Introduction
The last decade has seen a significant reduction in measles morbidity and mortality, in line with the World Health Organization's (WHO) goal of measles elimination. However, measles still accounts for a significant disease burden in children worldwide [1–Reference Muller3], and recently, measles outbreaks have been reported in many developed countries [Reference Wichmann4–Reference Wichmann9].
During 2006–2007 more than 12 000 measles cases, mostly in children, were reported in Europe, indicating further need for improvement [Reference Muscat10, Reference Kremer and Muller11]. In 2001–2008, 557 cases and 38 outbreaks were reported in the USA, with the peak incidence rates in infants and toddlers [Reference Parker Fiebelkorn6]. Commonly, individuals who contract measles (often related to international travel) transmit it to susceptible children residing in communities in which under-immunization prevails [Reference Meissner, Strebel and Orenstein12, Reference Obaro and Palmer13].
In Israel, the two-dose measles-mumps-rubella (MMR) vaccine schedule, together with high overall national immunization coverage, has resulted in a considerable decline in measles incidence [Reference Slater, Anis and Leventhal14–16]. However, in 2003 and 2004 two measles outbreaks emerged in Jerusalem, each of about 100 cases, mostly in young unimmunized children in ultra-orthodox communities [Reference Stein-Zamir17]. A further, larger measles outbreak emerged in 2007 in Jerusalem among ultra-orthodox communities, following importation of the disease. Between August 2007 and June 2008, 992 measles cases were reported in the Jerusalem district, accounting for two-thirds of the national incidence [Reference Stewart-Freedman and Kovalsky18–Reference Anis20].
The preponderance of measles cases in very young children led us to focus on the <5 years age group. We studied the age-specific incidence trends of measles, comparing the previous outbreaks (2003–2004) to the current one (2007–2008) and performed a case-control study in a search for risk markers in young children who contracted measles during the outbreak.
METHODS
Measles outbreak investigation
Measles is a notifiable disease in Israel by law; physicians and laboratories must notify the local District Health Office of all measles cases.
Case definition
Cases were defined on the basis of clinical presentation, laboratory findings and epidemiological characteristics [21].
A clinical case was defined as having generalized rash for ⩾3 days, temperature ⩾38·3°C and cough, coryza or conjunctivitis. A confirmed case was a clinical case with either laboratory confirmation (positive measles IgM antibody) or an epidemiological link to another case (two epidemiologically linked cases were considered confirmed.) A clinical case without laboratory confirmation or an epidemiological link to another case was classified as probable. A case of a febrile illness with exanthem was defined as suspected.
Laboratory investigation
Laboratory confirmation was defined as a positive measles IgM antibody test. Serological tests are performed at the laboratories of the health maintenance organizations. Virus was isolated from urine; RT–PCR was used for virus detection in urine and throat swabs and genotyping were performed at the Ministry of Health's central national virology laboratory in accordance with WHO standardized protocols [Reference Stein-Zamir17].
Laboratory tests were performed mainly at the beginning of the outbreak to ascertain the diagnosis of measles. Since the populations involved are socially and geographically distinct and definable, the epidemiological links between cases were very readily discernible, so that laboratory confirmation of the diagnosis of measles was not required in most cases. In the setting of a community-wide outbreak other causes of viral exanthems (e.g. rubella) were not routinely sought.
Epidemiological investigation
The epidemiological investigations were based on data collected from interviews (based on a case investigation questionnaire) with measles cases, their parents and the treating physician. Data were also collected from hospital files and from the health records within the well-baby clinic computerized database. The variables collected included the demographic characteristics, clinical and laboratory features, date of disease onset, hospitalization, cases in the household, cases' immunization status (for measles containing vaccine and other routine childhood vaccinations) and registration in a well-baby clinic. Well-baby clinics in Israel are designated as providers of preventive health services, hence, routine childhood immunizations are provided exclusively at the local public well-baby clinics in the district. All children are entitled to these services free of charge.
Population
The Jerusalem district's population numbered 879 700 in 2007 [22]. Children aged <1 year, 5 years and <15 years comprised 2·7%, 12·8% and 35·1%, respectively, of the district's population [23]. The measles cases in the Jerusalem district were stratified into age groups and specific incidence rates were calculated for the 2003–2004 outbreaks and the 2007–2008 outbreak.
Outbreak control
Outbreak control policy included administration of the MMR vaccine to those aged between 6 and 12 months within 3 days of their exposure to a case, and to susceptibles aged ⩾1 year, at any time following exposure. Children aged <6 months were given immunoglobulin (Ig) within 6 days of exposure to a case. Ig was also given to children aged 6–11 months within 4–6 days of exposure to a case. In addition, individuals with contraindications to MMR vaccine (immunocompromised vaccinees and pregnant women) received Ig.
Since most of the cases were children with mild disease, with very few becoming ill following post-exposure immunization, our data did not allow an assessment of the effect of post-exposure vaccination on the clinical course of the disease.
The case-control study
The study was performed in a town where the first measles cases were reported. The town (Beth Shemesh, BS), near Jerusalem, has a population of 77 000 (2008 census). A case was defined as a child aged <5 years who contracted measles between August 2007 and June 2008. Two controls per case were selected from the Jerusalem district newborn registry. (All births nationally are notified by law to the Ministry of the Interior, this information is then recorded in the newborn registry of the Ministry of Health.) Controls were matched by child's age (born within 14 days of the birth date of the case) and home address (children living in the same street at the time of the outbreak). This matching approach was chosen since each of the town's neighbourhoods tends to be homogeneous in terms of religious observance and socioeconomic status.
Every child in the study (cases and controls) was individually checked against both the newborn registry and the well-baby clinic computerized databases. Each of the controls was checked against the notifications on cases to ensure that they were, indeed, not infected with measles throughout the study period. Furthermore, during the period of the outbreak all children in the control group who attended the well-baby clinic for any routine visit were questioned specifically by the clinic nurses as to whether they had displayed clinical features of measles. In fact, none of the control children had shown clinical signs of the disease.
The variables, obtained from the Jerusalem district newborn registry, included age, gender, birth weight, child's birth order in the family (firstborn, second, third, etc.), maternal age and whether Israeli-born, and number of siblings.
The information obtained from the well-baby clinics' computerized database included registration in a well-baby clinic, age at registration, whether examined by a paediatrician, age at examination, growth evaluation (including last recorded percentile) and detailed immunization history.
The immunization schedule (in 2007) for the first year included three doses of hepatitis B virus (HBV) vaccine at ages 0, 1, and 6 months; four doses of diphtheria-tetanus-acellular pertussis-polio-Haemo-philus influenzae B vaccine (DTaP-IPV-Hib) at ages 2, 4, 6 and 12 months and MMR at 12 months (second dose at age 6 years). We evaluated the coverage for the third dose of HBV, the third and fourth doses of DTaP-IPV-Hib and the first dose of MMR. Data on the immunization with the first dose of MMR vaccine of siblings of cases and controls were also collected from the well-baby clinics' databases.
The study was conducted in accordance with the relevant Ministry of Health regulations.
Statistical analysis
Data analysis was performed with SPSS® and WINPEPI® software [24, Reference Abramson25]. Continuous variables were compared by Student's t test; categorical variables were compared by χ2 test. Proportions were compared by odds ratio (OR), rates by rate ratio (RR); with 95% confidence intervals (CIs). A multiple logistic regression model was utilized for comparison of cases and controls. Cumulative curves were plotted for cases and controls proportions of registration in a well-baby clinic and selected immunization coverage. A P value ⩽0·05 was considered significant for all comparisons.
RESULTS
The first three measles cases, originating from London, were diagnosed in August 2007. An outbreak in the Jerusalem district ensued, lasting several months and subsiding in June 2008 following an immunization campaign. Altogether, 992 cases were reported in the district (65% of the cases nationally), whereas the district's population is 12·2% of the country's population. The outbreak control measures included mass post-exposure vaccination of susceptibles. From August 2007 to June 2008 some 19 500 doses of MMR vaccine over and above the number given in a comparable ‘non-outbreak period’ (August 2006 to June 2007, about 28 000 vaccine doses) were administered in the Jerusalem district – an increase of about 70% in MMR vaccine administration.
Most patients were children from ultra-orthodox communities in the Jerusalem district; 720 (72·6%) were aged <15 years, 426 (42·9%) were aged <5 years and 127 (12·8%) were infants aged <1 year. Children aged <5 years had a higher age-specific incidence compared to persons aged ⩾5 years (374·2/100 000 vs. 72·9/100 000). The peak incidence rate shifted from children aged 1–4 years in the 2003–2004 outbreaks to infants aged <1 year in the 2007–2008 outbreak (Fig. 1). The incidence rate increased tenfold in infants (533·8 vs. 50·1/100 000, RR 10·7, 95% CI 5·8–19·7, P=0·0001), and fourfold in those aged >1 year. The highest incidence was observed in infants aged 6–12 months (916·2/100 000), which was significantly higher than in children above or below that age group; in the 0–6 months age group the incidence was 151·3/100 000 and in the 1–4 years age group it was 332/100 000. Infants aged 9–12 months were most prominently affected (1704·8/100 000).
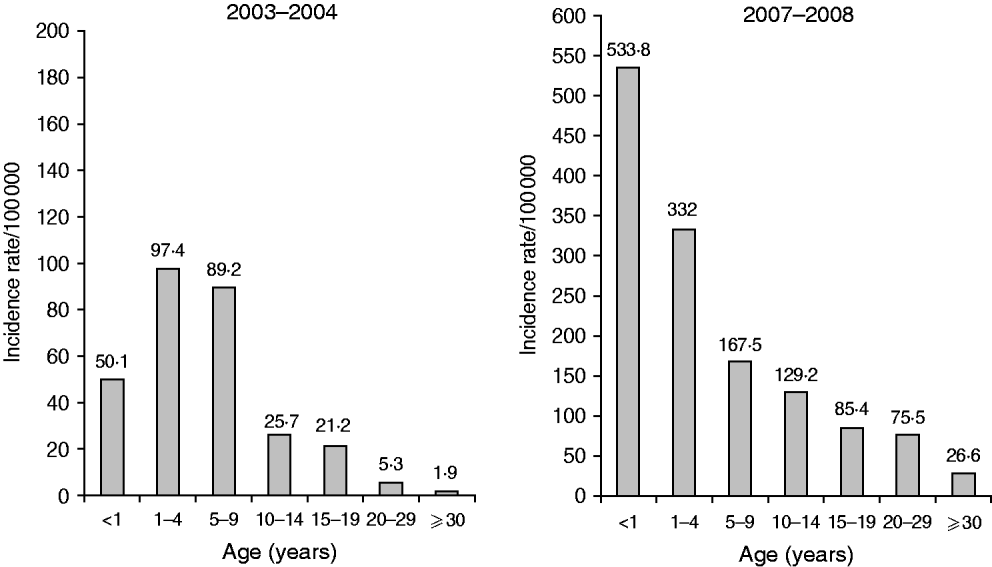
Fig. 1. Age group-specific incidence rates of measles per 100 000 population, Jerusalem district 2003–2004 and 2007–2008.
Children aged <5 years comprised 41·1% of the cases in 2003–2004 similar to 42·9% in 2007–2008. The distribution in the age subgroups (0–6 months, 6–12 months, 1–4 years) changed, being 4·3%, 7·7% and 88% in 2003–2004 vs. 4·2%, 25·6% and 70·2%, in 2007–2008, respectively. The proportion aged 6–12 months increased from 7·7% to 25·6% (OR 4·2, 95% CI 1·9–11, P=0·0001).
The case characteristics in the 2007–2008 and 2003–2004 outbreaks are presented in Table 1. The proportion of males was higher in 2007–2008. The proportion of hospitalizations was higher in 2007–2008 and varied with age, being 17·6% in children aged <5 years, 5·1% in children aged 5–14 years and 27·6% in persons aged >15 years. No difference was found regarding median age, laboratory confirmation (14·3% in 2003–4, 15·8% 2007–8) and vaccination status.
Table 1. General characteristics of measles cases in the Jerusalem measles outbreaks in 2003–2004, 2007–2008
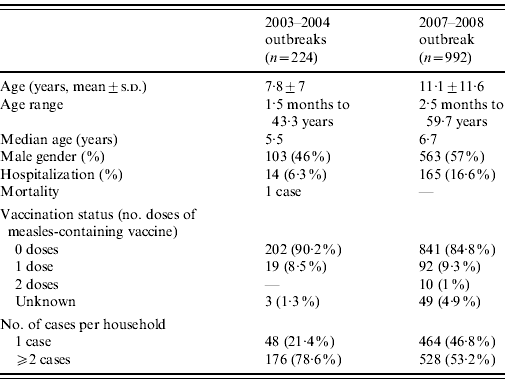
In the 2003–2004 outbreaks a single measles case occurred in 21·4% of affected households, compared to 46·8% of households in 2007–2008. Of the 127 infants aged <1 year, 90 (70·9%) were the only case in the household; only 20 (22·2%) were firstborn children. Households with infants aged <1 year were more likely to have a single case compared to households without infants (OR 3·7 95% CI 2·6–5·3, P=0·0001).
The case-control study
The first measles case in BS was a 3½-year-old toddler, reported on 8 August 2007, after exposure to an imported case. He attended an ultra-orthodox junior school; within 2 weeks all nine unimmunized classmates and the unimmunized teacher contracted measles. None of the other 26 previously immunized classmates contracted measles.
The measles cases aged <5 years reported in BS (n=74), were compared to 148 age- and neighbourhood-matched controls (Table 2). Notably, 32·4% were aged <1 year and 27% were infants aged 6–12 months. No differences were found with regard to the number of children in the household, maternal age and country of birth, child's birth weight (3359±476 g vs. 3341±434 g, cases and controls) and child's last recorded weight (9·3% and 13·1% below the 10th weight percentile in cases and controls, respectively).
Table 2. General characteristics of measles cases and controls
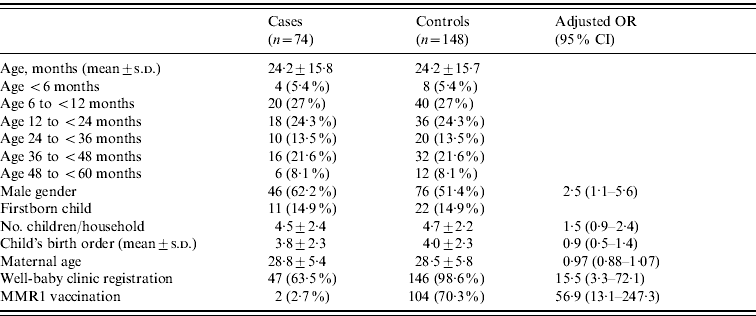
OR, Odds ratio; CI, confidence interval.
We employed a multiple logistic regression model for comparison between cases and controls. The variables included in the model were: child's age group, gender, number of children in the household, child's birth order, maternal age, well-baby clinic registration and MMR1 vaccination. Of these variables, three were found to be significant and were included in the final model (Table 2): MMR1 vaccination (higher odds in unvaccinated), registration in a well-baby clinic (increased odds in non-registered) and gender (higher odds in males). These three variables accounted for 62% of the variability between the cases and the controls.
Compliance with routine well-baby clinic healthcare (Fig. 2)
Cases were less likely to be registered in a well-baby clinic (63·5% vs. 98·6% in controls, P=0·0001, Fig. 2 a). If the child was registered, it was at an older age (6·32±10·4 months vs. 2·6±3·6 in controls, P=0·0001). Cases were less likely to have been examined by a paediatrician (40·5% vs. 77%, P=0·0001).
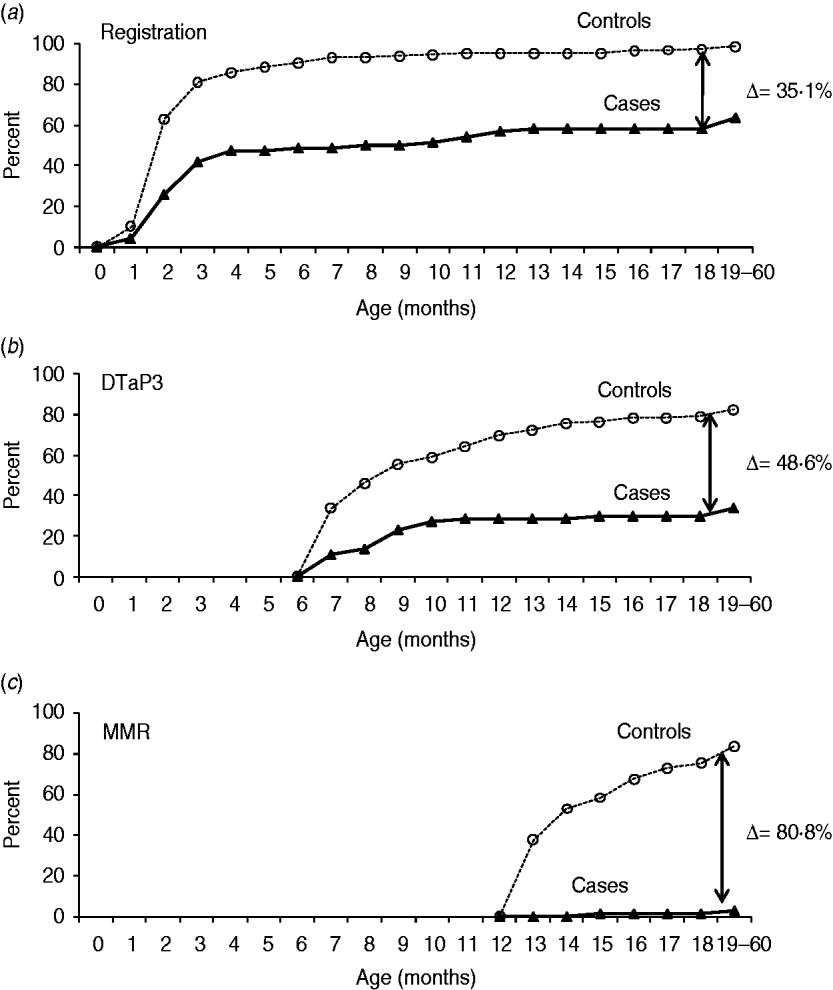
Fig. 2. Cumulative curves showing (a) well-baby clinic registration and (b) immunization coverage (percent) of selected vaccines: DTaP-IPV-Hib 3 scheduled for 6 months and (c) MMR1 scheduled at 12 months for cases and controls, by age.
Data on immunization coverage of selected vaccines (the third dose of DTaP and the first dose of MMR) are presented in Figure 2(b, c). The differences between cases and controls for well-baby clinic registration, DTaP3 and MMR1 coverage were 35·1%, 48·6% and 80·8% respectively (all P<0·001). The immunization coverage in cases was lower than for controls for all vaccines. For the third doses of HBV and DTaP-IPV-Hib it was 37·8% and 33·8%, respectively, compared to 81·1% and 82·4% in controls. At age ⩾12 months a mere 10·8% of cases had received the fourth dose of DTaP-IPV-Hib and only two (3·1%) children had received the first dose of MMR, compared to 59·2% and 83·8%, respectively, of controls. The average age at immunization was older for cases than for controls. Documented refusal to receive MMR vaccine was recorded in the well-baby clinic medical files of seven cases and in none of the controls. Since all routine immunizations are given in the well-baby clinics, all immunization-related data are obtained from those databases only.
Effect of birth order and clinic registration (Fig. 3)
For measles cases the child's rising birth order was inversely associated with registration in a well-baby clinic (χ2=7·17, P=0·007). The proportion of measles cases that were registered in a well-baby clinic declined from 81·8% for a firstborn child to 43·5% for those who were fifth-born and above, compared to 100% and 96·2%, respectively, in controls.
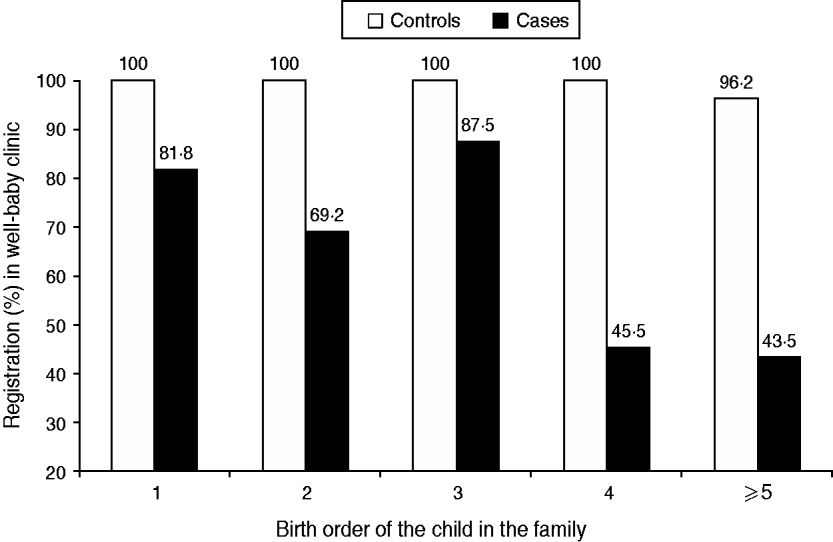
Fig. 3. Well-baby clinic registration (percent) of cases and controls, according to the child's birth order in the family.
Siblings of cases and controls (Fig. 4)
The total number of siblings was 740, of whom 238 were siblings of measles cases and 502 were siblings of controls. Only 47·9% (114/238) of cases' siblings had received the first dose of MMR vs. 94·4% (474/502) of controls' siblings (OR 18·4, 95% CI 11·4–29·9, P=0·0001).
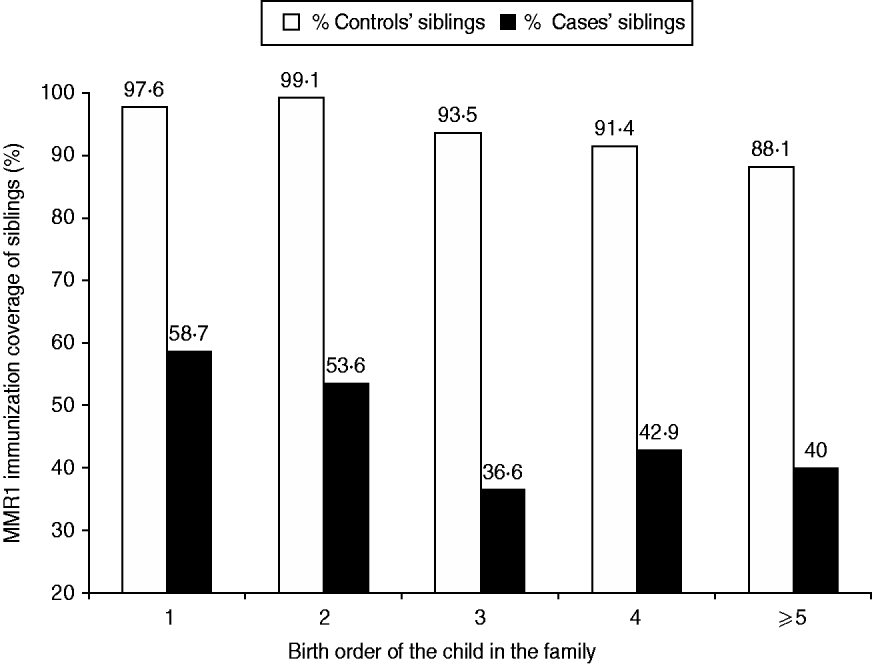
Fig. 4. MMR1 immunization coverage (percent) in the siblings of cases and siblings of controls, according to the child's birth order in the family.
For siblings of cases, the higher the child's birth order, the less likely was he/she to have received MMR (χ2=5·23, P=0·02). The proportion immunized with MMR declined from 58·7% in firstborn siblings to 40% in siblings who were fifth-born and above, compared to 97·6% and 88·1%, respectively, in siblings of controls.
DISCUSSION
Measles is a highly contagious disease, and prevention of infection and transmission necessitates sustainable herd immunity. The global progress towards measles elimination in the last decade has been significant and interruption of transmission has been documented in many countries [Reference de Quadros26]. However, as long as unimmunized specific population subgroups still remain, repeated penetration and spread of measles outbreaks are anticipated, especially affecting young unvaccinated children.
The 2007–2008 measles outbreak followed disease importation and was the largest in Israel in the past decade [Reference Anis20]. It emerged in the Jerusalem district among ultra-orthodox communities and remained largely confined to that population, possibly attesting to adequate herd immunity in the general population.
We reported two smaller measles outbreaks in 2003 and 2004 with 94% of nationally reported cases being in the Jerusalem area among the ultra-orthodox communities [Reference Stein-Zamir17, 27]. The ultra-orthodox communities tend to have large families, often in overcrowded living conditions under mediocre socioeconomic circumstances.
Increased incidence in infants
The most prominent feature of the 2007–2008 outbreak was the sharp increase in measles incidence in infants, especially those aged 6–12 months. While in the 2003 and 2004 outbreaks few infants were involved, in the 2007–2008 outbreak 12·1% of the patients were aged 6–12 months. The peak incidence was observed in infants aged 6–12 months (916·2/100 000) and specifically in those aged 9–12 months (1704·8/100 000, ~1·7%). That age group is below the current recommended age for the first dose of MMR vaccine (12 months), and hence protection against measles depends on the immunity derived from maternal passively transferred antibodies and on the external herd immunity in the community.
The US Centers for Disease Control and Prevention [Reference Parker Fiebelkorn6] reported a total of 557 confirmed cases and 38 outbreaks of measles during the post-elimination years 2001–2008. Ninety cases (16%) were infants aged <1 year. Of children in the 1–4 years age group, 45 (51%) were aged 12–15 months. The highest age-specific incidence was observed in infants aged 6–11 months.
Protection by maternal antibodies and timing of initial immunization dose
Maternal antibodies against measles are passively transferred to infants. The rising incidence rates of measles we found in infants aged 6–12 months may indicate waning of maternally derived protection. Decay of maternally derived measles antibodies in an Israeli population was reported by Dagan et al. [Reference Dagan28]. They concluded that infants aged >6 months may be inadequately protected, even in a well-immunized population. A prospective study from Belgium [Reference Leuridan29] in 2006–2008 demonstrated early waning of maternal antibodies, so that at age 6 months more than 99% of infants of immunized women and 95% of infants of naturally immune women had lost maternal antibodies, resulting in early susceptibility to measles. A study from France [Reference Gagneur30] showed that maternal measles-neutralizing antibodies decrease markedly, so that 90% of infants are unprotected against measles after age 6 months.
Infant protection against measles can be optimized both by increasing herd immunity through widening vaccine coverage and by lowering the age of routine immunization. Evidence of low maternally derived measles antibody levels in Europe led to calls for re-evaluation of immunization policies [Reference Johansen and Lopalco31] and consideration of scheduling an earlier age for the first dose of MMR vaccine. Recently, Metcalf et al. [Reference Metcalf32] studied the effect of epidemiological changes, demography and immunization coverage on the favourable age for the first dose of measles vaccine. High birth rates and low immunization coverage both favour early immunization at age 9–11 months. The presence of maternal antibodies, which can interfere with the infant's response to the vaccine, should also be taken into account when determining the timing of the first dose of measles immunization [Reference Leuridan and Van Damme33].
Risk markers
In the case-control study we evaluated possible early signs that may presage non-immunization against measles at age 12 months. The proportion of measles cases registered in a well-baby clinic was 63·5% vs. 98·6% for controls. The coverage for the third dose of HBV and DTaP/IPV/Hib in cases was 37·8% and 33·8%, respectively, compared to 81·1% and 82·4% in controls. A mere 10·8% of cases had received the fourth dose of DTaP/IPV/Hib and only two (3·1%) children had received the first dose of MMR, compared to 59·2% and 83·8%, respectively, in controls. Moreover, the average child's age at immunization was older for cases than for controls. Both cases and controls resided in ultra-orthodox neighbourhoods that were homogenous in terms of socioeconomic and religious features, with large households. However, utilization of preventive health services for children differed. While some two thirds of the cases were registered in a clinic, only one third complied with immunizations scheduled at 6 months, and at 1 year the compliance dropped to almost zero. The proportion of clinic registration in cases and measles vaccine coverage in their siblings, declined with rising birth order. The decreasing compliance with the child's age and the birth order may attest to a ‘burn-out phenomenon’, implying that some parents with large families may find it burdensome to follow the recommended scheduled well-baby visits. The immunization database in the well-baby clinic ‘flags’ children who default on immunizations. In those cases the nursing staff will attempt to contact the family by phone, or pay a home visit, to determine the reason behind the non-immunization and endeavour to encourage attendance at the clinic for catch-up immunization. With the recent introduction of the internet-based National Immunization Registry in Israel [Reference Stein-Zamir34] the identification and recall of these children will become even more effective.
A study in Baltimore, USA [Reference Guyer35] in the 1990s showed a decrease in preventive health visits from 80% in infants aged 3 months to <40% at 7 months. Immunization coverage was 71% for DTP1, 39% for DTP3, and 53% for MMR vaccine. A study performed in Philadelphia, USA [Reference Feemster36] in search of risk factors for late initiation of immunizations found rising birth order (two and above) to be one of the risk factors. In a study from Germany [Reference Poethko-Müller37] measles immunization coverage was low in children having three or more siblings. A study from Korea [Reference Jeong38] showed that risk indicators for under-immunization included parental factors, such as unawareness of immunization and immunization schedules, and the birth order of the child in the family – younger siblings being at greater risk.
In an orthodox Jewish community in northeast London [Reference Henderson, Millett and Thorogood39] it was shown that the uptake of preventive health programmes was usually low in that community and information on vaccine dangers tended to spread via community rumours, rather than through the media. Other factors affecting immunization coverage include parental concerns about MMR vaccine specifically [Reference Smailbegovic, Laing and Bedford40]. Safety concerns, personal experience, and religious beliefs all play a role in attitudes towards immunization [Reference Kennedy and Gust41]. In our study we showed that those non-compliant with MMR immunization were also non-compliant with other immunizations, and the problem was not concern regarding MMR vaccine specifically.
Our study has several limitations. As in all similar situations of outbreaks of communicable diseases, there is presumably a certain degree of underreporting, legal requirements notwithstanding. This may have skewed our findings to some degree, although with the widespread media campaign that was instituted – both within the general and the medical communities, the number of unreported cases was probably quite low. Second, the data we accessed did not enable us to determine the mothers' immunization status – whether they had been immunized against measles, had suffered from the disease or had been exposed to it. The mother's immune status is a very important factor in affording the infant immunity in the first months of life. Another important area that requires further elucidation is the reason or reasons behind the non-registration of infants in the well-baby clinics. Although the measles cases and controls were very similar populations – age-matched, living in the same street (and hence of similar sociological, educational and religious backgrounds), with similar-sized families, mothers of similar ages, etc. – one group coped with the recommended schedule of well-baby clinic visits, whereas the other did so only partially, if at all. Furthermore, the child's birth order had a significant impact on compliance in measles cases and their siblings, but not in the very closely matched control group. Further studies, perhaps using qualitative investigational methods, may provide us with a better understanding of these significant differences.
In summary, a markedly high increase in measles incidence was observed in infants aged 6–12 months (mainly in those aged 9–12 months), who are below the recommended age for MMR1 immunization. This finding requires further evaluation with regard to optimal timing of the first measles vaccine dose. Who are the children at risk for measles? The risk markers for measles included non-registration in a well-baby clinic, default on previous immunizations (due before MMR1) and rising birth order. In high-risk groups, such as Jewish ultra-orthodox communities, non-immunization with MMR will almost inevitably lead to measles upon exposure. Encouraging appropriate utilization of well-baby services and prevention of dropouts can have a significant effect on immunization coverage and community health.
ACKNOWLEDGEMENTS
The authors thank the medical and nursing staff of Jerusalem district well-baby clinics, physicians in community clinics and paediatric hospital departments.
DECLARATION OF INTEREST
None.








