INTRODUCTION
Cardiac emergencies in pregnancy and the postpartum period are rare but often life-threatening. An emergency physician’s differential diagnosis for chest pain in the peripartum patient often includes serious etiologies such as pulmonary embolism or acute myocardial infarction (MI). A lesser-known but important consideration on the differential for MI is that of a spontaneous coronary artery dissection (SCAD). In pregnant women, SCAD is defined as pregnancy-associated spontaneous coronary artery dissection (PASCAD). This condition is the most common cause of pregnancy-associated MI and is reported as the cause of MI in 24% to 35% of all women younger than 50 years.Reference Tweet, Gulati and Hayes 1 , Reference Saw 2 These patients may present to emergency rooms, yet this diagnosis receives limited attention among the emergency medicine community.Reference Ghaffarian, Furin and Bassett 3 Although PASCAD can be seen at any trimester in pregnancy and up to months postpartum, the majority of cases have been reported in the third trimester or early postpartum period defined as within 6 weeks of delivery.Reference Saw 2 , Reference Rose, Gedela, Miller and Carpenter 4 Abnormal electrocardiogram (ECG) changes, elevated troponins, and regional wall motional abnormalities on echocardiography are all diagnostic findings of PASCAD, which can be ultimately confirmed with coronary angiography.Reference Tweet, Gulati and Hayes 1 , Reference Aliyary, Mariani and Verhorst 5 Failure to immediately address this condition can lead to acute heart failure, cardiogenic shock, and death.Reference Goland, Schwarz, Siegel and Czer 6
CASE REPORT
We describe a 33-year-old female who presented with shortness of breath and crushing central chest pain in the first 36 hours postpartum after her fifth uncomplicated spontaneous vaginal delivery of a healthy baby. She complained of nausea and shortness of breath and described 15 minutes of 10/10 crushing central chest pain without radiation.
On exam, she was afebrile with a heart rate of 81, a blood pressure of 171/108, and was saturating 100% on room air. On auscultation, there was good air entry bilaterally and normal heart sounds with no murmurs. No pain or edema was found in her lower extremities. Her ECG is shown in Figure 1. Her chest pain and shortness of breath improved after approximately 15 minutes with only mild retrosternal discomfort lasting a further 2 hours.
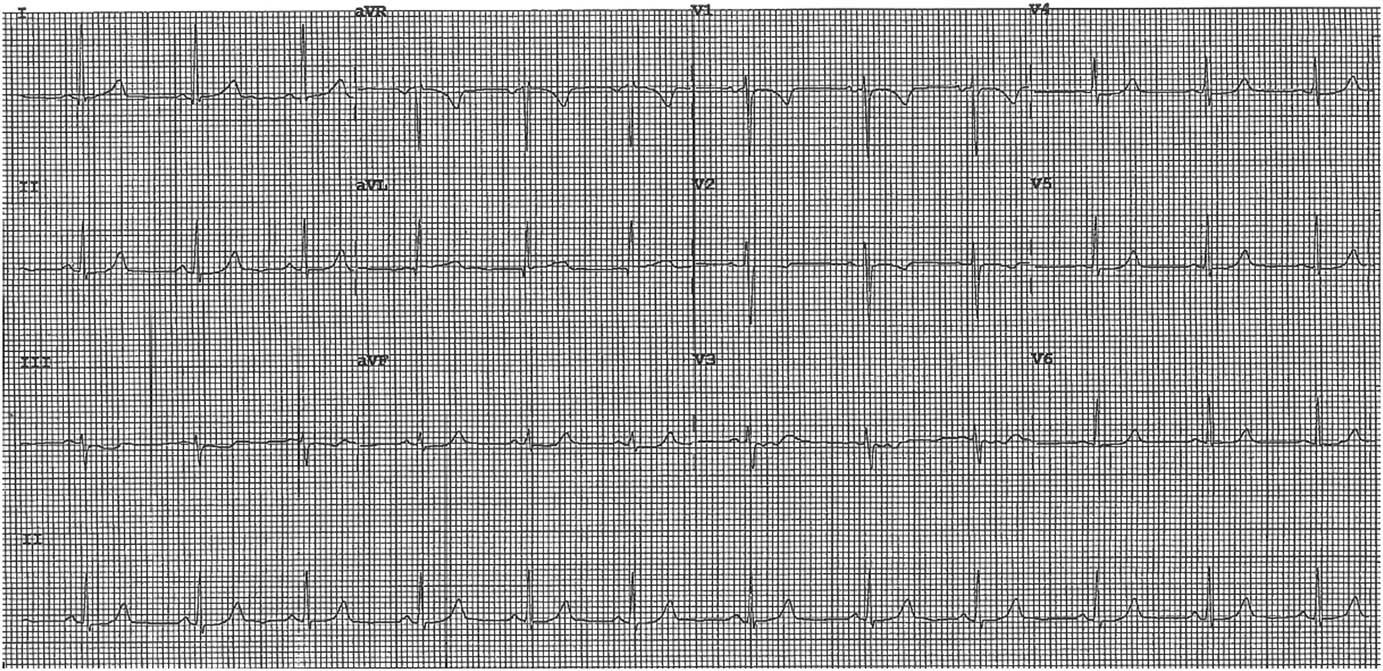
Figure 1 ECG on Presentation.
Laboratory results showed a hemoglobin of 87 and an initial troponin of 20 ng/L (reference range <14 ng/L normal). Her repeat troponin 7 hours later was elevated at 1080 ng/L, and she was treated with acetylsalicylic acid (ASA) 160 mg, clopidogrel 300 mg, and referred to a cardiologist.
Subsequent to her referral, she had a recurrent episode of chest pain lasting 30 minutes. Her ECG at this time revealed dynamic 0.5-mm ST segment depressions in leads V2-V3 with T-wave inversion in leads V2 (Figure 2). She was subsequently transferred to the Coronary Care Unit.
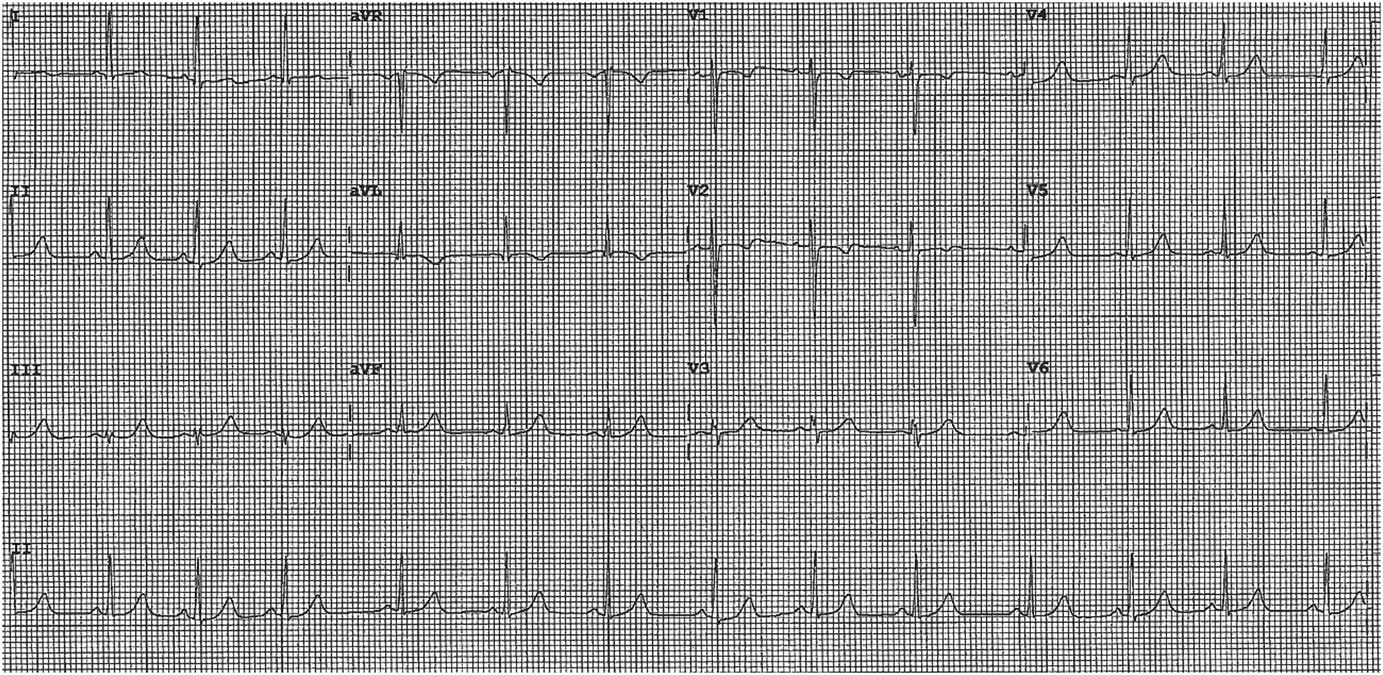
Figure 2 ECG Subsequent to Referral.
She received intermittent labetalol doses for blood pressure fluctuations and underwent cardiac catheterization. A coronary angiography revealed a decreased left anterior descending (LAD) flow consistent with a coronary artery aneurysm secondary to SCAD. She had a saccular aneurysm in the proximal LAD, 50% occlusion in the mid LAD, and 20% in the distal LAD. This was managed medically with blood pressure control (labetalol 200 mg bid) and dual antiplatelet therapy (ASA 81 mg, clopidogrel 75 mg) for 12 months. A bedside echocardiogram prior to discharge showed distal wall motion abnormalities in the distal inferoseptal area. The ejection fraction and valvular function were grossly normal, and there was no pericardial effusion present. She had no further episodes of chest pain and was asymptomatic at the time of discharge.
DISCUSSION
SCAD is considered a rare condition accounting for approximately 0.1% to 4% of all acute coronary syndrome (ACS) pathologies in the general population.Reference Saw 7 Its wide clinical presentations range from being asymptomatic to the more typical angina to sudden cardiac death. SCAD is defined as an intramural hematoma within the coronary artery that compresses the true lumen and must be unrelated to trauma or medical instrumentation such as coronary catheterization. Expansion of this false lumen by increased pressures may lead to subsequent myocardial ischemia and infarction.
It is well known amongst clinicians that women often present with atypical features of ACS. Although this condition is a relatively infrequent cause of ACS in the general population, SCAD proportionally causes a higher number of MIs in young women.Reference Saw, Aymong and Mancini 8 , Reference Marcoff, Popescu and Leidig 9 Furthermore, acute MI is a significant cause of mortality and morbidity in peripartum women.Reference Rose, Gedela, Miller and Carpenter 4
Remarkably, SCAD has been described as accounting for 24% to 35% of MI in women under the age of 50 and is suspected to have an even higher prevalence in women with pregnancy-related MI.Reference Saw 2 In pregnancy-related cases of MI, SCAD has been described as the most common mechanism and the underlying cause in 40% or more of cases.Reference Havakuk, Goland, Mehra and Elkayam 10 It has been identified as more common than atherosclerosis, thromboembolism, vasospasm, or Takotsubo syndrome in this population. Additionally, PASCAD is reported to have a higher acuity and worse prognosis than non-pregnancy-related SCAD cases.Reference Saw 2 Forty percent of PASCAD cases present as sudden death or die within hours of the onset of symptoms.Reference Marcoff, Popescu and Leidig 9 However, those who survive this acute phase and receive proper diagnosis and treatment generally have a good prognosis.Reference Marcoff, Popescu and Leidig 9
This condition is predominately seen in young healthy females with no traditional risk factors for coronary artery disease and typically in the postpartum period.Reference Tweet, Gulati and Hayes 1 , Reference Teruzzi, Calligaris and Ravagnani 11 , Reference Pabla, John and Mccrea 12 Women account for approximately 82% to 90% of SCAD cases.Reference Saw, Aymong and Mancini 8 , Reference Tweet, Hayes and Pitta 13 SCAD has also been associated with fibromuscular dysplasia, connective tissue disease, and episodes of extreme emotion or exertion.Reference Tweet, Gulati and Hayes 1 Multiparity appears to be a risk factor, as does a previous history of SCAD.Reference Marcoff, Popescu and Leidig 9 It is often seen at the end of pregnancy or early postpartum period where it is thought that the hormonal changes and hemodynamic stresses of pregnancy weaken the coronary artery wall and contribute towards its etiology.Reference Goland, Schwarz, Siegel and Czer 6
The wide clinical spectrum of PASCAD ranges from unstable angina to sudden cardiac death, but the primary presentation is often seen as non-ST elevation and ST-elevation MI.Reference Tweet, Gulati and Hayes 1 Classical signs and symptoms follow an ACS pattern and include chest pain, dyspnea, diaphoresis, and/or nausea. Initial diagnostic testing includes ECG and cardiac biomarkers. However, because these patients are often young and otherwise healthy, the diagnosis of PASCAD may be delayed or overlooked if repeat or serial testing is not considered. Therefore, clinicians must be aware of a potentially evolving ACS in this population and order serial ECGs and troponin levels to avoid missing or delaying a diagnosis of PASCAD.Reference Tweet, Gulati and Hayes 1 Abnormal ECG changes, elevated troponins, and regional wall motional abnormalities on echocardiography are all diagnostic findings of PASCAD, which can be ultimately confirmed with coronary angiography.Reference Tweet, Gulati and Hayes 1 , Reference Aliyary, Mariani and Verhorst 5
MANAGEMENT
Currently, no evidence-based management guidelines exist for SCAD or PASCAD due to its limited literature and lack of randomized control trials.Reference Rose, Gedela, Miller and Carpenter 4 Early intervention for these patients is imperative to allow for optimal outcomes. Blood pressure and heart rate control to limit vessel shearing is a critical part of the initial management.Reference Rose, Gedela, Miller and Carpenter 4 It appears unclear whether standard ACS pharmaceutical agents, such as ASA or clopidogrel, are indicated; yet patients often receive these initially because they may offer some protection and minimal harm. However, more potent agents, such as glycoprotein IIb/IIIa inhibitors, are not routinely used because they carry a higher bleeding risk and potential for extending dissection.Reference Rose, Gedela, Miller and Carpenter 4 , Reference Yip and Saw 14 Additionally, the role of new P2Y12 antagonists, such as prasugrel and ticagrelor, is also unclear.Reference Yip and Saw 14
The risks and benefits of anticoagulation in PASCAD are controversial. The risk of coronary artery dissection extension is met with potential resolution of a false lumen thrombus improving patency of the true lumen. Heparin agents started empirically have been discontinued once coronary artery dissection is declared on angiography to avoid the worsening of intramural hematoma.Reference Rose, Gedela, Miller and Carpenter 4 , Reference Yip and Saw 14 Moreover, thrombolytic treatment in PASCAD can be harmful and is not recommended because it may expand the dissection and worsen coronary vasospasm.Reference Rose, Gedela, Miller and Carpenter 4 , Reference Pabla, John and Mccrea 12 As a result, when PASCAD is highly suspected, urgent coronary angiography is crucial because PASCAD medical management differs from standard ACS therapy.Reference Yip and Saw 14 However, in remote centres without routine access to primary percutaneous coronary intervention (PCI), thrombolysis may need to be considered for ST-elevation MI patients.Reference Yip and Saw 14 Yet, because PASCAD has been described as the most common mechanism of pregnancy-associated MI and documented in over 40% of cases, thrombolytic therapy should only be considered after the risks and benefits have been assessed and when little other option exists in an unstable patient (Figure 3). In an analysis of 120 PASCAD cases by Havakuk et al., thrombolytic therapy was used in 10 cases and followed by either PCI (3 cases) or coronary artery bypass grafting (CABG) (5 cases).Reference Havakuk, Goland, Mehra and Elkayam 10
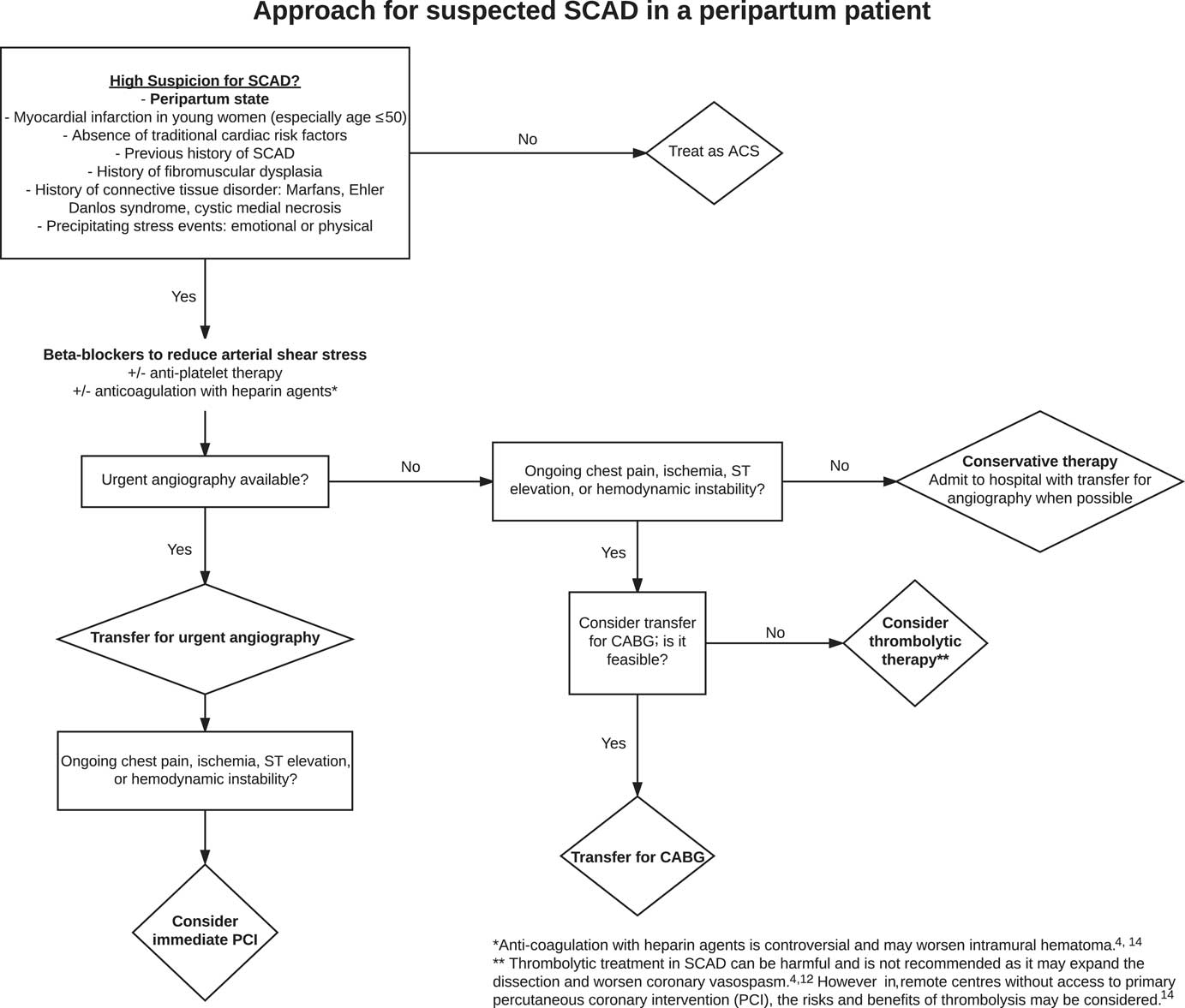
Figure 3 An Approach to PASCAD.
When ACS is recognized in a peripartum patient, urgent coronary artery catheterization should be performed to confirm a diagnosis of PASCAD and determine appropriate therapeutic strategies for management.Reference Tweet, Gulati and Hayes 1 , Reference Rose, Gedela, Miller and Carpenter 4 , Reference Aliyary, Mariani and Verhorst 5 Urgent angiography is often required for both the diagnosis of PASCAD and to rule out acute plaque occlusion. Emergency physicians need to have an early suspicion for potential PASCAD in female patients presenting with ACS symptoms and arrange for urgent coronary artery catheterization or a prompt transfer to a centre where this capability exists.
Treatment strategies are decided based on the extent and character of dissection detected on angiography and can include medical therapy, stenting for revascularization, or CABG. Hemodynamically stable patients with a single-vessel disease without progression, without a left main coronary artery involvement or danger to a large area of myocardium may be medically managed with close observation.Reference Marcoff, Popescu and Leidig 9 In contrast to atherosclerotic disease, PCI is associated with elevated rates of complications, including the risk of further dissection or subsequent emergency CABG.Reference Tweet, Gulati and Hayes 1 Complications with PCI can occur even in cases where there has been normal or near-normal coronary artery blood flow.Reference Tweet, Gulati and Hayes 1 , Reference Rose, Gedela, Miller and Carpenter 4 Thus, PCI or coronary stenting is not typically performed.
CONCLUSION
Chest pain or an ACS equivalent in a young peripartum woman without traditional cardiac risk factors should prompt an emergency physician to consider PASCAD.Reference Ghaffarian, Furin and Bassett 3 , Reference Pabla, John and Mccrea 12 This diagnosis is often overlooked and needs to be added to the list of serious etiologies for chest pain in a young patient such as pulmonary embolism, MI, or aortic dissection. SCAD is an important cause of ACS in women, accounting for 24% to 35% of MI in women under the age of 50.Reference Saw 2 , Reference Saw, Aymong and Mancini 8 Furthermore, PASCAD has been described as the most common mechanism of pregnancy-associated MI and documented in over 40% of cases.Reference Havakuk, Goland, Mehra and Elkayam 10 Rather than predominately thinking about pulmonary embolism in this population, one needs to add PASCAD to the differential diagnosis.
Immediate blood pressure and heart rate control are necessary to limit vessel sheering until urgent coronary angiography can be performed to confirm the diagnosis. Prompt arrangements for urgent coronary catheterization need to be made, because it is crucial to the diagnosis and to help avoid potentially harmful treatments. When compared with atherosclerotic disease, unique concerns exist in PASCAD. Thrombolytic treatment may be harmful and is not recommended, and PCI can result in the iatrogenic propagation of further coronary dissection.Reference Rose, Gedela, Miller and Carpenter 4 , Reference Movahedian, Leon, Sibai and Moussa 15
TAKE HOME POINTS
“Take Home” clinical messages for emergency physicians include the following:
1) SCAD as a cause of MI should be on the differential diagnosis of serious etiologies for chest pain in a young patient (i.e., pulmonary embolism/ACS/aortic dissection/SCAD).
2) Chest pain in a young peripartum female without traditional cardiac risk factors should prompt an emergency physician to think beyond pulmonary embolism and consider MI, which includes PASCAD in the differential.
3) Management of suspected PASCAD: Because anticoagulation with heparin is controversial with the risk of worsening intramural hematoma, thrombolytics also potentially harmful and currently not recommended, the emergency management of suspected PASCAD needs to place emphasis on prompt recognition and an urgent transfer to a primary angiography centre for coronary artery catheterization. This allows an individual risk benefit decision on the best treatment strategy by vascular or cardiac surgeons and cardiologists.Reference Rose, Gedela, Miller and Carpenter 4 , Reference Movahedian, Leon, Sibai and Moussa 15
Competing interests: None declared.





