Malnutrition is considered a typical feature of cerebral palsy (CP) as its incidence has been documented in 46–90 % of these children(Reference Soylu, Unalp and Uran1), with occurrence generally increasing in line with severity(Reference Rogers2). This is believed to relate to the commonly encountered feeding difficulties caused by a variety of factors including dysphagia, gastro-oesophageal reflux, problems with oral motor control and self-feeding ability(Reference Sullivan, Lambert and Rose3).
Impaired growth is another well-documented feature of CP(Reference Samson-Fang and Stevenson4). Much literature surrounds the relationship between malnutrition and growth with other health outcomes such as changes in healthcare requirements and participation in society(Reference Samson-Fang, Fung and Stallings5) as well as bone health(Reference Henderson, Kairalla and Abbas6, Reference Henderson, Kairalla and Barrington7) and immunological challenges(Reference Sullivan, Lambert and Rose3) in these children.
Percutaneous gastrostomy feeding is thought to assist children to correct growth impairments due to malnutrition(Reference Escott-Stump and Troy8). Many studies(Reference Shapiro, Green and Krick9–Reference Sullivan, Juszczak and Bachlet15) have found improvements with enteral feeding, notably in weight z-scores, although minimal changes have been noted for height z-scores. Improvements in height z-scores have however been documented in eight out of thirty-five children in one study(Reference Rempel, Colwell and Nelson16), although this was much less frequent than improvements in weight z-scores (twenty-four out of thirty-five) in the same children.
Many aspects of human physiology are interrelated. Sufficient macronutrients such as carbohydrates, fat and protein, as well as adequate micronutrients such as vitamins, minerals and trace elements, are all needed for the body to function in an integrated manner. Insufficiencies or imbalances in any of these components cause disturbances in this delicate balance(Reference Jackson, Warrell, Cox and Firth17).
Protein is important for growth, immune function and recovery from disease, as well as skeletal respiratory muscle function, which all become severely impaired when levels are low. Taking this into consideration, we decided to investigate actual protein consumption and a variety of other markers, which may relate to protein status in children with marked CP either orally or enterally fed and compare with controls. Other parameters considered include height, weight and BMI, plasma albumin, creatinine, urea and urate, as well as C-reactive protein as a marker of inflammation as this may affect some of the former variables.
Experimental methods
Children were recruited from the Royal Children's Hospital, Brisbane, Australia, as part of a larger observational study. Their characteristics are outlined in Table 1. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Ethics Committees of the Royal Children's Hospital (2007/117), the University of Queensland (2006000409) and Cerebral Palsy League of Queensland (2008/2009 1021). Written informed consent was obtained from all subjects' parents or guardians. Exclusion criteria included coexistence of specific chronic diseases such as renal, cardiac or identified metabolic disorders, as well as gastric resection, which may otherwise confound results.
Table 1 Participant characteristics
(Mean values, standard deviations and 95 % confidence intervals)
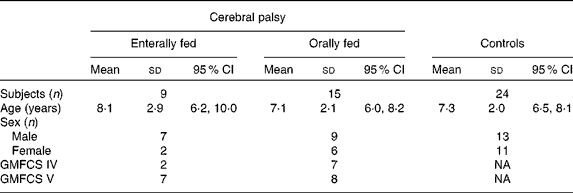
GMFCS, gross motor classification scale; NA, not applicable.
The parents of the children collected a three consecutive day dietary duplicate of all food consumed by their child. When the children were at school or in other care, instructions were given to return any unused portions of the food, so their intake sample could be adjusted accordingly. The food composite was then macerated and homogenised, then subsamples analysed for the direct quantity of protein. A sample of all fluids consumed was collected separately, along with their quantity, and calculated along with the food quantities, to give an accurate representation of consumption values.
For protein analysis, the food samples were digested in sulphuric acid with the added catalyst of copper sulphate. After digestion, the samples were analysed for N content using a Buchi Kjeldahl digestor unit (Buchi K438; Schweiz, Switzerland). The levels of N in the samples were used to calculate protein levels by using a converting factor of 6·250(Reference Thompson, Owen and Wilkinson18). Protein analyses were carried out with standard reference materials and the laboratory in-house reference materials for quality control and assurance. Energy intake was obtained from the same food samples via bomb calorimetry analysis.
Protein intakes of enterally fed children were calculated using food panel information provided by the relevant product companies, along with the volumes consumed per d by each child. All total values are reported as a percentage of minimum requirement for age and sex, as outlined by the National Health and Medical Research Council nutrient reference values for Australia and New Zealand(19). For energy intakes, values are reported as a percentage of estimated average requirement for age and sex, also based on the above reference source.
Fasting blood samples were collected from participants first thing in the morning and lithium heparin plasma was available for albumin, urea, urate, creatinine and C-reactive protein assays. These were measured simultaneously on the Beckman Coulter Synchron Clinical System (Synchron LX; Clinical Systems, Brea, CA, USA), with the creatinine quantification via a modified Jaffé method(Reference Junge, Wilke and Halabi20).
Z-scores were calculated for values which are known to vary with age and sex, such as height, weight, BMI, albumin, urate and creatinine. These were calculated from the Centers for Disease Control (2000) growth data (EPI INFO software; Centers for Disease Control, Atlanta, CA, USA) and previously published data(Reference Soldin, Brugnara and Wong21), respectively. As both groups of children with CP were non-ambulatory, an estimated height was calculated based on a mean and predictions of stature from measurements of upper arm length, lower leg length and knee height(Reference Spender, Cronk and Charney22).
Statistical analyses were conducted using Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA), with comparison of means between CP and control children obtained via t tests. P values between orally and enterally fed children were calculated via a post hoc analysis. P values below 0·05 were considered statistically significant. As no previous data exist in this area, a sample size of sixteen was selected, as it would detect a statistically significant difference if the means between the groups varied by 1 sd.
Results
Comparisons of group means are presented in Table 2.
Table 2 Resulting data expressed as comparisons of the group means
(Mean values, standard deviations and 95 % confidence intervals)

CP, cerebral palsy; EAR, estimated average requirement.
Mean values were significantly different from those of the control group: *P < 0·05, **P < 0·01, ***P < 0·001.
Anthropometric, height and weight z-score correlations are presented in Table 3.
Table 3 Anthropometric, height and weight z-score correlations
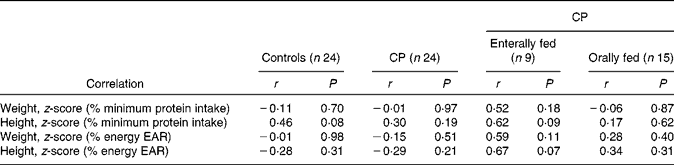
CP, cerebral palsy; EAR, estimated average requirement.
In the present cohort of orally fed children with CP, anthropometric data demonstrate significantly reduced z-scores for weight ( − 1·92 (sd 1·59)), height ( − 1·68 (sd 1·0)) and BMI ( − 1·06 (sd 1·62) kg/m2) compared with the control group and are in agreement with previously published normative datasets. The enterally fed group, however, showed improved z-scores, compared with orally fed children, for weight ( − 0·73 (sd 1·24)) and BMI (0·52 (sd 1·01)), but not height ( − 1·75 (sd 0·90)). This was due to lower weight z-scores in the orally fed group, which also reached statistical significance when compared with the enterally fed group.
Calculation via the D'Agostino-Pearson Omnibut test for normality indicated no significant deviations from normal distribution within each of the three groups. However, when combining the groups of children with CP, normality was not established for energy due to the large differences found between orally and enterally fed children's intakes.
Analysis via ANOVA revealed protein intakes to be significantly higher in the control group, compared with both orally and enterally fed children (P = 0·005). Energy intakes were also significantly different between enterally and orally fed children v. controls (P = 0·006).
In relation to other biochemical variables, which may be indicative of protein metabolism, significant differences were found between children with CP and controls for urate (P = 0·002) and creatinine (P < 0·001). Differences were also found between enterally v. orally fed children and controls for albumin (P < 0·001) and urea (P = 0·08) via ANOVA, although in the latter, significance between the means was reached for enterally fed children v. controls only (P = 0·03). Inflammatory marker C-reactive protein was also measured and found not to be increased in any of the groups. This latter component was included as an indicator of the acute-phase inflammatory response due to the potential of this phenomenon to decrease albumin levels irrespective of other parameters and hence potentially confound resulting data.
Discussion
The present anthropometric data support other previously published data(Reference Shapiro, Green and Krick9–Reference Sullivan, Juszczak and Bachlet15) and demonstrate differences in weight z-scores but not height z-scores when comparing enterally and orally fed children. The large standard deviation found in the CP group is affected by one of the orally fed children, with a weight z-score close to zero, having previously been administered enteral feeds which may have caused the unusually large weight z-score, compared with others in the orally fed group. Similarly, the enteral group contained a child who was partially enterally and orally fed, and as such had a much lower weight z-score than other children in this group. Height z-score standard deviation was closer to 1·0.
No significant correlations were found between weight z-score and either percentage of minimal requirement of protein or percentage of recommended daily energy intake in any of the groups. Interestingly, height was more closely related to protein intake in both control (P = 0·08) and enterally fed children (P = 0·09), while height was also more closely correlated with energy intake in the enterally fed group (P = 0·07). These measures may, however, reach significance with a larger sample size.
Compared with a reference population of age- and sex-matched typically developing children, a study of forty children with marked CP documented lower energy expenditure and high body fat content in children particularly if they were enterally fed(Reference Sullivan, Alder and Bachlet23). The authors have also highlighted the potential risk of overfeeding with available enteral feeds due to a potential shift from negative to positive energy balance that enhances this fat accumulation. A longitudinal study had similar findings which showed that children with severe motor impairment gained more fat than muscle over time and supported the theory that weight gain in these children may be mainly comprised of fat gain(Reference Ohata, Tsuboyama and Haruta24). A number of other smaller studies have also shown an increase in fat accumulation after gastrostomy insertion(Reference Sanders, Cox and Cannon10, Reference Isaacs, Georgeson and Cloud12, Reference Rempel, Colwell and Nelson16). This may explain the typical increases in weight but not height z-scores.
Significantly reduced body protein levels for age and height have been previously noted in fifty-nine children with marked CP using γ-neutron activation analysis(Reference Arrowsmith, Allen and Gaskin25). The advantage of this method is that it is a direct measure of body N and is not affected by other factors such as hydration status(Reference Baur, Allen and Rose26). Frequently utilised body composition assessment which uses measures of total body water with assumed hydration factors to equate lean tissue mass may suffer from bias due to an abnormal hydration(Reference Roubenoff, Kehayias and Dawson-Hughes27). Hyperhydration has been reported in children with malnutrition(Reference Shetty, Davies and Cole28). This has been attributed to a decrease of plasma proteins, mainly in the form of albumin, which act to maintain oncotic pressure within the blood vessels and hence assist with the resorption of fluid from interstitial spaces(Reference Mergner, Trump and Rubin29). Subsequent increases under these circumstances may increase total body water, falsely elevate lean body mass and hence total body protein estimations, when calculated via methods which utilise assumed hydration factors(Reference Arrowsmith, Allen and Gaskin25). A variety of studies have found mixed results in the calculation of total body water(Reference Bandini, Schoeller and Fukagawa30–Reference Berg and Isaksson32), which may be the result of confounding malnutrition. Each of these studies also had children with varying severities of CP, which is likely to have affected the levels of confounding from potential malnutrition issues. Assumption relating to the hydration of the fat-free mass in a number of children with CP may not be valid, and this could affect the ability to accurately predict their lean mass and hence total body protein, from such measures.
Bone health in these children is also a concern as there is a tendency for spontaneous fractures as a result of osteopaenia(Reference Leet, Mesfin and Pichard33), which has been attributed to nutritional(Reference Henderson, Lin and Greene34) and non-nutritional(Reference Henderson, Lark and Gurka35) factors. Protein plays an important role in Ca homeostasis as it affects both its intestinal absorption(Reference Kerstetter, O'Brien and Insogna36) and retention in the body(Reference Hunt, Johnson and Fariba Roughead37). A recent well-designed study by Arrowsmith et al. (Reference Arrowsmith, Allen and Gaskin38) demonstrated no significant increases in total body protein percentage for age or bone mineral content for age or height, after enteral feeding for a median of 19·4 months. When these parameters were compared with height-matched, as opposed to age-matched, controls, significant increases were found with increases from 83 to 99 %(Reference Arrowsmith, Allen and Gaskin38).
Although all of the groups in the present study were consuming protein within current recommendations, recent evidence has suggested that these recommendations may actually be underestimating human protein requirements by approximately 40–50 %(Reference Elango, Humayun and Ball39). In the past, N balance studies were utilised which have been documented to suffer from errors. These are believed to have occurred via an overestimation of N intake, coupled with an underestimation of N excretion, which in all lead to a shortfall in inference of the actual protein requirements(Reference Board40). The new method which is fast becoming accepted determines amino acid requirements via an indicator amino acid oxidation method which involves feeding a range of test intakes and measuring the response in oxidation. Here, the increasing intake of the limiting amino acid will linearly decrease the oxidation of this particle until no further change is detected. This point indicates when requirement has been reached with the entire process reflecting the amino acids increased incorporation into protein(Reference Elango, Ball and Pencharz41). From these later studies, values for some of the essential amino acids such as leucine, lysine, threonine and valine appear to be up to two times higher than originally thought(Reference Elango, Ball and Pencharz42). The National Health and Medical Research Council's current recommendations for children based on a population's estimated average requirement and an individual's recommended daily intake safe requirements are of 0·78 and 0·94 g/kg per d for boys and 0·61 and 0·87 g/kg per d for girls, respectively. As determined by the indicator amino acid oxidation, new requirements are suggested to be 1·35 and 1·6 g/kg per d, which demonstrate the extreme inadequacy of current recommendations(Reference Elango, Humayun and Ball43).
If these newer estimations were used in the present study group to ascertain the percentage of minimum protein requirements, orally fed children would on average be consuming 140 (sd 60) %, enterally fed children 119 (sd 32) % and control children 208 (sd 80) %.
Creatinine is often utilised as a surrogate measure for muscle mass(Reference Gibson44), and as such may be an indicator of total body protein status. In the present CP groups, creatinine was found to be significantly lower than in control children. This may also be explained in part due to notable inactivity, which has been shown to increase protein catabolism and therefore down-regulate protein synthesis(Reference Guadagni and Biolo45). Recent studies have shown that increasing protein intakes by 15–16·5 g of essential amino acids may reduce inactivity-related loss of skeletal and myocardial muscles in both young(Reference Paddon-Jones, Sheffield-Moore and Urban46) and old(Reference Ferrando, Paddon-Jones and Hays47) subjects. The quality of the protein has also been demonstrated to affect protein synthesis during inactivity(Reference Antonione, Caliandro and Zorat48). In typically developing adolescents, protein intakes greater than recommended have also been associated with positive health indicators such as waist circumference, insulin resistance and TAG levels(Reference Adamson, Daratha and Bindler49).
Albumin is the most abundant protein in human plasma and represents 40–60 % of total protein. As plasma levels depend on protein intake, albumin is often used as a surrogate to assess nutritional status(Reference Burtis and Ashwood50). Hypoalbuminaemia is associated with impaired protein metabolism, infection or stress, impaired hepatic function or toxic damage(Reference Jackson, Warrell, Cox and Firth17). The present data show significantly decreased levels of albumin in our enterally fed group compared with the other two groups. Urea is also found to be reduced in our enterally fed group. A low-protein diet and starvation cause a decrease in the synthesis of urea(Reference Pagana, Pagana and Dennison51) as particular amino acids are favoured for recycling, a process which aids in decreasing further protein catabolism(Reference Torun, Shils, Shik, Ross, Caballero and Cousins52). A low reading of urea may be indicative of inadequate protein intake, although it may also occur in severe liver disease(Reference Heimburger, Shils, Shike, Ross, Caballero and Cousins53).
Urate is a nitrogenous compound that is a catabolism product of the DNA building block purine(Reference Pagana, Pagana and Dennison51) and as such may give an indication of the state of protein nutriture. A low level of urate has been identified in Alzheimer's disease and associated with increased progression of cognitive decline. This is thought to be due to its function as one of the major antioxidants in plasma(Reference Irizarry, Raman and Schwarzschild54). The children with CP in the present study had significantly reduced levels of plasma urate, as well as many with high-level cognitive impairment, when compared with their typically developing counterparts.
Limitations to the present study include the relatively small number of participants due to its nature as a pilot study. However, significant differences between the groups were documented and should be reason to further investigate the disparities, given the presented literature. Future studies in larger samples should focus on methods which directly determine total body protein and compare with different levels, quality and composition of intakes, particularly in children with eating disorders and also those solely reliant on artificial nutrition.
Conclusion
Children with CP are well documented to have issues with malnutrition, growth and development. Given the importance of protein in all aspects of physiology and growth, adequacy of current recommendations warrants further investigation, particularly in those who are permanently administered standardised enteral formulas which adhere to these potentially inadequate guidelines.
The intricate workings of body biochemistry and the need for this to be adequately balanced call for nutrition focus in these children to be expanded beyond the commonly held isolated theories of energy intake and expenditure affecting growth and development.
Acknowledgements
N. S. is supported by a Royal Children's Hospital Foundation PhD scholarship and the University of Queensland. R. B. is supported by the National Health and Medical Research Council Career Development award, Smart State Fellowship and the University of Queensland. P. S. W. D., L. V. and N. S. are supported by the School of Medicine, the University of Queensland. U. T. is supported by Queensland Health, Centre for Public Health Sciences. N. S. was the project coordinator and the main author of this manuscript. N. S. provided research assistance for the project. U. T., Pam Kahlon and Hans Yates performed the laboratory analyses. P. S. W. D., L. V. and R. B. supervised the project and revised the manuscript. There are no conflicts of interest to declare.





