Introduction
Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998) proposed the new genus Loofilaimus, with L. phialistoma from Iran as its only species; they provided a detailed morphological description, including excellent line illustrations. When describing it, the authors were aware of the peculiar combination of very unusual traits that characterized their new taxon, discussed its intricate taxonomical position, and finally erected a new family, Loofilaimidae, accommodated under Tylencholaimoidea. Interestingly, no further information about Loofilaimus was available after its original description, not even any record. Thus, no advance in the study of this enigmatic genus has occurred over last 25 years.
A nematological survey conducted to explore the dorylaimid diversity of Iran yielded a new population of L. phialistoma, otherwise an easily recognizable species. The finding of fresh material offered the chance to undertake new studies, especially SEM observations and sequencing. Thus, this contribution aims to update its morphological characterization, to reassess previous information, and, more importantly, to explore its evolutionary relationships with an integrative approach, combining morphological and molecular data.
Material and Methods
Sampling and processing of nematodes
Soil samples were collected in natural habitats of East Azerbaijan province, northwestern Iran. Nematodes were extracted using the tray method (Whitehead & Hemning Reference Whitehead and Hemning1965), handpicked under a stereomicroscope, killed by adding hot FPG (4:1:1, formaldehyde: propionic acid: glycerine) solution, transferred to anhydrous glycerine according to De Grisse (Reference De Grisse1969), and mounted on permanent glass slides to allow handling and observation.
Morphological and morphometrical study
Nematodes were measured and photographed using an Eclipse 80i microscope (Nikon, Tokyo, Japan) equipped with differential interference contrast optics, a drawing tube (camera lucida), and a DS digital camera. Morphometrics include Demanian indices and other measurements and ratios, shown in Table 1, and the remaining form part of the literal description of the species.
Table 1. Morphometrics of Loofilaimus phialistoma Jairajpuri, Ahmad & Sturhan, Reference Jairajpuri, Ahmad and Sturhan1998.

Measurements in μm except L in mm, and in the form: mean ± s.d. (range)
1 After Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998).
2 Calculated from original illustrations and/or other morphometrics.
Scanning electron microscopy (SEM)
Specimens preserved in glycerine were selected for SEM observation according to Abolafia (Reference Abolafia2015). The nematodes were hydrated in distilled water, dehydrated in a graded ethanol-acetone series, critical point dried, coated with gold, and observed with a Zeiss Merlin microscope (5 kV) (Zeiss, Oberkochen, Germany).
DNA extraction, PCR and sequencing
Following morphological confirmation, DNA was extracted from two fresh specimens of L. phialistoma, as described by Subbotin et al. (Reference Subbotin, Waeyenberge and Moens2000). The D2–D3 expansion segments of 28S rDNA were amplified using the forward D2A (5′-ACAAGTACC GTGAGGGAAAGTTG-3) and reverse D3B (5’-TCG GAAGGAACCAGCTACTA-3) primers (De Ley et al. Reference De Ley, Felix, Frisse, Nadler, Sternberg and Thomas1999; Nunn Reference Nunn1992). Each polymerase chain reaction (PCR) mixture, with a final volume of 30 μL, contained 3 μL of DNA template, 15 μL 2x Master mix (Ampliqon, Denmark), 1 μL of each forward and reverse primers (10 pMol/μL), and 10 μL ddH2O. Reactions were carried out in a Thermal Cycler (Hybaid, Ashford, Middlesex, UK) with an initial denaturation step of 95 °C for 4 min followed by 33 denaturation cycles of 94 °C for 30 sec, annealing for 30 sec at 57 °C, extension at 72 °C for 90 sec, and a final extension at 72 °C for 10 min. The quality of DNA targets was checked by electrophoresis of 4 μL from each PCR product in 1% agarose gel containing ethidium bromide. The PCR products were visualized and photographed under UV light, and the length of each PCR product was measured by comparison with the Low DNA Mass Ladder (Invitrogen, Carlsbad, CA, USA). The PCR products were purified and sequenced directly for both strands using the same primers with an ABI 3730XL sequencer (Bioneer Corporation, Seoul, South Korea). The newly obtained sequences were submitted to GenBank database under accession numbers OQ831921 and OQ831922 as indicated on the 28S phylogenetic tree.
Phylogenetic analyses
For phylogenetic relationships, analysis was based on 28S rDNA fragments containing gene coding for 28S rRNA. The newly obtained sequences were manually edited using Chromas 2.6.6 (Technelysium, Queensland, Australia) and aligned with another 28S rDNA sequences available in GenBank using the ClustalW alignment tool in MEGA7 (Kumar et al. Reference Kumar, Stecher and Tamura2016). Poorly aligned regions at the extremes were removed from the alignments using MEGA7. The best-fit model of nucleotide substitution used for the phylogenetic analysis was statistically selected using jModelTest 2.1.10 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012). The phylogenetic tree was generated with the Bayesian inference method using MrBayes 3.2.6 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Mononchus truncatus Bastian, 1865 (AY593064) was chosen as the outgroup. Analysis under the General Time Reversible plus Invariant sites plus Gamma distribution (GTR+I+G) model was initiated with a random starting tree and run with Markov Chain Monte Carlo (MCMC) (Larget and Simon Reference Larget and Simon1999) for 1 x 106 generations. The tree was visualized and saved with FigTree 1.4.4 (Rambaut Reference Rambaut2018).
Results
Morphological description (Figures 1–3, morphometrics in Table 1)
Adult. Adults are moderately slender to slender (a = 29–41) nematodes of small size, 0.73–0.99 mm long. The body is cylindrical, visibly tapering towards the anterior end, but not so towards the posterior extremity as the tail is short and rounded in both sexes. Upon fixation, habitus is almost straight or slightly curved ventrad, to an open C shape. The cuticle tylencholaimid is almost smooth when observed with light microscope (LM), but under SEM bears very fine transverse striation, which is two-layered, with both layers displaying the same thickness, ca 1 μm, throughout the body, with an inner layer not especially irregular nor detached from the outer one, and radial refractive elements that are difficult to discern. The lateral chord is 8–12 μm wide, occupying 29–46% of the mid-body diameter. The lip region is continuous with the adjoining body, with totally amalgamated lips, and its anterior margin is conspicuously sunken and appreciably refringent. SEM observations (with due caution, as the specimens examined were not in perfect condition) include: a wide, circular oral field, consisting of a somewhat protruding peripheral ring annulus, two or three incisures concentric with the peripheral ring, situated inside and further down, and a central and slightly triangular oral plate bearing three short radial incisures; two circles of (inner and outer) labial papillae close together, located in weakly protruding lobes close to the peripheral ring; and cephalic papillae at an appreciable distance behind the labial ones, button-like and with a distinct pore at their centres. Amphid fovea are pouch-like, with a small, pore-like aperture, ca 1 μm in diameter when observed with LM. SEM observations were of poor quality, but the amphid aperture was apparently circular, small, and at an appreciable distance behind the cephalic papillae. The cheilostom is a short, truncate cone, with thick and slightly sclerotized walls ending in perioral thickenings. The odontostyle is comparatively short and robust, somewhat refractive in the specimens herein examined, 3.3–4.3 times as long as wide, 0.5–0.6 times the lip region diameter long, and 0.65–0.80% of the total body length; its aperture is 2.5–3.5 μm, ca one-half of the odontostyle total length. The guiding ring is simple, but distinct, and located at 4.5–5.5 μm or 0.4–0.5 times the lip region diameter from the anterior end. The odontophore is linear, lacking any differentiation, but its junction to the pharyngeal lining is enveloped by a conspicuous spindle-shaped swelling. The pharynx is entirely muscular, very gradually enlarging (in fact, it is difficult to distinguished both pharyngeal sections) into a basal bulb that occupies ca one-fourth (21–27%) of the total neck length; gland nuclei are apparently situated as follows (but it was difficult to appreciate their precise location in the specimens examined): DN = 78–84, S1N = 87–90, S2N = 93–95. The nerve ring is located at 76–92 μm or 54–58% of the total neck length from the anterior end. The cardia is short, rounded, and conoid, 6.5–11 x 7–10 μm. The caudal region is similar in both sexes, short and rounded, even slightly swollen or clavate in some specimens.

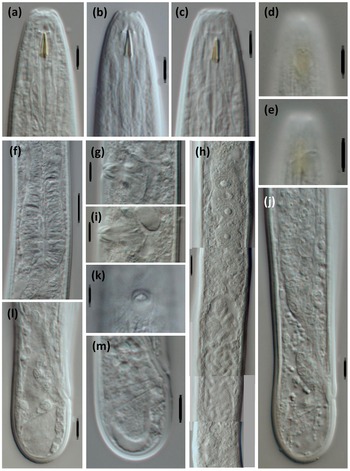
Figure 1. Light micrographs of Loofilaimus phialistoma Jairajpuri, Ahmad & Sturhan, Reference Jairajpuri, Ahmad and Sturhan1998 (Female). (a-c) Anterior region, in lateral median view; (d, e) Anterior region, in lateral surface view; (f) Pharyngeal bulb; (g, i) Vagina; (h) Anterior genital branch; (j) Posterior body region; (k) Vagina, in ventral view; (l, m) Caudal region. [Scale bars: a–e, g, i–m = 5 μm; f, h = 10 μm]

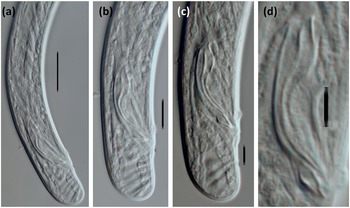
Figure 2. Light micrographs of Loofilaimus phialistoma Jairajpuri, Ahmad & Sturhan, Reference Jairajpuri, Ahmad and Sturhan1998 (Male) (a) Posterior body region; (b, c) Caudal region and spicules; (d) Spicule and lateral guiding piece. [Scale bars: a = 10 μm; b-d = 5 μm]

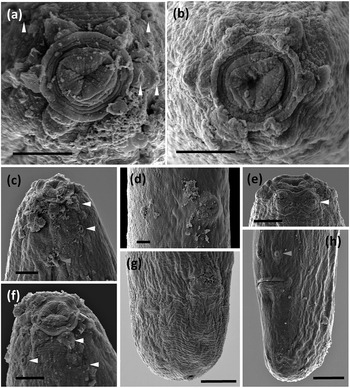
Figure 3. SEM micrographs of Loofilaimus phialistoma Jairajpuri, Ahmad & Sturhan, Reference Jairajpuri, Ahmad and Sturhan1998. (a, b) Lip region, in face view; (c) Lip region, in lateral view; (d) Vulva, in ventral view; (e, f) Lip region, in ventral view; (g) Female, caudal region; (h) Male caudal region; (Arrow head pointing at labial papillae (white), cephalic papillae (yellow), tentative postion of amphid fovea apertura (green), and male pre-cloacal genital papillae (blue). [Scale bars: a–f = 2 μm; g, h = 5 μm.]
Female. The genital system is diovarian, with both branches equally developed; the anterior is 115–167 μm or 14–19% of body length, and the posterior is 132–169 μm or 16–19% of body length. The ovaries are relatively small, often not reaching the sphincter level, 38–89 μm, with oocytes first arranged in two or more rows and then in a single row. The genital tract is poorly differentiated, at least in the specimens herein examined; thus, the following description should be taken with some caution. The oviduct is 54–104 μm or 1.9–3.9 body diameters long, apparently consisting of a distal portion made of prismatic cells and developed pars dilatata, usually with sperm cells inside. A weak narrowing is hardly appreciable, separating oviduct and uterus, but a distinct sphincter is not visible. The uterus is a simple tube-like structure, 46–68 μm or 1.6–2.5 body diameters long, often with abundant sperm cells inside. The vagina is 11–15 μm long, extending inwards to one-half (44–54%) of the body diameter: the pars proximalis is 6.5–10 x 3.5–5 μm, with almost straight walls surrounded by weak circular musculature; the pars refringens consists of (in lateral view) two triangular to ovoid sclerotized pieces 2.5–4 x 1–3 μm, with a combined width of 4–5 μm; the pars distalis is visibly sclerotized and partially embraces the pars refringens. The vulva is very small and pore-like. The prerectum is 1.9–4.2 and the rectum is 1.4–1.8 anal body diameters long.
Male. The prerectum is 3.6–4.7 and the cloaca is 1.9–2.3 anal body diameters long. An adcloacal pair of genital papillae is located at 6–7 μm from the cloacal aperture, and there is no ventromedian supplement. Spicules are appreciably atypical, with slender walls and a very fine median piece, 4.5–6.0 times as long as wide, and 1.3–1.7 times as long as the body diameter at the level of cloacal aperture. The head is very short, at 2–4 μm, as long as wide, and occupying 8–15% of the total length. The median piece is 1 μm thick; the hump is conspicuous, located at 6–9 μm or 22–30% of the total length; the curvature is 131–150º. A lateral guiding piece is 5–7.5 μm long, 2.2–3.0 times longer than wide, and visibly tapering at its posterior portion.
Molecular characterization
Two sequences were obtained from the 28S rDNA fragment of the rRNA gene, one with 933 bp from a female specimen and the other with 948 bp from a male specimen, both identical in a fragment in common with 929 bp.
Locality and habitat
The species was recovered in Northwestern Iran, East Azerbaijan province (GPS coordinates: 37°20’02’’ N, 47°48’62’’ E; altitude: 1778 m above sea level). It was recovered in soil samples around overgrown moss and algae on slightly salty wet rock. It was collected on 06 November 2017.
Discussion
Comparison with type population
The above description fits what is known about such specimens, but details of some morphological features have been added and/or refined, and a more extended morphometrical study is herein provided. Morphologically, a few trait differences deserve further comment. First, some elements of the lip region are now described with more accuracy. In fact, SEM shows a very unusual pattern in the dorylaims: a sunken oral field delimited by a ring annulus, an oral aperture in the centre of an almost triangular plate, etc. Second, the posterior location of the amphid aperture is confirmed, but the fovea is pocket-like rather than cup-like, with a very small, pore-like opening ca 1 μm in diameter. Third, the cheilostom design is now better illustrated and explained. Fourth, the description of the vagina is more complete and precise. Thus, the morphological characterization of the species has been extended and updated.
Table 1 presents a morphometrical comparison of the Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998) population with the new one herein studied. Most measurements and ratios are identical or widely overlap. Nevertheless, a few observed differences warrant further explanation. The most important discrepancy involves the lip region diameter, which was 8 μm in the earlier population vs 10.5–12 μm in the new one. This is certainly because, in the original description, it was measured at the level of the outer labial papillae, whereas the new one was assessed at the level of the amphid aperture. In fact, Jairajpuri et al.’s (Reference Jairajpuri, Ahmad and Sturhan1998) original Figure 1C and Figure 1D are fully comparable to our Figures 1A–C, with similar relative sizes of the lip region and odontstyle. Other minor differences in a-ratio (36–41 vs 30-34 in females) and female tail length (22–23 vs 18–21 μm) are regarded as simple intraspecies variations.
Given these findings, the identity of the material examined is not in doubt, with this being only its second observation, 25 years since its original description.
New insights into the evolutionary relationships of Loofilaimus
Morphological study. Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998) emphasized the “unusual combination of some morphological characters” that characterized Loofilaimus, among them: the peculiar shape of its lip region, the posterior location of the amphid aperture, a strongly muscular pharynx with poorly demarcated basal portion, and a total absence of ventromedian supplements. At least two of these traits should be interpreted as autapomorphies. On the one hand, the morphology of the lip region (i.e., visibly sunken and with sclerotized inner (anterior) lining) has a peculiar design when observed by SEM. Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998) and Andrássy (Reference Andrássy2009) considered it similar to that found in Mylodiscus Thorne, Reference Thorne1939, but, as Coomans and Loof (Reference Coomans and Loof1978) admirably explained, the lip region of this genus is disc-like, offset by constriction and with well demarcated lips, hardly comparable to that observed in Loofilaimus. On the other hand, and if possible, more importantly, the design of the pharynx is totally exceptional within dorylaims as, with an exception (see below), the numerous taxa having a pharyngeal bulb (i.e., members of Leptonchidae, Longidoridae, Mydonomidae, and Tylencholaimellidae) always present with a very slender and weakly or not muscular anterior pharyngeal section. In fact, the Loofilaimus pharynx in some aspects resembles that described in several rhabditid representatives, for instance, the genus Drilocephalobus Coomans & Goodey, Reference Coomans and Goodey1965. The posterior location of the amphid aperture, at an appreciable distance behind the level of the outer labial and cephalic papillae (and even the level of the guiding ring) might be a third autapomorphic trait. Conversely, the absence of ventromedian supplements, although somewhat rare, sporadically occurs in some dorylaimid taxa, for instance a few representatives of Thorniidae and Leptonchidae, and it should be regarded as an infrequent apomorphy.
Very rightly, Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998) drew attention to the resemblance of Loofilaimus and Bertzuckermania Khera, Reference Khera1970, another enigmatic genus, with neither having been recorded after its original description in a freshwater habitat of India. Nonetheless, they only stated (p. 19) that both genera differed “in the structure of cheilostome, odontostyle and oesophageal musculature,” with no further comment. Unfortunately, some aspects of the Bertzukermania diagnosis remain obscure, but available information about it suggests a tentative closer relationship with Loofilaimus than originally thought. First, both monospecific genera display a very similar general morphology (almost straight habitus, rounded lip region, diovarian females, short and rounded tail in both sexes, absence of ventromedian supplements) and morphometry (for instance, body length 0.70–1.00, c = 38–48 in Bertzuckermania). Second, and very interestingly, Bertzuckermania is characterized by an entirely and strongly muscular pharynx with a somewhat pyriform basal bulb occupying less than one-fourth of the total neck length. Third, its diagnosis describes a (Khera, Reference Khera1970: p. 144) “…mouth surrounded by a cuticularized stomal ring,” and Khera’s original Figure 1C and 1D illustrate the presence of a ring annulus around the oral aperture, a very similar image to that herein obtained with SEM for Loofilaimus. Thus, the possible coincidence of two remarkable autapomorphies in both genera supports a tentative close evolutionary relationship among them. For the rest, both genera significantly differ in the nature of their cheilostom (vs a simple truncate cone in Bertzuckermania), odontostyle (vs very slender), and the location of their amphid aperture (vs in front of the level of guiding ring, as usual in dorylaims).
Andrássy (Reference Andrássy2009) proposed new ideas about Loofilaimus kinship, placing it in Thorniidae, Thorneellinae. Thorniid taxa are characterized by some features similar to those observed in Loofilaimus: a rounded lip region with amalgamated lips in general; two circles of labial papillae close together; a small amphid aperture; a short caudal region that is rounded in both sexes; and no or very few ventromedial supplements. Nevertheless, Loofilaimus differs in its sunken oral field delimited by a ring annulus, cheilostom (vs simple, with no differentiation in Thorniidae), pharynx (vs with the two typical sections about equally sized), and, less importantly, the presence (vs absence) of pars refringens vaginae. Thus, the two relevant autapomorphic features of Loofilaimus do not occur in thorniid genera, particularly the unusual pharynx design.
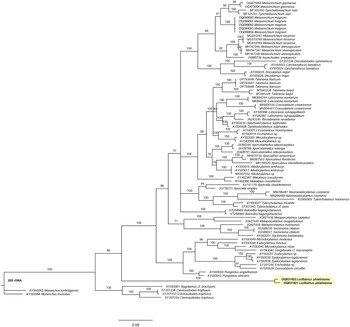
Molecular analysis. This is the first study of Loofilaimus sequences. As derived from the analysis of the D2-D3 28S-rDNA gene, the evolutionary relationships of Loofilaimus are presented in the molecular tree of Figure 4. Interestingly, but not surprisingly, the two sequences analysed do not show any close relationship with any other dorylaimid taxon. Conversely, they form a separate small subclade, with the Dorylaimina subclade as its sister group, both having in common 96% similarity. They are also 96% similar to the Nygolaimina subclade after this clustering: ((Nygolaimina + (Loofilaimus + Dorylaimina)).

Figure 4. Bayesian Inference tree from the newly sequenced Loofilaimus phialistoma Jairajpuri, Ahmad & Sturhan, Reference Jairajpuri, Ahmad and Sturhan1998 from Iran based on sequences of the 28S rDNA region. Bayesian posterior probabilities (%) are given for each clade. Scale bar shows the number of substitutions per site. New sequences in bold.
Integrative approach. Both morphological and molecular data support the idea that Loofilaimus is a very special dorylaimid taxon, certainly representing a restricted evolutionary trend. A closer relationship with the Dorylaimina clade is also obvious. On the one hand, the presence of a small but distinct odontostyle and the absence of cardiac glands separate it from the taxa forming part of the suborder Nygolaimina, which, as known, is characterized by a mural tooth as a stomatal protruding structure and three large cardiac glands around the pharyngo-intestinal junction (cf. Andrássy Reference Andrássy2009; Coomans Reference Coomans1964; Heyns Reference Heyns1968; Jairajpuri & Ahmad Reference Jairajpuri and Ahmad1992). On the other hand, its position in the 28S tree supports this hypothesis as well.
When proposing Loofilaimus, Jairajpuri et al. (Reference Jairajpuri, Ahmad and Sturhan1998) hesitated as to the taxonomical position of Loofilaimus, either as a member of Dorylaimoidea or as a representative of Tylencholaimoidea; they provided arguments for and against one and the other, decided to create a new family to accommodate it, and, finally, classified it under Tylencholaimoidea. Our results confirm the difficulty in establishing robust evolutionary relationships with other Dorylaimina representatives. Unfortunately, Andrássy’s (Reference Andrássy2009) proposal to integrate Loofilaimus into Thorniidae cannot be tested as the 28S gene has not been sequenced for any member of this family so far. Thus, the family Loofilaimidae should be maintained, and the genus Bertzuckermania should be provisionally transferred to it.
Acknowledgements
The Spanish authors appreciate the use of the equipment of “Centro de Instrumentación Científico-Técnica (CICT)”, University of Jaén. We thank Amparo Martínez-Morales for her assistance in obtaining SEM scans of the new species herein described.
Financial support
This project was supported by the University of Jaén, Spain, through the research program ‘PAIUJA 20121/2022: EI_RNM02_2021.’
Competing interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.