INTRODUCTION
The anthropo-zoonosis tularemia is caused by the facultative intracellular bacterium Francisella tularensis which can cause a wide variety of clinical syndromes ranging from asymptomatic infection to severe, sometimes fatal disease [Reference Sjöstedt and Brenner1, Reference Ellis2]. The microbiological diagnosis is difficult due to the unusual growth requirements of F. tularensis and the limited availability of serological assays or new molecular tools [Reference Splettstoesser3]. For this reason and also due to the non-specific clinical symptoms, the diagnosis is often delayed or missed and the number of human cases might be severely underestimated. In Europe, the distribution of endemic foci varies significantly [Reference Tärnvik, Priebe and Grunow4]. While high numbers of cases are reported annually in Finland and Sweden, less numerous cases in France, Hungary, Turkey, Austria and Czech Republic as well as only sporadic cases are noticed from countries like Germany, Switzerland, Italy, Spain or Greece [Reference Ellis2, 5, Reference Anda6]. In Germany, the incidence in the last four decades was extremely low with only 1–5 reported human cases per 80 million inhabitants per year [5].
In 2004 and 2005 tularemia re-emerged in Germany. An airborne outbreak in humans affecting a group of hare hunters occurred in the county of Darmstadt-Dieburg [Reference Hofstetter7]. Additionally, two epizootics were noticed in the county of Goettingen, Lower Saxony, where several non-human primates were infected resulting in a significant number of fatalities [Reference Splettstoesser8, Reference Mätz-Rensing9]. Both affected counties were not regarded as typical endemic foci of F. tularensis. In 2007, the highest number of human tularemia cases was reported for almost 50 years.
Historically, the first cases in Germany were recognized in 1937 [Reference Jusatz10]. From 1949 to 1959 repeated outbreaks in humans involving hundreds of cases occurred in three main areas: Brandenburg/Mecklenburg-Western Pomerania (Northeastern Germany), Schleswig-Holstein (Northern Germany), and Bavaria (Southern Germany). From the late 1950s onwards, a sharp decline in human infections occurred [Reference Jusatz10].
Unfortunately, reliable information on seroprevalence in humans in different geographical regions of Germany, permitting the identification of risk factors, does not exist. Only one single study, published in 2004 and comprising 6632 sera from a cross-sectional investigation of a representative sample of the general German population gave evidence that the seroprevalence in Germany might be as high as 0·2% [Reference Schmitt11]. In addition to the occurrence of the first airborne outbreak of human tularemia in Germany in 2005 [Reference Hofstetter7], there is also growing evidence of the re-emergence of tularemia in the last decade in Southern European (Portugal, Spain, Turkey) and Central European (Denmark, France, Austria, Bulgaria, Croatia, Former Republic of Yugoslavia) countries, respectively [Reference Anda6, Reference de Carvalho12–Reference Reintjes16].
In the present study we present the results of a population-based, cross-sectional health survey of the city population of Leutkirch, Baden-Wuerttemberg. We compared our data to previous results obtained from studies or reports from Germany and several other European countries, thereby summarizing for the first time the sparse data available on the incidence of tularemia in different countries in Europe.
METHODS
Design of the seroprevalence study
Briefly, 4000 of 12 475 inhabitants of an urban population were randomly selected from the registry of inhabitants and invited to participate in the study. Of the 4000 invited participants, 107 were ineligible because of having moved from the area without a forwarding address, resulting in a sample of 3893 subjects. A total of 2445 persons aged 10–65 years participated in the study leading to a participation rate of 62·8% [Reference Haenle17]. For tularemia analysis, serum samples were available from 2416 participants.
Each interview was conducted by a trained interviewer. The standardized questionnaire included personal data (e.g. date of birth, gender, education, current work, diseases), health and social behaviour (e.g. sports activities, nutrition, alcohol consumption), as well as assumptive risk factors for infection with F. tularensis (e.g. pets, forest and garden work or leisure activities).
Serological testing
A screening ELISA was used to detect F. tularensis-specific anti-LPS antibodies as described recently [Reference Schmitt11]. ELISA results below the mean optical density (OD) plus 1 standard deviation (s.d.) calculated from 1149 negative sera collected in Germany were estimated as ‘negative’. Results above the mean OD plus 3 s.d. were assumed as ‘positive’, whereas all results between these two values were taken as ‘borderline’. Positive as well as borderline sera were additionally tested with a confirmative immunoblot [Reference Schmitt11]. Sera were considered to be positive if they showed a typical LPS ladder at a dilution of 1/500.
Data acquisition on tularemia in Europe and Germany
In many European countries including Germany, human tularemia is a reportable disease. Additionally, most countries notify human cases as well as the occurrence of tularemia in the animal population to the World Organization for Animal Health (Office Internationale des Epizooties; OIE). Therefore we extracted all relevant data on notified cases from the handiSTATUS II databank (http://www.oie.int/hs2/report.asp). In Germany, monthly updated data on the incidence of human tularemia can be directly obtained from the webpage of the Robert Koch Institute, Berlin (http://www3.rki.de/SurvStat). Almost complete historical data on the occurrence of F. tularensis infections in humans were recently reported based on the yearly reports to the German health authorities from 1949 to 2001 [5].
Statistical methods
Absolute and relative frequencies were calculated for qualitative factors in the descriptive statistical analysis, while, for quantitative factors, mean and standard deviation, as well as the median, minimum and maximum were determined. Statistical analysis was performed using SAS version 8.02 (SAS, Heidelberg, Germany).
Ethical agreement and informed consent
The study met the international agreements of the revised version (2000) of the World Medical Association Declaration of Helsinki regarding ethical principles for medical research involving human subjects and was approved by the ethics committee of the State Medical Chamber Baden-Wuerttemberg. Written consent was obtained from each study participant.
RESULTS
Employing a highly sensitive and specific combination of two standardized immunoassays [Reference Schmitt11], a total of 56 sera out of 2416 samples gave positive results indicating a seroprevalence of 2·32%. This result differs significantly (P<0·01) from the 0·23% positive samples (out of 6673 sera) reported in a nationwide study performed in 1998 [Reference Schmitt11].
Stratification of the data indicated putative risk factors like ‘hunting’ (seroprevalence 6·25%) or ‘working as a farmer’ (3·94%) (Table 1). In German residents, seroprevalence was 2·30% (50/2162), whereas in participants of other nationalities seroprevalence was 3·70% (5/134). Due to the small number of positive samples in these subgroups, statistical tests were not performed. The analysis of age distribution showed no significant cumulation in any particular age group (Fig. 1).
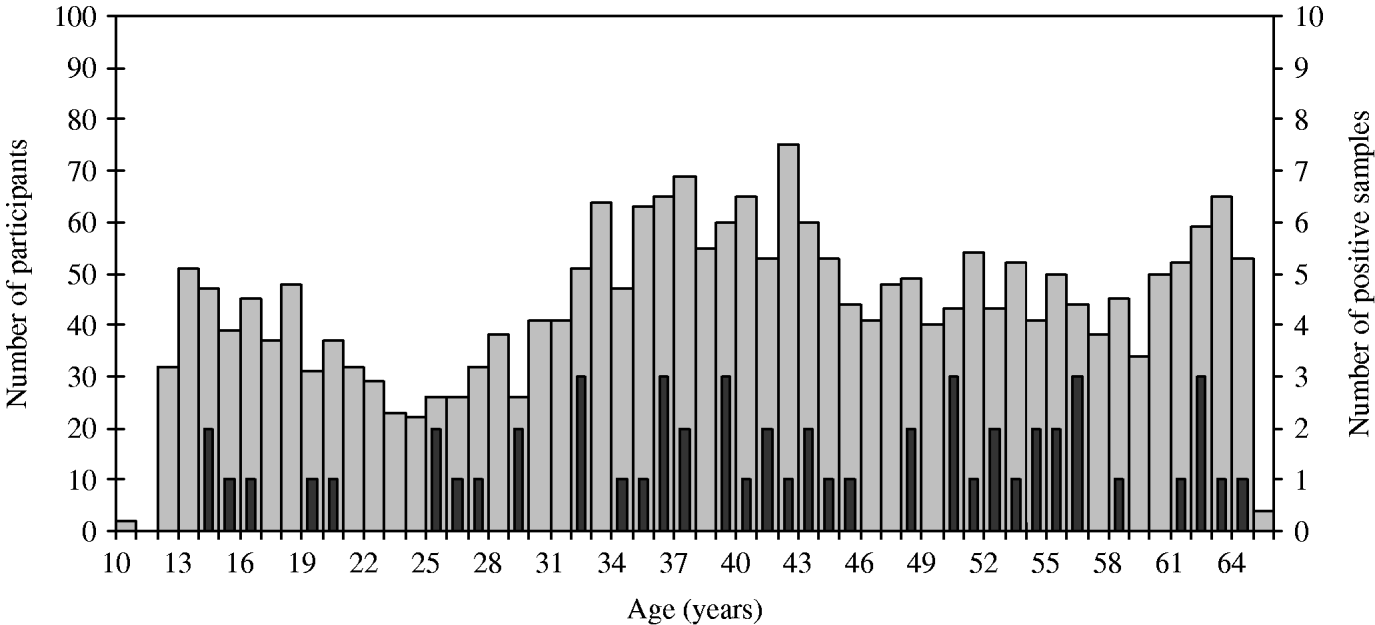
Fig. 1. Age distribution of the Leutkirch study group (![]() ) and of seropositive samples (
) and of seropositive samples (![]() ).
).
Table 1. Distribution of positive sera in different strata or putative risk groups. In some groups, the sum of positive and negative samples differs from the total number described in the text. This is due to missing data in some of the questionnaires
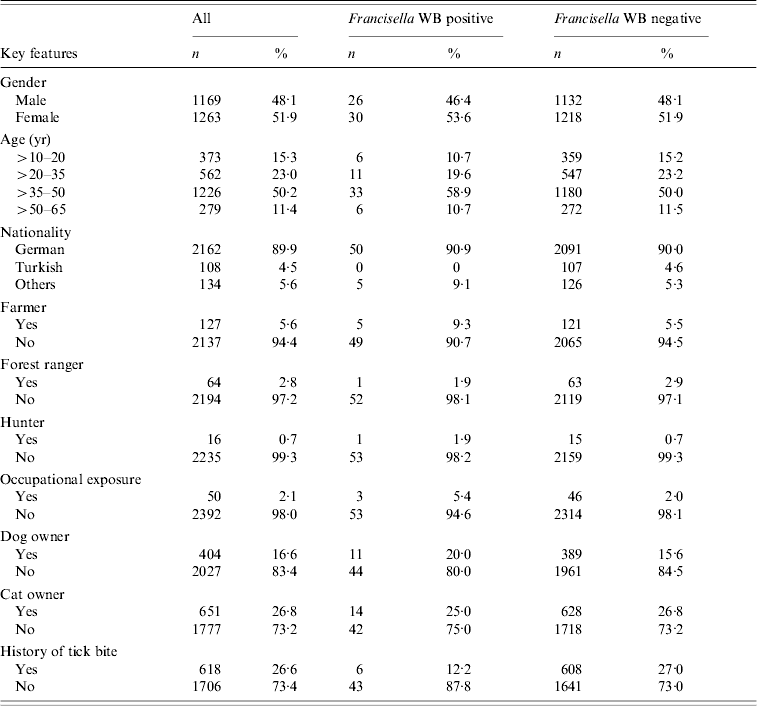
WB, Western blot.
Neither immigrants from Austria (0/12) nor from Turkey (0/107) showed positive results in tularemia serology. Markedly, seroprevalence differed clearly in terms of time of residency. In people living for more than 10 years in Leutkirch, seroprevalence was only 1·90% whereas prevalence varied between 4·30 and 7·90% in people living for only 1–3 or 3–5 years in this city.
Gender, outdoor activities, and exposure to ticks or pets did not appear to be associated with a higher risk for a positive test result.
The assessment of the re-emergence of tularemia in Germany by the analysis of recently notified cases through national surveillance introduced in 2001 revealed a total of 32 human cases from 2001 to 2006 (Fig. 2; http://www3.rki.de/SurvStat). In contrast to our results of the seroprevalence study, almost 75% of all infections affected males.
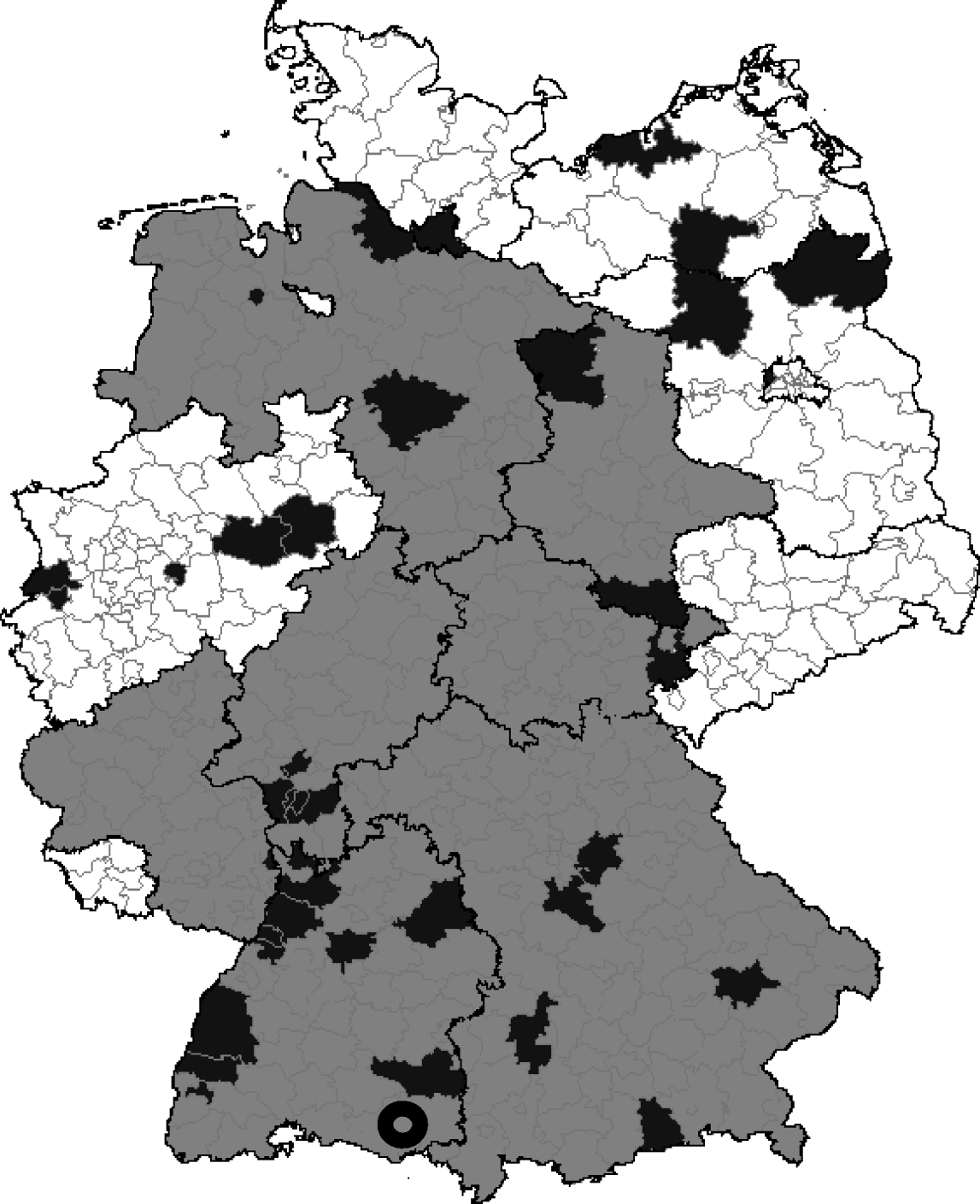
Fig. 2. Spatial distribution of reported Francisella tularensis infections in humans since 2001 (counties, black areas) and recovery of F. tularensis from rodents, hares or monkeys by PCR or culture (federal states, grey areas). Black circle (![]() ) denotes the study area for the seroprevalence study (city of Leutkirch). (Source modified from: SurvStat, http://www3.rki.de/SurvStat, 02.03.2008.)
) denotes the study area for the seroprevalence study (city of Leutkirch). (Source modified from: SurvStat, http://www3.rki.de/SurvStat, 02.03.2008.)
In the time period between 1974 and 2005, 93 serologically confirmed cases were notified in 15/16 federal states. The highest number of infections reported were in Baden-Wurttemberg (n=18), Hesse (n=14), North Rhine-Westphalia (n=13), and Bavaria (n=10), but in none of these cases was F. tularensis cultured or directly detected by PCR or immunological methods [5].
In contrast to that, the follow-up of all positive F. tularensis laboratory reports at the Bundeswehr Institute of Microbiology, Munich (2004–2007) showed that F. tularensis could be identified by culture and/or PCR in samples from at least seven different federal states (Fig. 2). This included the first direct detection of F. tularensis holarctica in a dead hare in the state of our seroprevalence study. Before 2004, tularemia in wildlife was last reported to the OIE in 1992 (http://www.oie.int/hs2/report.asp). A significant increase in the number of reported human tularemia cases occurred in 2007 compared to the last 50 years. In 2007, 21 confirmed cases were notified, including 11 cases in the federal state of Baden-Wurttemberg, where our study was performed.
Most European countries report cases of tularemia in humans, livestock, and wildlife to the OIE. In our comparative analysis we focused on human cases in the time period 1996–2004. Data from 2005 and 2006 are not yet available. Twelve European Union (EU) member states reported no human cases at all, although it should be noted, that Ireland as well as Portugal did not register tularemia at the national level until 2003. Additionally, data from Belgium and The Netherlands were incomplete. In the remaining 15 EU member states the cumulative incidence differed significantly between single countries (Table 2). Most clearly, the incidence was highest in the Scandinavian states of Finland and Sweden with cumulative numbers of more than 37 and 20 cases/100 000 inhabitants within the 9-year period, respectively. Marked incidences in this time period were also reported from Bulgaria (3·04/100 000), Hungary (7·41/100 000), Slovakia (7·92/100 000) and Czech Republic (5·58/100 000). Although Germany directly borders the latter country, the reported incidence in Germany was only 0·03/100 000. Besides the United Kingdom, where only one imported case had been notified, only Poland, Italy and France showed results similar to those from Germany. In all four countries the cumulative incidence was only 0·5–10% of the values reported from the other European countries (Table 2).
Table 2. Incidence of human tularemia cases in the EU member states from 1996 to 2004 (no data are available for 2005 and 2006)
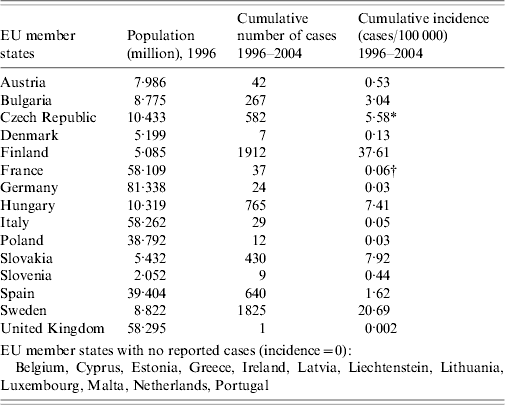
Data were accumulated from the annual reports of each country to the World Organization for Animal Health (http://www.oie.int/hs2/report.asp).
* No data 1999–2000.
† No data 1996–2002.
From the 28 European countries which are not members of the EU, only very fragmentary data were available. Here, Norway (1·85/100 000), Croatia (1·74/100 000) and the former states of Yugoslavia, Serbia and Montenegro (1·58/100 000) reported considerably high incidences of human cases. These reports are consistent with data scientifically published from these states.
DISCUSSION
The epidemiology of tularemia in Germany is characterized by three main enigmatic features: its sharp decline in the late 1950s, its irregular cycle and the irregular geographic appearance or persistence [5, Reference Jusatz10]. Whereas between 1949 and 1959 several outbreaks involving more than 500 patients had been described from the peninsula of Eiderstadt, the Baltic Sea region of Mecklenburg-Western Pomerania and the upper Main region near Wuerzburg, the number of human cases dropped to less than 10 patients in the following years [Reference Splettstoesser8]. Until 1989, no significant difference in tularemia incidence between the Federal Republic of Germany and the German Democratic Republic was apparent. After the reunification of Germany, which allowed free crossing over of deer, boars and other wildlife, less than five human cases occurred annually. Even from these cases, more than 40% were assumed to be imported.
Up to this point, no methodical investigation in the German population had been described. To address this deficit, sera from a comprehensive cross-sectional study comprising a representative sample of the German population were obtained and serologically tested. Because seroprevalence studies of rare infectious diseases are often hindered by the lack of highly specific diagnostic tools, we developed a combination of a screening ELISA and an immunoblot which proved to be suited to the performance of epidemiological studies in human tularemia. From a total of 6632 serum samples from individuals between the ages of 18 and 79 years, specimens from 15 (0·23%) individuals tested positive for F. tularensis-specific antibodies by ELISA and confirmatory Western blot [Reference Schmitt11]. These data provided some evidence that tularemia might be more abundant than is presumed from officially reported cases. Shortly after these data were published, two epizootics were recognized in the vicinity of Goettingen in Central Germany. The retrospective analysis of both outbreaks which occurred in non-human primate facilities and involved more than 20 animals, showed that the infection was passed to the monkeys by the indigenous rodent population in a region where tularemia had never been previously reported [Reference Splettstoesser8, Reference Mätz-Rensing9]. Within the next 12 months, the first airborne outbreak of human tularemia in Germany was notified in Darmstadt, federal state of Hesse. Here, at least 11 hunters were affected, of which one died [Reference Hofstetter7]. In 2006 and 2007, F. tularensis was definitely confirmed in hares from Thuringia, Rhineland-Palatinate, Hesse, Bavaria and Baden-Wurttemberg (W. D. Splettstoesser, unpublished data).
From this latter federal state the highest number of human cases was reported during the last three decades [5]. For this reason, we took the opportunity to analyse the sera derived from the Leutkirch study which was originally performed to estimate the frequency of Echinococcus multilocularis infections in this population [Reference Haenle17]. Leutkirch is located in the Southwest of Germany (47° 47′ latitude, 10° 01′ longitude). Data on elevation (655 m), regional mean annual air temperature (<9°C), and mean annual precipitation (1278 mm) (1961–2004) were obtained from the Federal Meteorological Service, Germany, but revealed no ecological risk factors associated with the endemic occurrence of F. tularensis [Reference Pikula18]. No data were available on hare or rodent populations or the presence of vectors like ticks. Because the study region in the vicinity of Leutkirch shared no geographical features associated with a higher risk of long-term persistence of F. tularensis in the environment [Reference Splettstoesser8, Reference Pikula18], we expected an equivalent seroprevalence as in the national survey (0·23%), or even less. To our surprise the frequency of positive samples exceeded this value by more than tenfold. The reason for this result is currently unknown, but as all results were generated in the same laboratory with the same test procedures, methodological reasons can be excluded.
Differences in stratified subpopulations gave further evidence that the seroprevalence of 2·32% was not due to a lack of specificity of the assay combination. Markedly, the seropositive rate seemed to be dependent on the duration of residency. A lower percentage in residents living for more than 10 years in the Leutkirch area would be well in line with the assumption that this area is a low-risk region. However, on the other hand, that would mean that the seroprevalence of tularemia might even be significantly higher than 0·23% or even 2·32% in other vicinities of Germany.
In Norway, tularemia is also a common disease in small rodent and hare populations, in which large outbreaks can be observed. In humans, the yearly number of cases is low, usually less than 10. Nevertheless, serological investigations on hunters and healthy schoolchildren indicate, with up to 4·70% positive results in the latter group, that F. tularensis low-grade infection is widespread [Reference Berdal19]. Similar data were reported from Austria and Poland, when either hunters or healthy forest workers were investigated [Reference Deutz20, Reference Rastawicki21]. Seropositivity was 3·0% in 149 hunters from the provinces of Styria and Burgenland [Reference Deutz20]. In the second study, the prevalence of antibodies to F. tularensis was evaluated in 480 serum samples obtained from healthy forest workers from different regions of Poland. IgA antibodies were detected in 4·60%, IgG antibodies in 3·80% and IgM antibodies in 2·70% of the serum samples [Reference Rastawicki21].
All studies summarized here, coincide with our results and are in accord with the assumption that tularemia may be misdiagnosed and underestimated, especially in areas where the incidence is historically assumed to be very low. This hypothesis is additionally supported by the analysis of the cumulative incidence in all European countries according to the OIE. It is unlikely that in Germany, which shares several geographical as well as ecological features with its neighbouring countries, e.g. France, Austria, or Czech Republic, the presence of F. tularensis in wildlife is really significantly lower than in those states.
In Germany as well as in most other European states, modes of transmission, location of endemic foci or even the definite animal host of F. tularensis are either not well understood or unknown. To gain more insights into the genuine distribution of this highly virulent pathogen, clinical awareness of the disease entity, molecular characterization and typing of recovered strains and the application of sophisticated geographical information systems have to be increased. This is of great importance due to the first indication that climate change might be associated with an extension of endemic foci and an increase of human and animal tularemia [Reference Deutz and Guggenberger22].
ACKNOWLEDGEMENTS
We thank Margot Ehrle, Kerstin Weiß, and Frank Feist for their excellent technical assistance in performing this study and all members of the EMIL Study Group for the initial work.
DECLARATION OF INTEREST
None.






