In rats, acute administration of an amino acid mixture lacking the catecholamine precursors tyrosine and phenylalanine decreases the availability of plasma tyrosine to the brain. This technique, known as ‘tyrosine depletion’, diminishes brain catecholamine synthesis and attenuates the dopamine-releasing properties of the psychostimulant drug amphetamine (Reference McTavish, Cowen and SharpMcTavish et al, 1999a ). The ability of amphetamine to release dopamine has been linked to its psychostimulant effects (Reference Sharp, Zetterstrom and LjundbergSharp et al, 1987), and the latter have been employed as a model for clinical mania (Reference Jacobs and SilverstoneJacobs & Silverstone, 1986). The present study was designed to explore the effects of tyrosine depletion in humans, where we predicted that a tyrosine-free amino acid mixture would attenuate subjective and objective psychostimulant effects of intravenous methamphetamine in healthy subjects and would diminish the intensity of the manic syndrome in acutely ill patients.
METHOD
Subjects
We studied 16 healthy volunteers (8 men and 8 women) with no previous history of psychiatric disorder or substance misuse. Their mean age was 28 years (range 21-45 years). We also studied 20 psychiatric inpatients (12 men, 8 women) of mean age 37 years (range 18-58 years) who met DSM-IV (American Psychiatric Association, 1994) criteria for manic episode in a bipolar 1 disorder. All had suffered previous episodes of mania. All subjects gave written informed consent to the study, which was approved by the local ethics committee.
Experimental design
Methamphetamine study
The volunteer study was conducted in a double-blind, randomised, crossover fashion. Subjects were tested on two occasions, at least one week apart, with the following regimes balanced for order across subjects: amino acid mixture balanced with tyrosine/phenylalanine (BAL) plus intravenous methamphetamine (0.15 mg/kg); and amino acid mixture deficient in tyrosine/phenylalanine (TYR-free) plus intravenous methamphetamine.
Based on Sheehan et al (Reference Sheehan, Tharyan and McTavish1996), the composition of the TYR-free mixture for male subjects was: isoleucine, 15 g; leucine, 22.5 g; lysine, 17.5 g; methionine, 5 g; valine, 17.5 g; threonine, 10 g; and tryptophan, 2.5 g. The BAL mixture contained additionally 12.5 g of tyrosine and 12.5 g of phenylalanine. Female subjects received drinks of the same composition but 20% less by weight of each amino acid than their male counterparts. This was to take account of the mean lower weight of the female subjects (see Reference Sheehan, Tharyan and McTavishSheehan et al, 1996). The amino acids were suspended in tap water that was flavoured with blackcurrant in order to disguise the unpalatable taste of the mixture. This amino acid mixture has been shown to produce significant lowering of plasma tyrosine and phenylanine, with a nadir of 3-5 h (Reference Sheehan, Tharyan and McTavishSheehan et al, 1996).
Subjects came to the laboratory at 08.30 a.m. on the morning of each test, having followed a low-protein diet (total protein content less than 20 g) for the preceding 24 h and fasted from midnight. The aim of this was to maximise the effect of the amino acid mixtures to increase protein synthesis, thereby ensuring maximum utilisation of body stores of tyrosine and phenylalanine following the TYR-free mixture (see Reference Delgado, Charney and PriceDelgado et al, 1990; Reference Smith, Fairburn and CowenSmith et al, 1997). Following their arrival at the laboratory, subjects were cannulated with an indwelling venous cannula and baseline blood samples were taken for amino acid and prolactin estimation. Subjects then completed baseline 10-cm visual analogue scales (VAS) for the following items (rating of 0=not at all; 10=most ever): ‘hungry’, ‘feeling good’, ‘alert’, ‘depressed’, ‘mind-racing’ and ‘buzz’. A measure of sustained attention was performed at baseline, prior to the ingestion of the allocated amino acid mixture. The VAS ratings were repeated at 30-minute intervals, together with the venous blood samples. The methamphetamine (0.15 mg/kg) was administered 4 h after ingestion of the amino acid mixture, when VAS ratings were continued at the increased frequency of 15-minute intervals for a further 2 h. Performance in the test of sustained attention was assessed again 15 min after methamphetamine administration.
Plasma was separated by centrifugation and stored at −30°C. Prolactin levels were measured using a standard immunoradiometric assay (reagents provided by Netria, London) with inter— and intra-assay coefficients of variation of 4.8% and 2.4%, respectively. The plasma concentrations of neutral amino acids (leucine, isoleucine, phenylalanine, valine, tryptophan, tyrosine and phenylalanine) were measured by an automated high-performance liquid chromatography (HPLC) system with fluorescence end-point detection and pre-column sample derivatisation adapted from the method of Furst et al (Reference Furst, Pollack and Graser1990). Norvaline was used as an internal standard. The limit of detection was 1.3 pg/ml and inter— and intra-assay coefficients of variation were 13% and 8%, respectively.
The measure of sustained attention was the Rapid Visual Information Processing (RVIP) from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Reference Robbins, James and OwenRobbins et al, 1994). In this task, subjects must respond to three-digit sequences (3-5-7, 2-4-6, 4-6-8) in a continuous stream of digits lasting for 7 min. In an effort to avoid ceiling effects due to practice at the task, digits were presented at the faster rate of 200/min, with nine target sequences per minute. The RVIP provides measures of speed of response (response latency), accuracy (target sensitivity) and inappropriate responding (response bias).
Patient study
Patients were allocated randomly and blindly to receive a single administration of either the TYR-free or BAL mixture described above and observed for the next 6 h. They were given the mixture as early as possible on the morning of the test day. Subjects did not fast prior to the study and prescribed medication and food were not withheld during the test day. Baseline clinical state was rated using the Young Rating Scale (Reference Young, Biggs and ZieglerYoung et al, 1978), whereas change during the study was measured with the Beigel Manic State Rating Scale, a scale designed to be sensitive to clinical changes over short time periods (Reference Beigel, Murphy and BunneyBeigel et al, 1971) before mixture administration and hourly for the following 6 h. The blinded rater also guessed at the mixture administered, based on a global clinical impression of change in mental state.
Statistical analysis
In the methamphetamine study, the primary VAS measure was ‘mind-racing’ because a pilot study indicated that this effect of amphetamine was the most sensitive to tyrosine depletion (Reference McTavish, McPherson and SharpMcTavish et al, 1999b ). For all VAS measures, the time of methamphetamine administration was used as a baseline. The VAS scores for each subsequent time point were measured as a change from baseline and these values were summed to give a measure of total response over the 2-h period following methamphetamine. Mean total responses for each VAS following the BAL v. TYR-free mixtures were compared using two-tailed paired t-tests. The amino acid measurements also were compared by paired t-tests (two-tailed), whereas prolactin levels were analysed with a two-way repeated-measures analysis of variance (ANOVA). The RVIP variables also were analysed using two-way repeated-measures ANOVA.
In the patient study, the Beigel scores were analysed as percentage change from baseline and compared using a repeated-measures ANOVA with ‘time’ (time of rating) as the main within-subject factor and ‘mixture’ (TYR-free or BAL) as the between-subject factor. A χ 2 test was used to analyse the prediction of the mixture received.
RESULTS
Methamphetamine study
Four hours after administration of the TYR-free mixture, mean (s.e.) plasma tyrosine levels had fallen substantially (54.0 (s.e. 42) to 18.8 (s.e. 2.5) nmol/l, P < 0.001). In contrast, after the BAL mixture the tyrosine levels increased (53.8 (s.e. 4.3) to 182 (s.e. 14.5) nmol/l, P < 0.001). The ratio of plasma concentrations of tyrosine+phenylalanine to other large neutral amino acids (a key determinant of tyrosine and phenylalanine brain entry; Reference PardridgePardridge, 1977) fell after both mixtures but the decrease was much greater (about 95%) after the TYR-free mixture than after the BAL mixture (about 40%). The mean (s.e.) ratio at 4 h was 0.008 (s.e. 0.002) after the TYR-free mixture and 0.120 (s.e. 0.01) after the BAL mixture (P < 0.001).
There were no significant differences on any of the VAS measures 4 h after ingestion of the two amino acid mixtures (P > 0.1). However, following administration of methamphetamine the subjective psychostimulant effects were lower following the TYR-free mixture than the BAL mixture and significantly so for ratings of ‘mind-racing’ and ‘buzz’, with a trend to significance on ‘alert’ (Fig. 1). Prior to administration of methamphetamine, plasma prolactin levels were significantly greater following the TYR-free mixture, as shown by a significant time × mixture interaction on the ANOVA (F=6.88, P < 0.001) (Fig. 2).
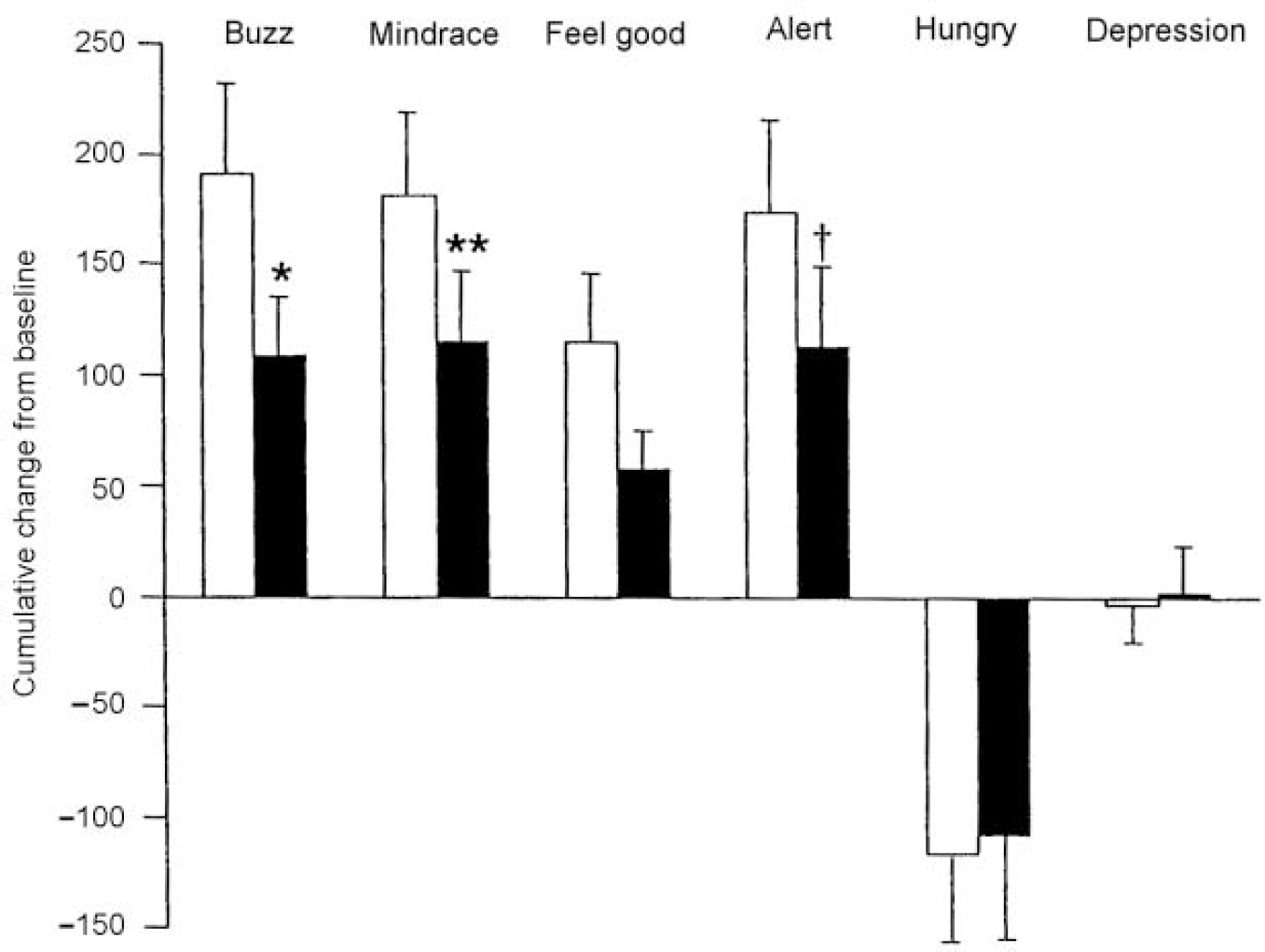
Fig. 1 Subjective ratings of mood and experience (using visual analogue rating scales) following methamphetamine (0.15 mg/kg i.v.). Dark bars: following administration of the TYR-free mixture; light bars: following administration of the BAL drink. These data are presented as the cumulative mean change from baseline ± 1 s.e. Statistical comparisons between the two amino acid mixtures are shown: † P=0.055, * P<0.05; ** P<0.001.
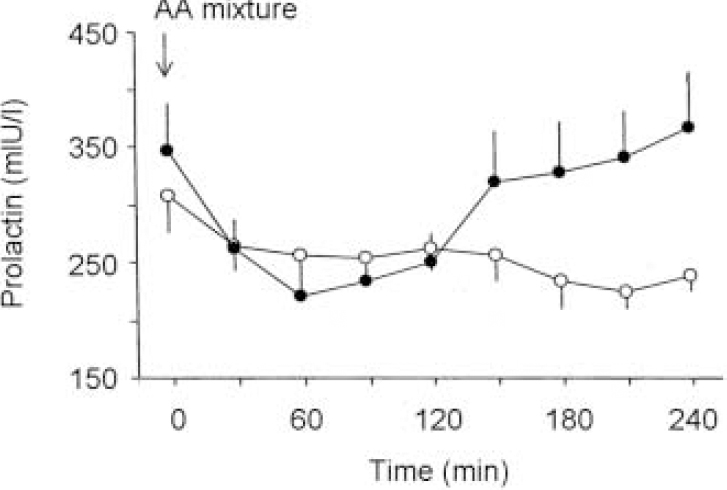
Fig. 2 Mean (s.e.) plasma prolactin (mU/l) in 16 healthy subjects following tyrosine and phenylalanine-free (TYR-free) (filled circles) or nutritionally balanced (open circles) amino acid (AA) mixture given at time zero. The prolactin concentrations after the TYR-free mixture are significantly higher (F=6.88, P<0.001, ANOVA).
Complete cognitive test data were available for 13 subjects. Methamphetamine was found to have significant main effects on RVIP in decreasing the response latency (F=29.28, P < 0.001), and increasing the target sensitivity (F=9.19, P=0.01), and response bias (F=6.60, P=0.025). The effect of methamphetamine on both response latency and response bias was attenuated by the TYR-free mixture, as shown by a significant interaction between time of test and mixture administered (Table 1). In particular, speed and response bias were increased significantly following amphetamine on the occasion when volunteers received the BAL mixture (P < 0.02) but not following the TYR-free mixture (P > 0.05).
Table 1 Effect of amino acid mixtures on cognitive responses to methamphetamine
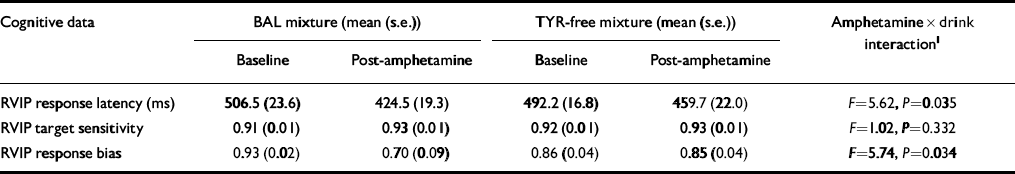
| Cognitive data | BAL mixture (mean (s.e.)) | TYR-free mixture (mean (s.e.)) | Amphetamine × drink interaction1 | ||
|---|---|---|---|---|---|
| Baseline | Post-amphetamine | Baseline | Post-amphetamine | ||
| RVIP response latency (ms) | 506.5 (23.6) | 424.5 (19.3) | 492.2 (16.8) | 459.7 (22.0) | F=5.62, P=0.035 |
| RVIP target sensitivity | 0.91 (0.01) | 0.93 (0.01) | 0.92 (0.01) | 0.93 (0.01) | F=1.02, P=0.332 |
| RVIP response bias | 0.93 (0.02) | 0.70 (0.09) | 0.86 (0.04) | 0.85 (0.04) | F=5.74, P=0.034 |
Patient study
The two treatment groups (TYR-free v. BAL) were well matched for baseline severity of manic symptomatology on the Young Scale (20.3 (s.e. 1.3) v. 19.5 (s.e. 2.0), age (37.4 (s.e. 4.4 and 37.0 (s.e. 2.6 years) and gender (70% v. 50% female). Both groups also were well matched for total daily dose of antipsychotic medication prescribed (400 (s.e. 41) v. 470 (s.e. 31) mg chlorpromazine equivalents per day). In the BAL group three subjects were taking mood stabilisers (one each on carbamazepine, lithium and valproate), as were three in the TYR-free group (two on lithium and one on lithium and valproate).
Relative to the BAL drink, administration of the TYR-free mixture significantly diminished clinical ratings of mania on the total Beigel Scale as revealed by a significant main effect of treatment in the ANOVA (F=4.5, P < 0.05; Fig. 3). In the TYR-free subjects there was a reduction in manic symptomatology of approximately 35% over the 6-h observation period; in contrast, subjects who received the BAL mixture showed little clinical change. This was confirmed by the global clinical impression, whereby prediction of the mixture administered by the blinded rater was correct for 75% of patients (χ2 test, P < 0.05).
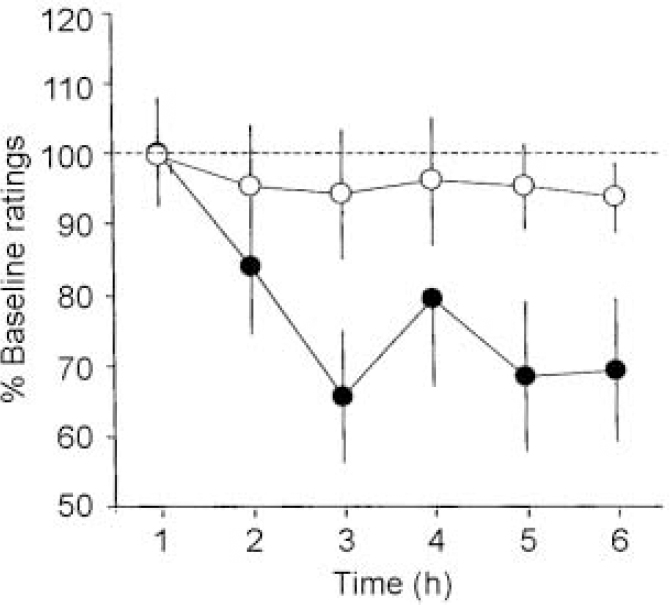
Fig 3 Clinical ratings of manic symptoms using the Beigel Manic State Rating Scale. Filled circles represent the scores of patients receiving the TYR-free drink; open circles represent the scores of patients receiving the BAL amino acid drink. Data are presented as the mean of percentage baseline ratings ± 1 s.e. Administration of the TYR-free drink reduced clinical ratings of mania significantly relative to the BAL drink (main effect of treatment: F=4.5, P<0.05).
In both controls and patients the amino acid drinks generally were well tolerated, with transient nausea being the chief side-effect. No subject vomited and there was no difference in side-effects between the two drinks.
DISCUSSION
Effect of tyrosine depletion on the psychostimulant effects of methamphetamine
The subjective psychostimulant effects of methamphetamine in the volunteers as measured by VAS ratings of ‘mind-racing’ and ‘buzz’ were significantly lower following the TYR-free mixture than the BAL amino acid mixture. A similar trend was seen with ratings of ‘alert’. These data suggest that the TYR-free amino acid mixture may decrease amphetamine-induced dopamine release in humans, as it does in rodents.
The VAS findings of the present study are similar to those obtained in a pilot investigation using oral amphetamine, except on that occasion amphetamine-induced decreases in VAS ratings of ‘depressed’ also were attenuated by the TYR-free mixture (Reference McTavish, McPherson and SharpMcTavish et al, 1999b ). In the current study, however, methamphetamine produced little decrement in the ratings of ‘depressed’ after either mixture (Fig. 1). In humans and animals, amphetamine-induced anorexia probably is mediated via increased release of noradrenaline in the lateral hypothalamus (Reference SilverstoneSilverstone, 1983; Reference Hoebel, Hernandez and SchwartzHoebel et al, 1989). The anorectic effect of methamphetamine was not diminished by the TYR-free mixture, which is consistent with the inability of tyrosine depletion to lower amphetamine-induced noradrenaline release in animal studies (Reference McTavish, Cowen and SharpMcTavish et al, 1999a ).
As well as decreasing the subjective effects of methamphetamine, the TYR-free mixture also attenuated some of the effects of methamphetamine on the RVIP test of sustained attention. After receiving methamphetamine, subjects displayed faster response times and were better at detecting targets but made more inappropriate responses. This is consistent with previous research on the psychostimulant effects of amphetamines (Reference KoelegaKoelega, 1993). The effects of methamphetamine on response time and inappropriate responding were attenuated significantly following the TYR-free mixture than the BAL mixture. In contrast, the effect of methamphetamine on target detection was not decreased by the TYR-free mixture. There is evidence from animal studies that the effect of amphetamines on ‘behavioural activation’ (psychomotor speed and impulsivity) is mediated by increased dopamine neurotransmission, whereas the improvement in target detection is mediated by increased noradrenergic function (Reference Cole and RobbinsCole & Robbins, 1992). These findings therefore offer further support that tyrosine depletion specifically attenuated the dopaminergic effects of methamphetamine administration.
Effects of tyrosine depletion on brain tyrosine availability and dopamine function
Ingestion of amino acid mixtures stimulates protein synthesis (Reference Biggio, Porceddu and GessaBiggio et al, 1976). We have shown previously that the TYR-free mixture significantly lowers plasma tyrosine, which probably represents utilisation of endogenous stores of tyrosine and its precursor phenylaline (Reference Sheehan, Tharyan and McTavishSheehan et al, 1996). Amino acids such as tyrosine and phenylalanine are taken up into the brain by a saturable netural amino acid transporter. This means that the brain availability of tyrosine and phenylalanine is determined by the ratio of their concentration in plasma relative to other neutral amino acids (Reference PardridgePardridge, 1977). This ratio was lowered by both mixtures but to a much greater extent by the TYR-free mixture. Thus, relative to the BAL mixture we would expect brain phenylalanine and tyrosine availability to be diminished substantially after the TYR-free mixture. It seems likely that the BAL mixture also reduced the availability of tyrosine and phenylalanine to some extent, making it a rather conservative control. A placebo control therefore would have been useful but was not included in the design because of the ease with which it would have been distinguished from the amino acid mixtures.
In rodents TYR-free amino acid mixtures, although lowering dopamine synthesis, do not decrease extracellular basal dopamine levels (Reference McTavish, Cowen and SharpMcTavish et al, 1999a ). In our subjects, however, plasma prolactin levels, which are determined by dopamine availability in the anterior pituitary (Reference CheckleyCheckley, 1980) were increased significantly by the TYR-free mixture. This suggests that the TYR-free mixture can lower basal dopamine release in humans. The TYR-free mixture in our study did not alter subjective VAS ratings taken just before administration of the methamphetamine, implying that the decrease in dopamine function is not sufficient to produce obvious subjective effects. In addition, we have found that the TYR-free mixture given by itself does not alter significantly the performance on the RVIP (Reference Harmer, McTavish and ClarkHarmer et al, 2001), suggesting a lack of effect on psychomotor function.
Tyrosine depletion and mania
We also obtained preliminary evidence that the TYR-free mixture is capable of attenuating the symptoms of acute mania. As is common in the in-patient treatment of manic illness, our subjects were receiving treatment with antipsychotic drugs at doses likely to produce a high degree of dopamine D2 receptor occupancy (Reference Chou, Zito and VitraiChou et al, 1996; Reference Kapur, Zipursky and JonesKapur et al, 2000). Despite this, they continued to experience clinically significant manic symptomatology that was diminished by tyrosine depletion.
The effect of tyrosine depletion to attenuate manic symptomatology was apparent soon after mixture ingestion. This time course is similar to the effects of tryptophan depletion, which is capable also of producing striking changes in mood over a few hours (Reference Delgado, Charney and PriceDelgado et al, 1990; Reference Smith, Fairburn and CowenSmith et al, 1997). We believe that tyrosine depletion produced a rapid reduction in manic symptomatology in the present study because it augmented the therapeutic effect of D2 receptor blockade, presumably by lowering pre-synaptic dopamine release. This effect might be particularly apparent at highly active synapses (Reference Wurtman, Hefti and MelamedWurtman et al, 1981) and therefore would make the reduction in neurotransmission at D2 receptors more complete. Alternatively, tyrosine depletion could act by decreasing neurotransmission at other dopamine receptor subtypes that are not subject to significant blockade by antipsychotic medication.
It will be important to determine whether the TYR-free mixture can produce antimanic effects in unmedicated patients and whether its antimanic activity can be sustained over clinically relevant time periods. In the current study, it was thought impractical to place patients on a low-protein diet prior to the amino acid drink, but differences in nutritional status could lead to variability in the effects of the TYR-free mixture. In addition, patients were studied early in their admission so relatively few were taking mood-stabilising drugs. Conceivably the effect of tyrosine depletion could be less in such subjects. These factors and the small number of patients studied mean that our findings should be regarded as pilot data that need to be extended.
Clinical implications
Taken together, our data suggest that the TYR-free amino acid mixture is capable of attenuating pathological increases in dopamine neurotransmission following amphetamine administration and in mania. These findings offer the intriguing possibility that nutritional strategies aimed at lowering tyrosine availability may be a novel means of augmenting the treatment of psychiatric disorders, such as schizophrenia and mania, in which overactivity of dopamine pathways may play a key role in pathophysiology (Reference Jacobs and SilverstoneJacobs and Silverstone, 1986; Reference LaruelleLaruelle, 1998). This application might extend to disorders of misuse of drugs such as amphetamine and cocaine, where increases in dopamine neurotransmission underpin both reinforcing effects and also perhaps cue-associated craving, an important factor in provoking relapse from abstinence (Reference Pilla, Perachon and SautelPilla et al, 1999).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Acute reduction in tyrosine availability to the brain may influence aspects of brain dopamine function in humans.
-
• Tyrosine depletion may have the ability to attenuate pathological increases in dopamine activity.
-
• Nutritional strategies that limit tyrosine availability may be useful adjuncts in the treatment of disorders associated with increased dopamine neurotransmission.
LIMITATIONS
-
• There is currently no direct evidence in humans that tyrosine depletion lowers dopamine neurotransmission.
-
• The effects of tyrosine depletion in the mania patients may be dependent on the presence of concomitant antipsychotic drug treatment.
-
• It is uncertain whether the dopamine-modifying effects of tyrosine depletion will persist with repeated treatment.
Acknowledgements
We thank G. Campling, M. Clements and J. Odontiadis for technical assistance and R. Hockney for nursing care.







eLetters
No eLetters have been published for this article.