Both dietary fibre and protein have been demonstrated to reduce postprandial glycaemia(Reference Karamanlis, Chaikomin and Doran1, Reference Pick, Hawrysh and Gee2). This suggests that the addition of fibre and protein to high-carbohydrate foods may assist with acute glycaemic control in type 2 diabetes. Lupin flour, derived from lupin endosperm, is a legume product containing 25–30 % fibre and 40–45 % protein, with negligible sugar and starch. Lupin flour has been successfully incorporated into a range of food products. However, its effects on glycaemia, particularly in type 2 diabetes, have not been widely studied.
Among non-diabetic adults, consumption of bread enriched with lupin flour reduced blood glucose levels, with a concomitant increase in postprandial insulin levels, in one study(Reference Hall, Thomas and Johnson3) but not in another(Reference Lee, Mori and Sipsas4). The incorporation of fibre from lupin into high-carbohydrate foods appears to have only weak effects on glycaemia. In a study of non-diabetic adults, postprandial blood glucose was unaffected and insulin was decreased compared with control(Reference Johnson, McQuillan and Sin5), while there were no effects on glucose or insulin in a study of adults with non-insulin-dependent diabetes mellitus(Reference Feldman, Norenberg and Voet6).
There is evidence that particular components of lupin may have anti-hyperglycaemic effects. Conglutin-γ, a protein contained in lupin seed, exerts an insulin-like action(Reference Terruzzi, Senesi and Magni7) and reduces glycaemia in hyperglycaemic rats in a dose-dependent manner(Reference Magni, Sessa and Accardo8). Lupin also contains various phytochemicals and amino acids that have been suggested to assist in the reduction of postprandial glycaemia(Reference Hall, Thomas and Johnson3). The unique combination of nutritive components in lupin raises the possibility that its effects on glycaemia may differ from other nutritional sources of protein and fibre. There is some evidence that glycaemia is affected differentially by different sources of protein and fibre(Reference Feldman, Norenberg and Voet6, Reference Jenkins, Wolever and Wong9). Soya is a legume, similar to lupin, and is a commonly used food ingredient. Soya-based products have been shown to reduce glycaemia in healthy adults(Reference Blair, Henley and Tabor10), although its effects have not been compared with lupin.
The aim of the present study was to assess the acute effects of a lupin-based beverage on glucose and insulin responses in type 2 diabetic subjects, and to compare these effects with those of a soya-based beverage matched for macronutrients, primarily fibre and protein. We hypothesised that the lupin and soya beverages would lower the acute glycaemic response compared with the control beverage. Additionally, we hypothesised that lupin, which contains conglutin-γ, would lower postprandial glycaemia more than soya.
Materials and methods
Participants
We recruited individuals with type 2 diabetes, aged 35–65 years, from the general population using newspaper advertisements. The diagnosis of diabetes was confirmed by either a fasting serum glucose ≥ 7 mmol/l, the participant's registration with the National Diabetes Services Scheme (which requires a medical diagnosis of diabetes), or a communication with the participant's general practitioner (with a review of relevant clinical data). Exclusion criteria included type 1 diabetes, duration of diabetes >10 years; insulin use; glycated Hb >9 %; change in body weight >10 % in the previous 6 months; cigarette use in the previous 6 months; daily ethanol consumption >30 g for females or >40 g for males; known allergy to lupin, nuts, soya, dairy, wheat or gluten; other major chronic illness; change in regular prescription medications in the previous 3 months. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Royal Perth Hospital Ethics Committee (Perth, WA, Australia). Written informed consent was obtained from all subjects. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN: 12609000375257).
Study design
In this randomised, controlled, cross-over trial, eligible participants attended three testing sessions, each 7–14 d apart. At each study visit, participants consumed a beverage containing glucose (control), glucose plus lupin (lupin) or glucose plus soya (soya). The test beverages were administered in a random order assigned by computer-generated random numbers concealed in opaque envelopes that were opened sequentially by the chief investigator. Participants were not told which beverage they received at each visit. Participants were requested to maintain their usual diet, physical activity and medications for the duration of their involvement in the study. To avoid a second-meal effect(Reference Wolever, Jenkins and Ocana11), participants were asked to consume the same meal on the evening before each visit. Participants were also asked not to consume alcohol or engage in vigorous physical activity for 24 h before each visit. Anti-hyperglycaemic medications were withheld on the morning of each study visit.
Participants attended each study visit at 08.00 hours following a 12 h fast. An intravenous cannula was inserted into the antecubital vein through which a single baseline blood sample was drawn after a rest period of 15 min from cannula insertion. Participants then consumed the test beverage over 5–10 min. Further blood samples were drawn at 15, 30, 45, 60, 90, 120, 180 and 240 min after beverage consumption. After the final blood sample was collected, the cannula was removed and participants were provided with a meal.
Test beverages
The three test beverages contained 50 g glucose (control), 50 g glucose plus lupin flour (lupin) or 50 g glucose plus soya protein and fibre isolates (soya). All three beverages were matched for total volume and carbohydrate and fat content, and the lupin and soya beverages were matched for protein and fibre content (Table 1).
Table 1 Ingredients and energy and nutrient composition of the control, lupin and soya test beverages
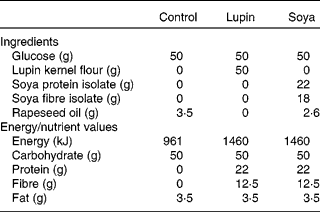
The beverages were prepared at the beginning of each test day. Glucose powder (Glucodin; Reckitt Benckiser, West Ryde, NSW, Australia) was dissolved in 50 ml of boiling water and prepared as a lemon-flavoured cordial (made with 0·5 g citric acid, 0·5 g tartaric acid and 3·5 ml lemon juice) in order to mask the flavour and improve the palatability of the beverages. This mixture, containing 50 g of glucose, was used as the base for all three test beverages. Following cooling of the glucose mixture to room temperature, water at room temperature was added to a total volume of 600 ml. To make the lupin beverage, 50 g lupin flour were added to the beverage. Lupin flour was finely milled from the dehulled kernels of Australian sweet lupin (Lupinus angustifolius) sourced from the Department of Agriculture and Food, Western Australia. To make the soya beverage, 22 g of protein from soya protein isolate and 12·5 g of fibre from soya fibre isolate were added to the 600 ml glucose and water mixture. Isolates of soya fibre (Fibrim 1020 IP) and protein (Supro XT 219D IP) were provided by Solae Australia (Chatswood, NSW, Australia). Rapeseed oil was added to the soya and control beverages to match the fat content of the lupin beverage. The final macronutrient content and ingredients in the beverages are shown in Table 1. The lupin and soya beverages were homogenised for 30 s before presentation to the participants in a translucent drink bottle. Immediately after finishing the beverage, each participant added a further 200 ml of water to the drink bottle and drank this to ensure that all the test ingredients were consumed.
Biochemical analyses
Venous blood was collected into serum tubes and centrifuged at 1500 g for 10 min at 4°C, and aliquots were stored at − 80°C until analysed. Glucose was measured by a hexokinase method using a fully automated analyser (Hitachi 917; F. Hoffmann-La Roche Ltd, Basel, Switzerland), with an inter-assay CV of < 3 %. Insulin was measured by ELISA (Boehringer Mannheim, Mannheim, Germany), with an inter-assay CV of < 2 %. C-peptide was measured using a solid-phase, two-site chemiluminescent immunometric assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) with an inter-assay CV of < 5 %. All samples from each individual were analysed in the same assay to minimise variability.
Statistics
Statistical analyses were performed using SPSS 15.0 (SPSS, Inc., Chicago, IL, USA) and STATA 10 (StataCorp, College Station, TX, USA). The primary endpoints of interest were the 4 h post-beverage serum glucose, insulin and C-peptide responses. Differences between baseline values and between peak values for each treatment were assessed using ANOVA with Tukey's adjustment for multiple comparisons. A random-effects linear model was fitted to observed data for each variable (glucose, insulin and C-peptide). Each model consisted of a random intercept and slope to account for individual participant variability due to within-participant correlations. The models also contained fixed-effects for treatment group, time as a categorical variable with nine categories (0, 15, 30, 45, 60, 90, 120, 180 and 240 min), treatment order and treatment period. Treatment order and period were removed from the final models. Mean post-beverage differences between treatments were assessed with a mixed-effects model that included a random intercept, main effects for treatment and main effects for time.
Results
Participant characteristics
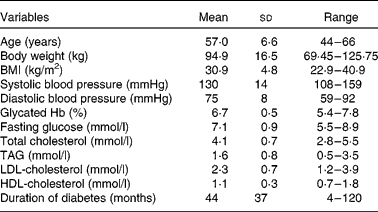
Of the twenty-nine eligible participants randomised to treatment, twenty-four (nineteen men and five women) completed the study (Fig. 1); three participants withdrew for personal reasons; one because of illness; one because of difficulty obtaining blood samples. The characteristics of the study participants are shown in Table 2. Of the twenty-four participants, eight (33 %) were on diet treatment alone for their diabetes, fourteen (58 %) were treated with metformin, one (4 %) was taking gliclazide and one (4 %) was prescribed both metformin and gliclazide. Other concomitant therapies included anti-hypertensive (79 %) and cholesterol-lowering (58 %) medications.

Fig. 1 Trial profile showing the number of participants at each stage of study recruitment and completion.
Table 2 Participant characteristics
(Mean values, standard deviations and ranges, n 24)
Serum glucose, insulin and C-peptide responses
At baseline, the mean fasting glucose (control, 7·4 (sd 1·1) mmol/l; lupin, 7·4 (sd 1·2) mmol/l; soya, 7·5 (sd 1·2) mmol/l), insulin (control, 14 (sd 7) mU/l; lupin, 13 (sd 7) mU/l; soya, 14 (sd 7) mU/l) and C-peptide (control, 1·1 (sd 0·4) mmol/l; lupin, 1·0 (sd 0·4) mmol/l; soya, 1·0 (sd 0·4) mmol/l) concentrations were not different according to the test beverage (P>0·5). The glucose, insulin and C-peptide concentrations measured over 4 h post-beverage consumption are presented in Fig. 2. The glucose response was lower for lupin and soya (P < 0·001) compared with control but was not significantly different between lupin and soya (P = 0·25; Fig. 2(a)). The insulin response was higher for lupin and soya compared with control (P < 0·001), and lower for lupin compared with soya (P = 0·013; Fig. 2(b)). The C-peptide response was higher for lupin and soya compared with control (P < 0·001), but the difference between lupin and soya did not reach significance (P = 0·07; Fig. 2(c)). Peak glucose concentrations were attained 45 min after ingestion of each beverage (control, 13·0 (sd 1·7) mmol/l; lupin, 11·6 (sd 1·7) mmol/l; soya, 11·1 (sd 2·0) mmol/l) and were lower for lupin and soya (P < 0·001) compared with control but not significantly different between lupin and soya (P = 0·13).
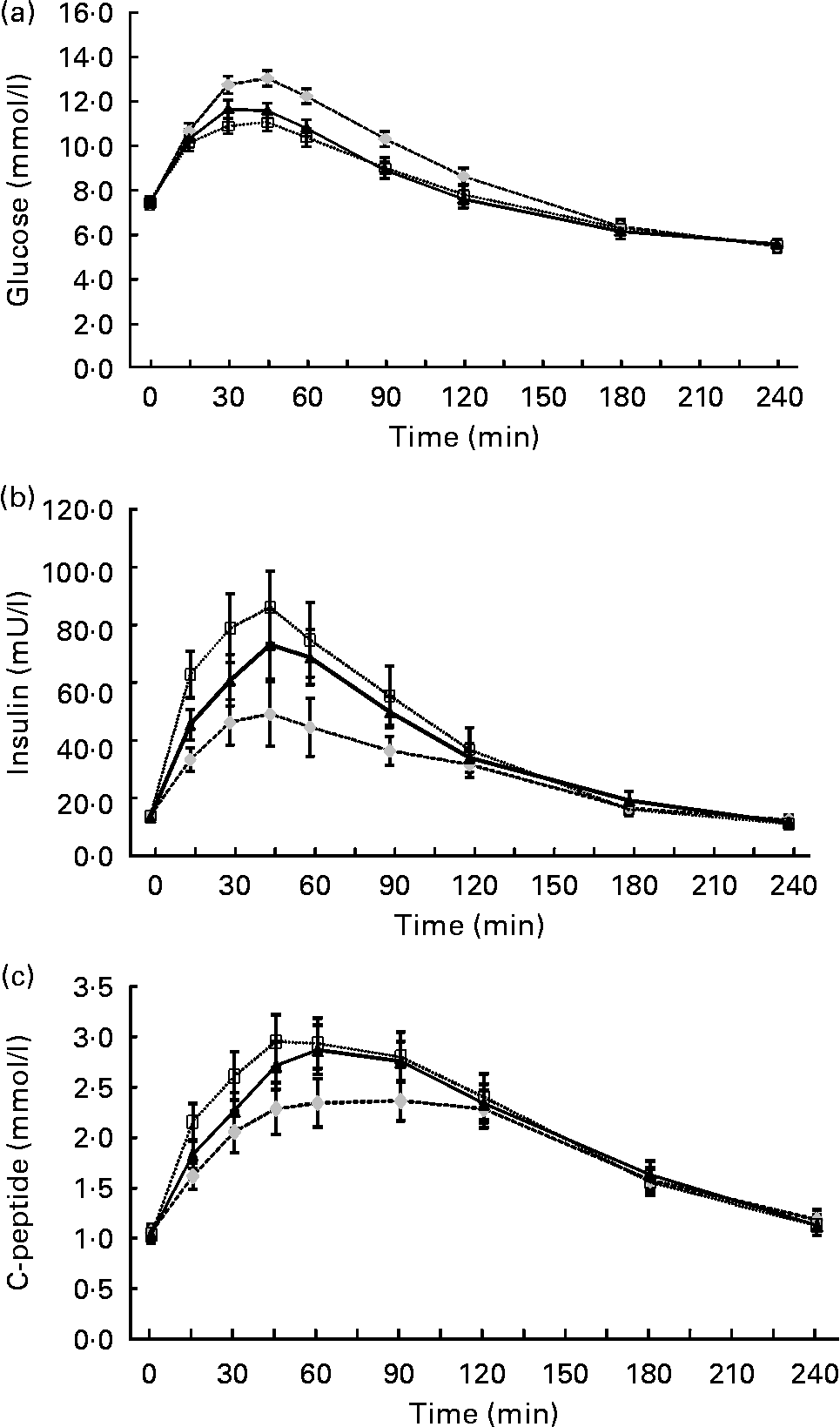
Fig. 2 (a) Serum glucose, (b) insulin and (c) C-peptide concentrations in 4 h after consumption of the control (![]() ), lupin (
), lupin (![]() ) and soya (
) and soya (![]() ) beverages (n 24). Values are means, with their standard errors represented by vertical bars (with treatment effects analysed using mixed models). Mean values of post-beverage glucose levels over 4 h were significantly different for lupin and soya compared with control (P < 0·001), but not significantly different between lupin and soya (P = 0·25). Mean values of post-beverage insulin levels were significantly different for lupin and soya compared with control (P < 0·001), and significantly different for lupin compared with soya (P = 0·013). Mean values of post-beverage C-peptide levels were significantly different for lupin and soya compared with control (P < 0·001), but not significantly different between lupin and soya (P = 0·07).
) beverages (n 24). Values are means, with their standard errors represented by vertical bars (with treatment effects analysed using mixed models). Mean values of post-beverage glucose levels over 4 h were significantly different for lupin and soya compared with control (P < 0·001), but not significantly different between lupin and soya (P = 0·25). Mean values of post-beverage insulin levels were significantly different for lupin and soya compared with control (P < 0·001), and significantly different for lupin compared with soya (P = 0·013). Mean values of post-beverage C-peptide levels were significantly different for lupin and soya compared with control (P < 0·001), but not significantly different between lupin and soya (P = 0·07).
Discussion
Consistent with our first hypothesis, the acute glycaemic response to a carbohydrate-rich beverage in individuals with type 2 diabetes was reduced with the addition of lupin and soya. Our second hypothesis was not supported – we found that both lupin and soya reduced glycaemia to a similar degree. Both lupin and soya increased insulin levels post-beverage compared with control, but lupin had a lesser effect compared with soya.
The present results are consistent with those reported by Hall et al. (Reference Hall, Thomas and Johnson3) in non-diabetic individuals. The reduction in glycaemia resulting from lupin and soya may be attributed to several factors, including an increase in viscosity, an increase in gastrointestinal solids, a delay in gastric emptying time, the presence of hypoglycaemic phytochemicals and the increased protein and/or fibre content in the legume-based beverages. As Hall et al. (Reference Hall, Thomas and Johnson3) observed, further research is needed to elucidate the role of each of these factors in reducing glycaemia(Reference Hall, Thomas and Johnson3).
It seems likely that the protein content of the lupin and soya beverages contributed significantly to their glucose-reducing effects. We postulate this based on the substantial increase in insulin and C-peptide in response to both lupin and soya that we observed. Dietary protein enhances insulin secretion in both healthy and diabetic individuals(Reference Karamanlis, Chaikomin and Doran1, Reference Gannon, Nuttall and Neil12). Supporting this, studies using isolated fibre from lupin(Reference Johnson, McQuillan and Sin5) and oat gum(Reference Braaten, Wood and Scott13) have demonstrated a decrease in insulin levels compared with a control meal. There are several mechanisms by which protein may attenuate postprandial hyperglycaemia; these include slowing of gastric emptying and intestinal delivery and absorption of glucose(Reference Karamanlis, Chaikomin and Doran1), and stimulation of insulin secretion via (i) an effect on incretin hormones(Reference Karamanlis, Chaikomin and Doran1) or (ii) an effect of amino acids on pancreatic β-cells(Reference Nuttall and Gannon14). We did not assess these specific mechanisms in the present study.
The present results suggest that there is no difference in the extent to which lupin and soya have a glucose-reducing effect, and this is probably due to similarities between lupin and soya with respect to properties of their protein and fibre. Few studies have directly compared the acute effects of protein from different sources on glycaemia. In one study, whey protein decreased glucose and increased insulin levels more than meat protein in those with type 2 diabetes(Reference Frid, Nilsson and Holst15). Gannon et al. (Reference Gannon, Nuttall and Neil12) studied the effects of protein from a variety of sources, including beef, turkey, gelatin, egg-white, cottage cheese, fish and soya, on glucose and insulin responses in type 2 diabetic individuals and concluded that, though there was variation in the magnitude of the effects, all resulted in raised insulin and decreased blood glucose levels (except egg-white). The reason that some studies do show differences, whereas others do not, may relate to the ability to detect very small differences. The present results are generally consistent with previous findings and indicate little, if any, difference between lupin and soya on the glucose response.
The dietary fibre content of lupin and soya may also have contributed to the reduction in glycaemia compared with control. Many studies have shown that soluble fibre improves glycaemia in type 2 diabetic individuals, probably by slowing gastric emptying and decreasing glucose absorption(Reference Pick, Hawrysh and Gee2, Reference Brennan16). However, lupin(Reference Evans and Cheung17) and soya(Reference Burkhalter, Merchen and Bauer18) contain predominantly insoluble fibre, and although insoluble fibre has been shown to reduce glycaemia in some studies(Reference Hamedani, Akhavan and Samra19, Reference Samra and Anderson20), only small effects on glycaemia have been shown in studies of healthy and diabetic individuals consuming lupin kernel fibre(Reference Johnson, McQuillan and Sin5, Reference Feldman, Norenberg and Voet6) and soya fibre(Reference Tsai, Mott and Owen21, Reference Tsai, Vinik and Lasichak22). Therefore, the glycaemic effects of the legume-based beverages are likely to be more attributable to their increased protein rather than increased fibre content.
Control of hyperglycaemia following food and beverage consumption is an important treatment goal in diabetes management. Postprandial glycaemia may contribute more than fasting glycaemia to overall glycaemic control, especially at lower glycated Hb levels(Reference Fonseca23–Reference Woerle, Neumann and Zschau25). There is now a large body of evidence, including observational prospective cohort studies, randomised controlled trials, and mechanistic studies in animal models, which supports the consumption of low-glycaemic index foods and diets for the prevention of type 2 diabetes and CVD(Reference Potenza, Gagliardi and Nacci26). Postprandial hyperglycaemia, by increasing oxidative stress, endothelial dysfunction and advanced glycation(Reference Potenza, Gagliardi and Nacci26), may also be an independent risk factor for CVD(Reference Cavalot, Petrelli and Traversa27). There is some evidence that therapies targeting postprandial glycaemia may reduce cardiovascular events(Reference Chiasson, Josse and Gomis28, Reference Hanefeld, Cagatay and Petrowitsch29). Therefore, dietary intake of legumes such as lupin and soya that reduce postprandial glycaemia may assist in the glycaemic management and prevention of diabetic complications and CVD. This may be most effective in early diabetes when β-cell function and endogenous insulin secretory capacity are less impaired. The subjects in the present study had diabetes of relatively short-to-medium duration, and the glycaemic effects of legumes may be more limited in patients with more advanced diabetes.
In the present study, we found that although lupin and soya resulted in the same glycaemic response to a fixed glucose load, lupin resulted in a significantly lower insulin response.
The importance and reliability of this difference in insulin secretion (between lupin and soya) for the long-term management of blood glucose in type 2 diabetic individuals and risk of CVD is not clear. However, it may have implications for the risk of type 2 diabetes and CVD. Hyperinsulinaemia is associated with increased risk of type 2 diabetes(Reference Brand-Miller, Hayne and Petocz30) and CVD(Reference Dickinson and Brand-Miller31). In addition, there is evidence that acute hyperinsulinaemia can cause endothelial dysfunction(Reference Hodge, English and O'Dea32), sympathetic hyperactivity(Reference Jarvi, Karlstrom and Granfeldt33) and reduced Na excretion(Reference Salmeron, Ascherio and Rimm34). In the longer term, these effects may contribute to endothelial dysfunction, hypertension and elevated risk of CVD.
It is unclear why the post-beverage insulin levels in the present study were lower with lupin than with soya. While it is possible that soya protein is more insulinotropic than lupin protein, the results may also be related to differences in the presentation of the soya and lupin proteins in the present study. Lupin protein was presented as part of lupin flour, whereas soya protein was presented as an isolate. It is possible that soya protein isolate may have been digested more quickly or had greater bioavailability than the lupin protein in the milled lupin flour. Whether a component of lupin flour, such as conglutin-γ(Reference Magni, Sessa and Accardo8), might have also contributed to reducing blood glucose levels through insulin-mimetic effects(Reference Terruzzi, Senesi and Magni7) merits further investigation. There have been no previous studies that have compared the effects of lupin and soya protein isolates on glycaemia and insulin levels.
It is not known whether the beneficial glycaemic effects of legumes are sustained in the long term. Population studies are consistent in demonstrating benefits of low-glycaemic index foods and low-glycaemic index diets for glycaemic control and insulin sensitivity in type 2 diabetes, and insulin sensitivity and risk of developing type 2 diabetes in healthy subjects(Reference Brand-Miller, Hayne and Petocz30–Reference Schulze, Liu and Rimm36). The evidence that short-term regular consumption of legumes(Reference Jang, Lee and Kim37, Reference Nestel, Cehun and Chronopoulos38) improves insulin sensitivity and glycaemic control is mixed, and may depend on the type of legume and the health status of the population studied. A recently published longer-term intervention study of postmenopausal Chinese women with pre-diabetes and untreated early diabetes found no effect of soya protein on glycaemic control and insulin sensitivity over 6 months(Reference Liu, Chen and Ho39). The evidence for the benefit of high-protein diets on insulin sensitivity and glycaemic control in type 2 diabetes is mixed. Some studies have reported improved insulin sensitivity, and others have reported poorer insulin sensitivity and diabetic control(Reference Boden, Sargrad and Homko40–Reference Samaha, Iqbal and Seshadri44).
A limitation of the present study is that lupin and soya were provided as beverages rather than solid foods. Although lupin flour is more often consumed in solid foods, it could be used to supplement or fortify beverages in the future. Soya protein, in particular, and fibre are already commonly used in ‘soya milks’. In the present study, it was necessary to use beverages in order to investigate the effects of lupin on the glucose and insulin responses to a fixed dose of carbohydrate. We have previously conducted an acute trial that examined the effects of a lupin-enriched meal of solid food (bread), which was energy matched with a control meal, on post-meal glucose and insulin responses(Reference Lee, Mori and Sipsas4). We observed a significant reduction in the glucose response and a decrease in the insulin response at a trend level (P = 0·06). However, given the large reduction in the provision of glycaemic carbohydrate, because lupin flour partially replaced wheat flour in bread, the impact of lupin flour itself, rather than the reduction in carbohydrate, on glucose and insulin responses was unclear. To understand the effects of lupin flour on glucose and insulin responses, it is necessary to match the dose of carbohydrate. The present findings provide evidence that lupin flour itself is responsible for the effects on both glucose and insulin independent of the glycaemic load.
We conclude that adding lupin or soya to an oral carbohydrate load reduces glycaemia acutely. Lupin has similar glycaemic effects to soya but with less insulin stimulation. The results of the present study add to our understanding of the acute effects of lupin and soya on glucose and insulin responses. It would be beneficial for other studies to examine the effects of conglutin-γ on glycaemia in human subjects. Investigation of the longer-term effects of regular consumption of a lupin-enriched diet on glycaemic control and insulin sensitivity is warranted in order to ascertain whether lupin may benefit glycaemic management in type 2 diabetic individuals.
Acknowledgements
None of the authors has any conflict of interest to declare. The present study was supported by the Western Australian Government, Department of Industry and Resources. E. R. D. contributed to the study concept and design, acquisition, statistical analysis and interpretation of the data, and drafting and revision of the manuscript. T. A. M. and J. M. H. contributed to the study concept and design, acquisition, statistical analysis and interpretation of the data, drafting and revision of the manuscript, and obtaining funding. G. T. C. contributed to the study concept and design, medical management of the study participants, interpretation of the data, and drafting and revision of the manuscript. A. E. B. contributed to the study concept and design, analysis and interpretation of the data and revision of the manuscript. R. J. W. contributed to the analysis and interpretation of the data, and revision of the manuscript. I. B. P. and S. S. contributed to the study concept and design, revision of the manuscript and obtaining funding.






