area for planting rubber trees. The first rubber seedlings were imported from Malaysia in the early 20th century and were subsequently planted on a large scale. Since 1952, rubber trees have been widely planted in various soil types across Hainan Island. As of 2020, the rubber planting area in Hainan Province reached approximately 530,000 hectares (Li et al. Reference Li, Dai, Zhu, Zheng, Yu and Hu2024).
Several scholars have undertaken relevant research on plant diversity within rubber plantations. For instance, Wang et al. (Reference Wang, Mishra and Yang2023) found that increased plant diversity in rubber plantations significantly enhanced soil biodiversity and ecosystem stability. Conversely, Chen et al. (Reference Chen, Ma, Yang, Xiao, Kou, Wu, Yun, Zaw, Nawan and Sengprakhon2021) discovered that the rapid and irrational expansion of rubber plantations led to a notable decline in the number of species. Furthermore, Liu et al. (Liu et al. Reference Liu, Liang, Tang, Jin, Guo and Siddique2023) demonstrated that integrative cultivation systems within rubber plantations significantly inhibit the growth of understory vegetation and lead to a sharp loss of understory vegetation diversity. In Hainan, studies on rubber plantation plant diversity have primarily focused on the influence of environmental factors or management practices. For example, Zeng et al. (Reference Zeng, Wang, Yang, Li, Wang and Xia2023) identified temperature and precipitation as comprehensive influencers on rubber plantation plant diversity, while Wang et al. (Reference Wang, Li, Yang, Ceng, Xia, Wang, Qi and Hao2022) emphasized the significant impact of management methods on understory plant diversity in rubber plantations. Herbaceous layer plants constitute a vital component of understory vegetation, and their diversity plays a critical role in the natural succession of forests. Rubber plantations, being artificial ecosystems, exhibit prolonged succession cycles and diminished diversity due to extensive human intervention (Hu et al. Reference Hu, Wang, Jiang, Yang, Yang, Li and Yang2023). Research on the plant diversity characteristics of the understory herb layer in rubber plantations reveals insights into the evolving traits of natural succession processes.
Since the establishment of National Park of Hainan Tropical Rainforest in 2021, a substantial portion of operational rubber plantations has been abandoned rubber plantation, including those within the Yinggeling Branch of National Park of Hainan Tropical Rainforest. The evolving traits of understory vegetation in these abandoned rubber plantations remain largely unexplored, leaving the evolutionary patterns of rubber plantations post-abandonment unknown. Hence, this study aims to investigate the plant diversity and community composition of the understory herbaceous layer during the natural succession of rubber plantations to shed light on this issue.
Materials and methods
Study area
The Yinggeling Branch of Hainan Tropical Rainforest National Park is located in the mountainous region of central and southern Hainan Island, spanning 18°49’30″—19°08’41″N and 109°11’27″—109°34’06″E and covers a total area of 861.7 km2. The area includes a controlled zone of 40,900 hectares and a core protected area of 45,200 hectares, encompassing Baisha County, Qiongzhong County, Wuzhishan City, Changjiang County, and Ledong County (Zhu et al. Reference Zhu, Qi, Yang, Li and Su2023). The region experiences a tropical maritime monsoon climate within ts mountainous terrain, with Yinggeling Peak reaching an altitude of 1812 m (Zhang et al. Reference Zhang, Yang, Xia, Wang, Wang, Ceng, Qi, Li, Chen, Tian, Li, Li and Liang2022). The average annual temperature in the Yinggeling Nature Reserve ranges from 20 to 24°C, with an average annual precipitation of 1800 mm and an annual evaporation rate of 1600 mm (Yang et al. Reference Yang, Huang, Chen, Wei and Chen2023).
Based on our preliminary survey, the total area of the Yinggeling Branch within Hainan Tropical Rainforest National Park is 88,177 hectares, with artificial forests covering 18,399 hectares, representing 20.9% of the total area. Within the general control area, artificial forests span 17,027 hectares, comprising 41.4% of the total general control area. This includes 5,874 hectares of rubber plantations, accounting for 34.5% of the plantation forests within the general control areas.
Methods
This study’s investigation area is situated at the peak management and protection station within the Yinggeling Branch of National Park of Hainan Tropical Rainforest. Sample plots were selected for field surveys along a gradient of different successional times (0, 3, and 7 years). Understory vegetation was surveyed using a sampling method. Three sample plots, each measuring 100 m× 100 m, were chosen from rubber plantations undergoing natural succession for 0, 3, and 7 years, with three 20 m × 20 m quadrats surveyed within each plot. Herbaceous layer plants beneath the rubber plantation canopy were surveyed within the quadrats, recording species name, average height, coverage, and abundance. Additionally, information regarding sample plots’ altitude, slope, aspect, soil type, and canopy coverage was recorded (Table 1).
Table 1. Information on rubber plantation plots in National Park of Hainan Tropical Rainforest, China

Note: “ZY-1,” “ZY-2,” and “ZY-3,” respectively, represent the 1st, 2nd, and 3rd plots in succession year 0; “TY-1,” “TY-2,” and “TY-3” represent succession 3, respectively. The 1st, 2nd, and 3rd sample plots in the year; “SY-1,” “SY-2,” and “SY-3,” respectively represent the 1st, 2nd, and 3rd sample plots in the 3rd year of succession.
Data analysis
Using a sampling method survey, diversity information regarding herbaceous layer plants beneath rubber plantations with varying succession periods was collected. This included investigating the types and number of individuals within the herbaceous layer (including woody plants, herbs, and ferns with a height ≤ 1 m). Statistical analyses of individual plants, including height and coverage, were conducted, and key values were calculated using relevant methods. Richness indices such as the Margalef index (dM), Shannon–Wiener index (H), Simpson index (P), Pielou index (E), and beta diversity index were computed based on the survey data.
-
(1) Important value = (relative frequency + relative density + relative significance)/3
-
(2) Margalef index:
 ${d_M} = {{{S - 1}}\over{{\ln N}}}$
${d_M} = {{{S - 1}}\over{{\ln N}}}$
-
(3) α diversity:
Shannon–wiener index calculation formula:
 ${H' = - \mathop \sum \limits_{i = 1}^S Pi\ln Pi}$
${H' = - \mathop \sum \limits_{i = 1}^S Pi\ln Pi}$
The Simpson index calculation formula:
 ${P=1 - \mathop \sum \nolimits_{i= 1}^S P{i^2}}$
${P=1 - \mathop \sum \nolimits_{i= 1}^S P{i^2}}$
The calculation formula of Pielou index:
 $$E = {{ - \sum\nolimits_{i = 1}^S P i\ln Pi} \over {\ln S}}$$
$$E = {{ - \sum\nolimits_{i = 1}^S P i\ln Pi} \over {\ln S}}$$
In the above four formulas, the number of species in the S-quadrat; Pi represents the relative density of the ith species; N is the sum of the numbers of all species in the quadrat.
-
(4) β diversity
Community β diversity was calculated using Sørensen community similarity
The formula for calculating the Sørensen index:
 $Cs=2c/\left( {a + b} \right)$
$Cs=2c/\left( {a + b} \right)$
where a and b are the number of species in each of the two quadrats, and c is the number of species common to the two quadrats.
All raw data recording, summary, statistics, and calculations are processed using Excel 2021. Use Origin 2021 software to conduct variance analysis on herbaceous plants under rubber forests with different succession years.
Results
Community composition of understory herbaceous layer
Plant composition of the herbaceous layer under the rubber plantation
This field survey documented a total of 175 plant species in the herbaceous layer of the rubber plantation, representing 149 genera and 75 families. Notably, species Gramineae and Rubiaceae families collectively comprised 46.45% of the total species diversity.
Among the surveyed species, three varieties from three families and three genera were identified. Notably, the understory herbaceous vegetation consisted of ferns and angiosperms, with no gymnosperms observed. There were 17 fern species, constituting 9.71% of the total, spanning across 10 families and 11 genera. Angiosperms dominated with 158 species, representing 90.29% of the total, distributed across 65 families and 138 genera. Additionally, our survey recorded 9 species of vines, accounting for 5.14% of the total species richness, spread across 6 families and 9 genera.
Analysis of the herbaceous layer of the rubber plantation revealed that the highest occurrence of tree seedlings, shrub seedlings, and herbaceous plants in the seventh year of succession. Furthermore, with increasing succession time, there was a notable increase in the proportion of tree seedling species within the community. While shrub seedling species initially decreased and then increased, peaking at 7 years of succession, the trend in herbaceous plants remained less evident (Figure 1).

Figure 1. Change trends of various life forms in plant communities in the understory herbaceous layer of rubber plantation at different succession times.
Species composition of the understory herb layer of rubber plantation
The survey results reveal that the herbaceous layer of the rubber plantation harbors 73 plant species in the initial stages of natural succession (0 years), encompassing 39 families and 67 genera. After 3 years of succession, the number of species increases to 115, maintaining the same family and genus count. At 7 years of succession, although the number of genera rises to 96 within 56 families, the overall species count remains at 101, compared to the 3-year. Despite a decline in species count from 2015 onward, it remains higher than the initial succession period (0 years) (Figure 2). Analysis reveals a trend of initial increase followed by a decrease in the number of plant species, families, and genera over the succession period. The 3-year mark demonstrates this trend prominently. The herbaceous layer exhibits the highest diversity in terms of plant families, genera, and species, while the initial 0-year stage showcases the lowest diversity. These findings highlight the significant influence of succession duration on the herbaceous layer’s plant diversity (Figure 2).
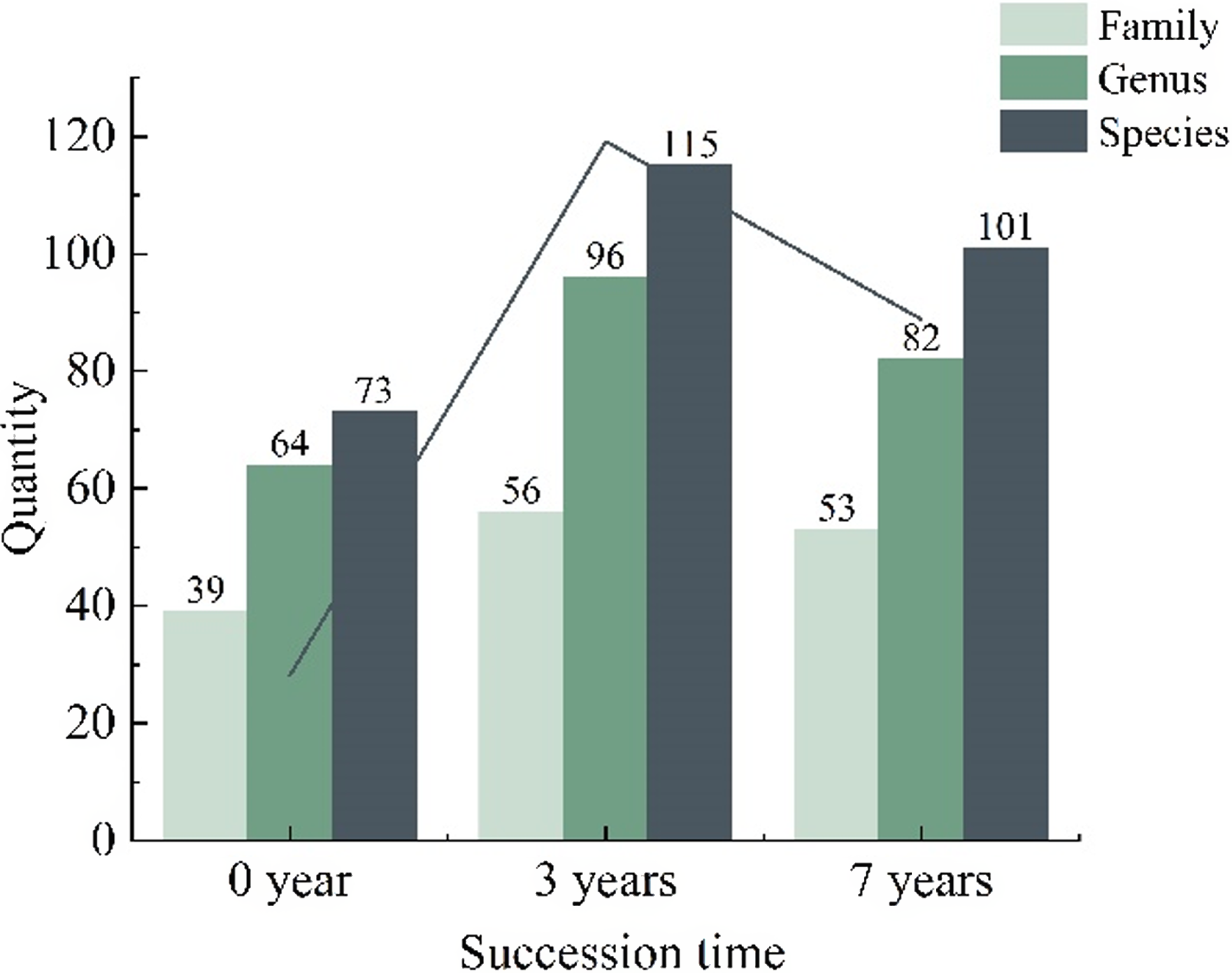
Figure 2. Composition of plant families, genera, and species in the understory herbaceous layer of rubber plantation at different succession times.
Predominant plant families and genera within the understory herbaceous layer of rubber plantations
The survey findings indicate a relatively limited number of species in the understory herb layer of rubber plantations undergoing 0, 3, and 7 years of natural succession, with the top six dominant families and genera outlined for each phase. In rubber plantations with 0 years of natural succession, the top five dominant plant families include Rubiaceae, Fabaceae, Gramineae, Moraceae, and Asparagaceae, comprising a total of 22 genera, representing 34.38% of the total genera, and 26 species, accounting for 35.62% of the total species. The predominant plant genera in the understory herbaceous layer of rubber plantations at this stage are Ficus, Melastoma, Alpinia, Pteris, Hedyotis, and Lasianthus, totaling 15 species, constituting 20.55% of the total species count (Table 2).
Table 2. Dominant families in the herbaceous layer of rubber forest understory at 0, 3, and 7 years of succession
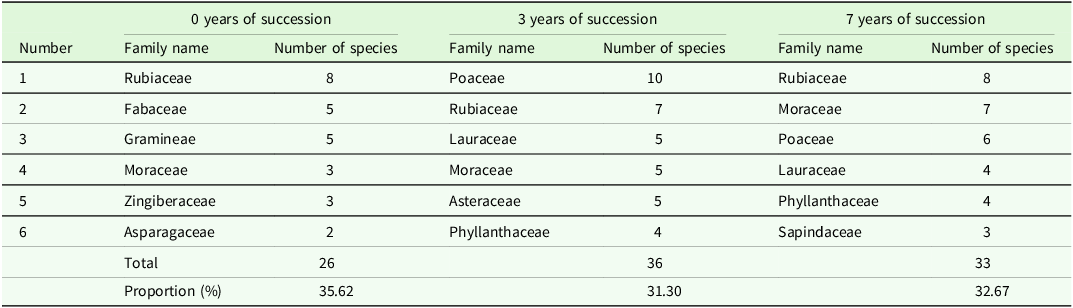
Table 2 illustrates the top six dominant plant families in the understory herbaceous layer of rubber plantations undergoing 3 years of natural succession: Poaceae, Rubiaceae, Lauraceae, Moraceae, Asteraceae, and Phyllanthaceae. These families encompass a total of 28 genera, representing 29.17% of the total genera, and 36 species, accounting for 31.30% of the total species count. Among the dominant genera are Ficus, Pteris, Cinnamomum, Mallotus, Melastoma, and Rubus, totaling 16 species and constituting 13.91% of the total species.
The top six dominant families in the understory herbaceous layer of rubber plantations undergoing 7 years of natural succession are Rubiaceae, Moraceae, Poaceae, Lauraceae, Phyllanthaceae, and Sapindaceae. Together, they encompass 24 genera, representing 29.27% of the total genera, and 33 species, accounting for 32.67% of the total species count. Among the top six dominant genera are Ficus, Lasianthus, Syzygium, Rubus, Hedyotis, and Pteris, totaling 9 species within the genus, constituting 18.81% of the total species (Table 3).
Table 3. Dominant genera in the herbaceous understory of rubber forests in years 0, 3, and 7 of succession

The dominant families in the understory herbaceous layer of rubber plantations undergoing natural succession for 0, 3, and 7 years primarily consist of Rubiaceae, Gramineae, Zingiberaceae, and Moraceae. Lauraceae and Phyllophyllaceae emerge as dominant families in forests with 3 and 7 years of succession and persist across rubber plantations for 20 years. Among the dominant genera in the understory plants of rubber plantations undergoing 0, 3, and 7 years of natural succession, Ficus and Pterospermum stand out, with Ficus being the predominant genus across all stages of succession. Notably, Melastoma is the leading genus among the understory herbaceous plants in rubber plantations with 0 and 3 years of natural succession and in forests with 3 and 7 years of succession. Meanwhile, Rubus prevails as the dominant genus in the understory herbaceous layer.
Species importance analysis
Table 4 presents statistics on the top ten species with significant importance values within the understory herbaceous layer community of rubber plantations at different stages of succession. Tetrastigma pachyphyllum emerges as the dominant species in rubber plantations undergoing natural succession for 0 years, the importance value is 6.95%. In forests with 3 years of succession, Eargrass stands out with an importance value of 5.05%. Microstegium fasciculatum takes precedence as the top species in the herbaceous layer of rubber plantations with 7 years of natural succession, exhibiting an importance value of 8.96%. Among the top ten species in rubber plantations with natural succession durations of 0, 3, and 7 years, Zingiberaceae and Gramineae plants are prominent. Notably, the Poaceae plant Microstegium fasciculatum ranked second in importance in the 0th year of succession, and by the 7th year of succession, it had become the best species of the herbaceous layer plant in the understory of the rubber forest.
Table 4. The top 10 species with the highest importance values in each plot

Note: “Pi” represents the species important value.
Species diversity analysis of the herbaceous layer community under the rubber plantations
The survey results indicate no significant difference in the Shannon–Wiener index, Simpson index, and Pielou index across the understory herbaceous community of rubber plantations with 0, 3, and 7 years of natural succession. However, there is a significant difference in the Margalef index between the 3-year and 7-year continuous periods. Interestingly, no significant distinction is noted in the Margalef index between the understory herbaceous layer of rubber plantations undergoing 3 and 7 years of succession (Figure 3). The Shannon–Wiener index, Simpson index, and Pielou index peak at 7 years of natural succession, while the Margalef index reaches its highest point at 3 years of natural succession.

Figure 3. Species diversity in the understory herb layer of rubber forests at different succession stages.
Similarity analysis of the herbaceous layer community under the rubber plantations
Table 5 illustrates the similarity of plant communities within the understory herbaceous layer of rubber plantations at varying stages of succession in the Yingge Ling Branch of Hainan Tropical Rainforest National Park. The highest similarity coefficient of 0.56 is observed between the understory herbaceous plants of rubber plantations with 0 and 3 years of natural succession, while the lowest similarity coefficient of 0.46 is noted between the plant communities of rubber plantations with 0 and 7 years of natural succession. Conversely, the similarity coefficient between rubber plantations with 3 and 7 years of natural succession is 0.50.
Table 5. Similarity of plant communities in the herbaceous layer of the rubber plantation understory under different succession times

Discussion
Changes in the number of species in the understory herb layer of rubber plantations at different succession times
A total of 175 plant species were recorded in the herbaceous layer of the rubber plantation during this study, spanning 149 genera and 75 families, with Gramineae and Rubiaceae accounting for 46.45% of the total. The number of plant species in the understory herbaceous layer exhibited an initial increase followed by a decrease. The peak was observed at 115 species during the 3rd year of succession, while the lowest count was 73 species during the initial year of succession. Additionally, as succession years increased, the proportion of tree seedling species within the community showed a rising trend. In contrast, shrub layer species demonstrated an initial decline followed by an increase, reaching their maximum at 7 years of succession. This trend is likely due to the cessation of artificial management of rubber plantations, which allowed the understory plant communities to recover naturally. Without human interference, the vegetation, especially herbaceous plants, gradually returned to their natural growth state. By the third year of natural recovery, the herbaceous plant community under the rubber canopy reached its dominant period. During this natural recovery, other shrubs, vines, and tree plant communities also recovered. After three years, the growth of these naturally recovered communities, such as tree seedlings and shrub seedlings, began to compete with the herbaceous community for light, water, nutrients, and space, even forming a strong comparative advantage.
Understory herbaceous plants in rubber plantations are notably sensitive to environmental shifts. The proliferation of shrubs and trees, leading to increased canopy density, impedes the growth of understory herbs, aligning with the findings of Xiao Jun et al. (Reference Xiao, Lei, Li, Ma, Yu and Xiao2023). Moreover, following natural management in rubber plantation succession, there was an increase in the number of understory plant species and tree and shrub seedlings, consistent with the research conducted by Lan Guoyu et al. (Reference Lan, Wu and Xie2014). This surge is attributed to the relatively barren and unsuitable soil surface during the initial 0 years of natural succession. As natural succession progresses, more species find suitable conditions for survival, corroborating the findings of Wei Jianxing et al. (Reference Wei, Chen, Zheng and Tian2023).
Diversity characteristics of the herbaceous layer community within the rubber plantation
Species richness serves as a vital indicator of community dynamics. The Shannon–Wiener index primarily measures the uncertainty of individual occurrence, with higher values indicating greater diversity. The Simpson dominance index suggests a more uneven distribution of different organism types within the biological community, highlighting the prominence of dominant organisms and their ecological functions. The Pielou evenness index reflects the distribution of individual numbers across all species within the rubber plantation. No significant differences were observed in the Shannon–Wiener index, Simpson index, and Pielou index of the herbaceous layer plant community under the rubber plantation at the three succession times. However, the Margalef index of the understory herbaceous layer of the rubber plantation with 0 years of succession was significantly lower than that of the other two succession times. The Shannon–Wiener index, Simpson index, and Pielou index reached their peaks at 7 years of natural succession in the herbaceous layer community under the rubber plantation, while the Margalef index peaked at 3 years. The reason why the Margalef index model is different from other indices is largely because the Margalef index is more sensitive to changes in species richness than other indices and has a stronger ability to distinguish changes in community diversity (Su et al. Reference Su, Zhang, Jia and Xue2017). Overall, the diversity index of the understory herbaceous layer community in rubber plantations with 0 years of succession was the lowest, whereas it was higher in forests with 7 years of succession. These findings are consistent with those of Chen Li et al. (Reference Chen, Huang, Lan, Tan, Yang and Wu2019), supporting the belief that plant diversity in rubber plantations after near-natural management aligns with this conclusion. The natural restoration of rubber plantations helps to increase the diversity of the understory herbaceous community.
Comparison of the herbaceous layer community within the rubber plantation across different stages of succession
The natural succession of rubber plantations over different years results in varying species counts and community similarities. The plant communities of rubber plantations that have undergone natural succession for 0 and 3 years exhibit the highest similarity, suggesting a considerable total species count. This may be attributed to the relatively short duration of natural succession, maintaining the dominance of herbaceous plants in the understory vegetation community. Conversely, the plant communities of rubber plantations with 7 years and 0 years of natural succession exhibit the lowest similarity, indicating fewer shared species. After 7 years of natural succession, native trees and shrubs have emerged in the understory vegetation community, while unsuitable herbaceous plants have retreated, resulting in changes in community composition. The similarity of plant communities in rubber plantations undergoing natural succession for 3 years and 7 years falls between the two extremes. Although the 7-year succession has initiated the transformation of rubber plantations into tropical rainforests, it is still in its early stages. Nonetheless, with reduced human interference and alterations in the understory microenvironment, the plant community in the herbaceous layer is undergoing changes driven by species competition. In ecology, plant diversity is considered a natural solution for forest restoration, carbon capture, and climate change. Increased plant diversity can also increase carbon sequestration by increasing plant productivity, plant biomass, defoliation, root biomass, and microbial biomass (Duan et al. Reference Duan, Fu, Nottingham, Domeignoz-Horta, Yang, Du, Wang and Li2023). This study utilizes spatial analysis to examine diversity differences among plants in the herbaceous layer of rubber plantations across different succession years. While this method is straightforward and practical, it lacks the depth of long-term temporal dimension. Future research endeavors should focus on continuous monitoring of plant diversity changes in the herbaceous layer of rubber plantations over extended period to provide a comprehensive understanding of successional dynamics.
Conclusions
There were 175 species of understory plants in 149 genera and 75 families in the Yinggeling area of Hainan Tropical Rainforest National Park, of which 46.45% of the total species were Poaceae and Rubiaceae. In the rubber understory with natural succession of 0, 3, and 7 years, the dominant families were Rubiaceae, Poaceae, and Moraceae, among which Ficus and Pterocarpus were the dominant genera. The plant species composition and importance values of the understory herbaceous layer in rubber plantations undergoing 0, 3, and 7 years of succession varied with the duration of succession. The highest species count was observed in the 3rd year of succession, whereas the diversity indices—Shannon–Wiener, Simpson, and Pielou—peaked at 7 years of natural succession, with significant differences noted in the Margalef index. In general, natural restoration has increased the species diversity of the understory herbaceous layer of the rubber forest. However, the changes in the community characteristics of the natural restored rubber forest require further analysis of factors such as woody plants, climate environment, and soil.
Data Availability
Data will be made available on request.
Acknowledgments
We are grateful to the financial support for this work from Horizontal Natural Science Project (RH2200005384). The authors thank the Editor and Reviewers for their valuable comments and constructive suggestions.
Author Contribution declaration
Chunyan Du: Field investigation survey and writing—original draft preparation; Donghai Li, Dongling Qi, and Xiaobo Yang: conceptualization, methodology, and reviewing and editing; Weifeng Wang: resource; Zicheng Zhu, Rentong Liu, Shaocui He, Xin Su, and Naiyan Shang: field investigation; and Chuan Yang and Qingmao Fu: reviewing.
Funding
The authors acknowledge the financial support for this work from Horizontal Natural Science Project (RH2200005384).
Declaration of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.











