n-3 PUFA are essential to a vast array of physiological processes. n-3 PUFA intake may play a preventive role for CVD, cognitive decline and some cancers in adults( Reference De Caterina 1 – Reference Stonehouse, Conlon and Podd 3 ). Among children, n-3 PUFA are required for normal cognitive and psychomotor development( Reference Uauy and Dangour 4 ). Long-chain n-3 PUFA, including EPA (20:5n-3) and DHA (22:6n-3), can be obtained from fish or by physiological conversion from the precursor α-linolenic acid (ALA, 18:3n-3), which is abundant in flaxseed, rapeseed and soyabean oils. In many populations where fish intake is low, ALA from vegetable oils should become the most readily available source of n-3 PUFA. Yet, for up to half of the world’s population, n-3 PUFA supply from vegetable oils is also critically limited. An estimated 3·1 billion people live in areas where fish availability is <400 g/week and ALA is <4 % of total vegetable oil( Reference Petrova, Dimitrov and Willett 5 ). In the Americas, Colombia is ranked as the country with the lowest n-3 PUFA intake as a percentage of total energy( Reference Hibbeln, Nieminen and Blasbalg 6 ). The problem is particularly severe in landlocked regions of these countries, where fatty fish is largely unavailable and the ALA content of the most widely consumed vegetable oils does not reach 4 %( Reference Baylin, Mora-Plazas and Cobos-de Rangel 7 ).
The effect on PUFA status of providing fish-oil supplements or altering the PUFA composition of the diet has been examined in randomized trials and controlled-feeding studies, respectively( Reference Hodson, Skeaff and Fielding 8 ). Nevertheless, supplementation may not be a sustainable public health intervention in the long term, especially in low- and middle-income countries where the prevalence of n-3 PUFA deficiency is highest. Furthermore, controlled-feeding studies may not provide a realistic estimate of the impact of achievable, modest dietary changes in free-living populations. Replacing cooking oils of low ALA content with ALA-rich vegetable oils at the household level could be an easy-to-implement, efficacious intervention to improve n-3 PUFA status. We conducted a randomized trial to determine the effect of providing families with a one-month supply of one of two cooking oils, sunflower oil v. soyabean oil, on whole-blood fatty acid composition of children from Bogotá, Colombia. We hypothesized that soyabean oil would result in higher whole blood ALA concentrations than sunflower oil at the end of the intervention.
Methods
Study design and population
We conducted a parallel, randomized intervention of cooking oils among sixty families in Bogotá, Colombia. Families were randomly assigned to receive a one-month supply of either sunflower oil (n30) or soyabean oil (n 30). These sixty families were randomly chosen among participants in an ongoing longitudinal investigation of nutrition and health in Colombia( Reference Arsenault, Mora-Plazas and Forero 9 ). The families in the cohort represent low- and middle-income families in Bogotá. Only families with a single child enrolled in the cohort were eligible to participate in the trial. In addition, eligibility was contingent on using vegetable oil at home as the main cooking fat. After applying these criteria, >80 % of the cohort’s families were eligible to participate( Reference Baylin, Mora-Plazas and Cobos-de Rangel 7 ). The minimal sample size required was determined with the use of a two-sided paired t test to detect a difference in ALA change between the two treatment arms of 0·10 % or more, assuming no effect of the intervention on the standard deviation, α=0·05 and statistical power of 80 %.
Experimental regimens
We aimed to compare the effect on PUFA status of two cooking oil types that were accessible to this population in local markets. Soyabean oil was chosen because of its high ALA content (5·7 %). Sunflower (0·2 % ALA) was selected as the comparison oil because it was one of the preferred cooking fats in this setting according to a preliminary study( Reference Baylin, Mora-Plazas and Cobos-de Rangel 7 ). In order to select the intervention oils, early in 2011 we purchased bottles of all cooking oils available in local markets around the city. These sixty-five oils comprised soyabean (n24), sunflower (n13), rapeseed (n 3), corn (n 1), soyabean/palm mixture (n6), soyabean/sunflower mixture (n2), sunflower/rapeseed mixture (n2) and other vegetable mixture oils unspecified by the manufacturer (n14). The fatty acid composition of these oils was analysed using gas–liquid chromatography, following the protocol described under ‘Laboratory methods’. According to results from these biochemical analyses, we chose the soyabean and sunflower oil brands with the highest concentrations of ALA and linoleic acid (LA, 18:2n-6), respectively, and with the lowest content of trans fat. The two oils ultimately selected had <1·5 % trans fat and distinct concentrations of essential n-3 and n-6 fatty acids (Table 1). Soyabean and sunflower oil bottles differed slightly in shape and external appearance, but the only indication of the oil type contained was on their commercial labels. Hence, we thoroughly removed these labels to achieve masking of participants to oil type during the course of the study.
Table 1 Fatty acid composition of experimental cooking oils
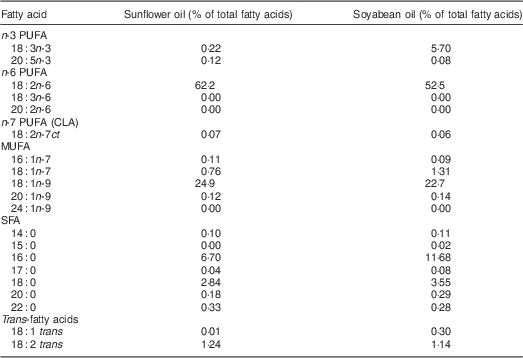
CLA, conjugated linoleic acid.
Recruitment and randomization procedures
We created a list of identification (ID) numbers from 1 to 60. Each number was randomly assigned to either sunflower or soyabean oil with the use of permuted block randomization in blocks of size equal to 4. Between June and July 2011, a trained research assistant recruited the families through a telephone call, assigned each family an ID number and made an appointment for a home visit within one week. All families selected agreed to participate. At these baseline visits, the study team members explained the aims and procedures of the study, answered any questions and obtained written informed consent from the mothers as well as verbal assent from the children to participate. The research was conducted in accordance with guidelines laid down by the Declaration of Helsinki. The study protocol and procedures were approved by the Ethics Committee of Javeriana University in Bogotá, and the Health and Behavioral Sciences Institutional Review Board at the University of Michigan.
Baseline evaluations
At the baseline visit, research assistants administered questionnaires to collect information on sociodemographic characteristics and cooking oil habits. The study dietitian administered a 24 h diet recall to the child, inquiring on intake of all foods and beverages during the prior 24 h following the US Department of Agriculture five-step multiple-pass method( Reference Conway, Ingwersen and Moshfegh 10 ). Anthropometric measurements from the mother and child were performed with the use of standard techniques( Reference Lohman, Roche and Martorell 11 ). Height was measured to the nearest 1 mm with wall-mounted Seca 202 stadiometers (Seca, Hanover, MD, USA) and weight to the nearest 0·1 kg with Tanita HS201 electronic scales (Tanita, Arlington Heights, IL, USA). Next, the research assistant obtained a capillary blood sample from the child, about 80 μl, by puncturing the palmar side of the left ring finger’s distal end with a disposable contact-activated sterile lancet and expressing the blood into an EDTA tube. The sample was placed in a cooler with dry ice and transported on the same day to Javeriana University in Bogotá, where it was cryopreserved at −80°C until transportation to the USA for analyses. Although samples could not be collected after an overnight fast in all children for practical reasons, all were obtained after at least 2 h from the last meal.
At the end of the baseline visit, research assistants delivered two bottles (approximately 3 litres each) of the oil to which each family had been randomly assigned, marked only with the family’s ID number. They measured the volume of oil currently in use at the household and instructed mothers to stop using this oil and replace it with the experimental oil for all food preparation purposes starting immediately. Mothers were also told not to reuse the experimental oils and to request additional oil from the team if the supply initially provided was exhausted. They were also asked to keep all containers of oil used during the study period.
Follow-up visit
A follow-up home visit was carried out after 4 weeks from the baseline visit. At this visit, research assistants administered a new 24 h diet recall to children, and obtained new samples of capillary blood from the children. In addition, the researchers administered a questionnaire to the mothers that inquired about compliance with the experimental oil and its acceptability. Researchers requested to see both the pre-intervention and the experimental oil containers and measured the remaining oil.
Laboratory methods
Fatty acids were quantified in the capillary whole-blood samples collected from the children at baseline and follow-up at Harvard University in the USA. Total fatty acids were chosen because they are responsive to short-term changes in intake as opposed to erythrocyte membrane fatty acids, which have a turnover rate >1 month( Reference Katan, Deslypere and van Birgelen 12 ). Fatty acids were extracted into isopropanol and hexane containing 50 mg of 2,6-di-tert-butyl-p-cresol as an antioxidant and transmethylated with methanol and sulfuric acid, as described by Zock et al.( Reference Zock, Gerritsen and Katan 13 ). After esterification, blood samples were evaporated and the fatty acid methyl esters were redissolved in isooctane and assessed by gas–liquid chromatography as previously described( Reference Baylin, Mora-Plazas and Cobos-de Rangel 7 ). Peak retention times were identified by injecting known standards and purity ranges were all above 99 % (NuCheck Prep, Elysium, MN, USA); Agilent Technologies ChemStation A·08·03 software was used for analysis. Fatty acids are expressed as percentage of total fatty acids in whole blood. The between-run CV of the most abundant fatty acids were low, about 7 % on average. For whole-blood ALA and LA, CV were, respectively, 8·5 % and 6·2 %. For long-chain PUFA CV were somewhat larger: 12·5 % for EPA; 15·6 % for docosapentaenoic acid (DPA, 22:5n-3); 26·7 % for DHA; 10·9 % for dihomo-γ-linolenic acid (DGLA, 20:3n-6); and 10·5 % for arachidonic acid (AA, 20:4n-6).
Data analyses
We compared baseline anthropometric and nutritional characteristics between the treatment groups. Children’s height-for-age and BMI-for-age Z-scores were calculated according to the WHO growth reference( Reference de Onis, Onyango and Borghi 14 ). Nutrient and energy intakes were estimated from the 24 h diet recalls using each food’s nutrient and energy composition values from the US Department of Agriculture’s Standard Reference food composition database, supplemented with data from manufacturers and published reports (Food Processor Software; ESHA Research, Salem, OR, USA), the Composition Table of Colombian Foods by the Colombian Institute of Family Welfare( 15 ) and the Composition Table of Foods from Costa Rica: Fatty Acids( Reference Monge and Campos 16 ).
The analytic strategy followed the intention-to-treat principle. Primary end points were changes in whole-blood fatty acids in the children from baseline to the end of the intervention. These changes were estimated using mixed-effects linear regression models for repeated measures with an unstructured variance specification( Reference Fitzmaurice, Laird and Ware 17 ). Differences and 95 % confidence intervals in change between soyabean and sunflower oils were estimated from these models. Tests with P<0·05 were considered statistically significant.
Because improved availability of cooking oil may lead to increased intake of fried foods, we examined changes in total energy intake and percentage of energy from saturated fat from randomization to the end of the intervention, according to the 24 h recalls. Compliance was assessed by calculating experimental oil disappearance as the difference between the total volume of oil dispensed and the volume remaining at the end of the study. In addition, we estimated disappearance of the pre-intervention oil and asked the mothers whether they had used any of it. Because the external appearance of the experimental oil containers differed slightly from each other, we assessed masking by asking mothers to guess which oil they had been receiving, at the end of the intervention. The proportion of correct guesses was compared between treatment arms with use of Fisher’s exact test. All analyses were carried out with the statistical software package SAS version 9·3.
Results
Children from participating families were 14·5 years old at recruitment, on average (range=10·7–18·3 years). Thirty-seven per cent were boys. There were no differences in demographic, maternal, dietary or household characteristics between treatment groups (Table 2); however, children in the soyabean oil arm had higher height-for-age and BMI-for age Z-scores than children in the sunflower arm. The most common oil in use at the time of study initiation was soyabean oil (53 % of families), followed by unspecified vegetable oil mixtures (23 %) and soyabean/palm oil mixtures (12 %). Only five families were using sunflower oil at recruitment, either alone or in a mixture with another vegetable oil. None of the families were using the same type and brand of oils selected as experimental regimens prior to enrolment. There were no differences in the fatty acid composition of the oils in use before randomization between the two treatment arms (see online supplementary material, Supplemental Table 1). The intervention lasted for a mean 28·2 (sd 0·9) d and 28·1 (sd 0·3) d in the sunflower and soyabean oil arms, respectively (range=28–32 d). The time from the last meal to the collection of the sample did not differ between groups at baseline or at follow-up.
Table 2 Characteristics of participating children and mothers from Bogotá, Colombia, according to cooking oil assignmentFootnote *
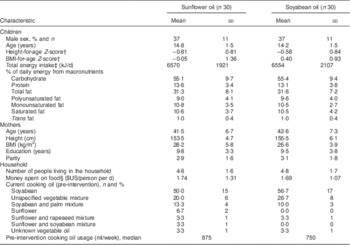
* Values are presented as means and standard deviations unless noted otherwise.
† According to the WHO growth reference for school-aged children and adolescents (5–19 years)( Reference de Onis, Onyango and Borghi 14 ).
‡ From a 24 h diet recall administered to the children prior to randomization.
§ Using an exchange rate of 1755 Colombian pesos=$US 1, as of July 2011.
Children from households assigned to soyabean oil experienced a statistically significant increase of 0·05 percentage points in whole-blood levels of ALA (P=0·03) from randomization to the end of the intervention (Table 3). In contrast, assignment to sunflower oil resulted in an ALA decline of 0·12 percentage points (P<0·0001); hence, the effect of soyabean compared with sunflower oil on ALA was 0·17 percentage points (P<0·0001). Mean DPA and DHA concentrations increased significantly in both groups to the same extent. While there were no statistically significant changes in whole-blood LA concentrations, long-chain n-6 PUFA including DGLA and AA increased significantly and by similar amounts in both groups. Soyabean oil resulted in a 0·04 percentage point increase in conjugated linoleic acid (CLA, 18:2n-7ct) whereas sunflower oil had no effect (P=0·03). Compared with children receiving soyabean oil, those in the sunflower oil arm experienced a greater decrease in whole blood-concentrations of n-7 MUFA. In addition, children in both groups experienced a significant reduction in whole-blood levels of oleic acid (18:1) as well as SFA palmitic acid (16:0) and behenic acid (22:0); these decreases did not differ significantly between oil assignment arms. None of the oils had significant effects on eighteen trans-fatty acids.
Table 3 Effect of sunflower and soyabean cooking oils on Colombian children’s fatty acid concentrations in whole blood
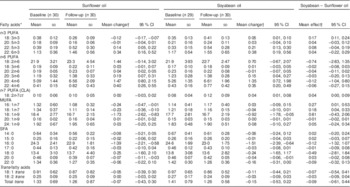
CLA, conjugated linoleic acid.
* Fatty acids are expressed as a percentage of total fatty acids in whole blood.
† Means and 95 % CI from mixed-effects linear regression for repeated measures. An unstructured covariance matrix was specified.
Finally, we examined the effect of the interventions on diet and compliance. None of the interventions resulted in increased total energy, saturated fat (Tables 2 and 4) or other macronutrients intake by the children. Only seven families (12 %) reported reusing the intervention oil on at least one occasion. Compliance with the intervention assignment was high. Families consumed 65 % of the oil volume with which they were provided and virtually none of their pre-intervention oil during the study period. The oils contributed <20 % of daily fat intake. There were no differences in compliance between cooking oil assignment arms. At the end of follow-up, only eight families (13 %) correctly guessed the oil type (sunflower or soyabean) to which they had been assigned. Acceptability of the intervention was high according to the mothers’ ratings of the oil’s taste and their satisfaction cooking with it. A majority of mothers expressed their willingness to replace their pre-intervention oil with the experimental oil and to use it in the long term in the absence of financial constraints (Table 4).
Table 4 Diet, compliance, masking and acceptability at the end of a randomized intervention of sunflower or soyabean cooking oil in Bogotá, Colombia, according to oil assignmentFootnote *
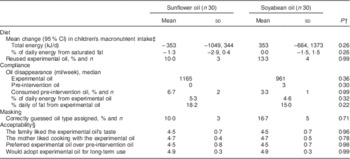
* Values are presented as means and standard deviations unless noted otherwise.
† From Wilcoxon rank-sum and Fisher’s exact tests for continuous and dichotomous variables, respectively.
‡ Differences in intake according to 24 h diet recalls administered before randomization and at the end of the intervention period. Estimates are from mixed-effects linear regression for repeated measures. An unstructured covariance matrix was specified.
§ Scores from Likert-type scales from 1=‘completely disagree’ to 5=‘strongly agree’.
Discussion
In the present randomized investigation, providing low- and middle-income Colombian families with a one-month supply of soyabean oil increased whole-blood ALA levels in their children, while providing sunflower oil decreased them. Whole-blood concentrations of very-long-chain n-3 and n-6 fatty acids increased in all children, irrespective of oil assignment.
A lowering of blood ALA resulting from sunflower oil intake had been reported in some( Reference Lasserre, Mendy and Spielmann 18 , Reference Valsta, Salminen and Aro 19 ) but not all( Reference Hussein, Ah-Sing and Wilkinson 20 ) previous investigations. In the present study, a sunflower oil-related decrease in blood ALA can be explained because about one-half of the families were using soyabean oil, a much richer source of ALA, before randomization. Soyabean oil increased blood ALA only modestly, possibly because a large proportion of families were already consuming it before the intervention. The increase may have been small also because the oils contributed only <20 % of daily fat intake.
Of note, both oils increased whole-blood concentrations of the very-long-chain n-3 fatty acids DPA and DHA. In the soyabean oil arm, increases in blood DPA and DHA coincided with a modest rise in the precursor ALA, while there was no change in EPA. In the sunflower oil arm, blood DPA and DHA increased despite a decline in ALA and no change in EPA, in contrast to previous dietary interventions of unconfined( Reference Valsta, Salminen and Aro 19 ) or confined( Reference Lasserre, Mendy and Spielmann 18 ) women where sunflower oil intake tended to decrease DHA in some blood lipid fractions. Although these findings are somewhat unexpected, there are a number of possible explanations. First, both intervention oils had a small amount of EPA. It is possible that increased availability of EPA led to a modest rise in blood DHA( Reference Kew, Mesa and Tricon 21 ) even in the absence of a measurable increase in whole-blood EPA. Second, in the sunflower arm, a decrease in blood ALA may have prompted upregulation of Δ6 desaturase expression to enhance DHA production from EPA. This inverse feedback effect has been described( Reference Nakamura and Nara 22 ). In the soyabean oil arm, the modest increase in ALA could have been sufficient to stimulate elongation and desaturation into very-long-chain n-3 fatty acids. It is difficult to determine whether blood ALA concentrations at baseline should be considered low, since no previous studies have reported whole-blood PUFA concentrations in school-aged children or their relationships with clinical end points. Mean whole-blood ALA of participants was comparable to that of adult Costa Ricans from a landlocked region where access to soyabean oil is adequate( Reference Baylin, Kim and Donovan-Palmer 23 ). Third, DHA intake from other sources could have increased in both groups during the intervention period by chance or as a result of enhanced availability of cooking oil in the household. Nevertheless, dietary analyses provided no evidence to support this explanation. Last, regardless of their statistical significance, these differences might also be due to chance; we noted that the assay CV for DPA and DHA were generally higher than those for other fatty acids.
Whole-blood concentrations of long-chain n-6 fatty acids DGLA and AA also increased in both groups from pre- to post-intervention, even in the absence of significant changes in blood levels of the substrate LA. In the sunflower oil group, the decrease in ALA could have enhanced competitiveness of LA as substrate to the common Δ6 desaturase enzyme, leading to rises in blood DGLA and AA. We noted decreases in oleic and palmitic acid blood levels in the two intervention groups. Both of these fatty acids have been quoted as additional substrates of Δ6 desaturase( Reference Lasserre, Mendy and Spielmann 18 , Reference Rioux, Pedrono and Legrand 24 , Reference Guillou, D’Andrea and Rioux 25 ). If this is true, reductions in oleic and palmitic acids could provide both LA and ALA a competitive advantage for the Δ6 desaturase that may result in increased production of both n-6 and n-3 very-long-chain fatty acids. Alternatively, a general upregulation of Δ6 desaturase may have resulted in increased production of both n-6 and n-3 very-long-chain PUFA, independent of one substrate’s availability relative to another. Finally, because the data are expressed as percentage of total fatty acids in blood, increases in some fatty acids might only represent changes relative to decreases in others.
There are several strengths to our investigation. Its randomized, prospective nature lends support to a causal role of the family’s cooking oil on the fatty acid status of children. This is particularly noteworthy considering that children were presumably exposed to other dietary fats outside the household environment. No previous investigation of cooking fats had focused on children. The study also highlights the potential effectiveness of a relatively simple dietary modification, namely changing a family’s cooking oil, over other interventions that may be less sustainable in the long term, including supplementation. A month’s supply of soyabean oil (4 litres) for one of these average families would currently cost about $US 8, generally less than the same volume of sunflower oil or vegetable oil mixtures. Promoting replacement of the latter with soyabean oil should be feasible through mass media campaigns and individual education at primary health-care contacts. The use of oils that were commercially available in this setting is an additional strength of the study. Acceptability and compliance with the interventions were high and there were no indications that total energy or energy intake from saturated fat increased as a result. There were no losses to follow-up.
There are also some limitations. Our initial studies in this population indicated that ALA supply was scarce in commercially available cooking fats( Reference Baylin, Mora-Plazas and Cobos-de Rangel 7 ) and led us to compare the effects of soyabean oil against those of sunflower oil, one of the most widely consumed vegetable oils at the time. Nevertheless, the availability of soyabean oil at relatively low cost increased substantially shortly before the beginning of the trial, to the extent that over half of participating families were using it by the time the study began. Because of this, the change in blood ALA observed in the soyabean oil group (0·05 percentage points) is likely an underestimate of the underlying effect of switching from an oil less rich in ALA (e.g. sunflower) to soyabean oil. In addition, the head-to-head comparison of sunflower v. soyabean oil may have become less relevant than determining the effect of changing pre-intervention oil to better quality options. Ideally, the trial could have been restricted to soyabean oil non-users, but this would have hampered the generalizability of results. Alternatively, a short standardization period in which all participants received the same oil pre-randomization could have been carried out; but cost and logistical complications prevented us from implementing such an option. While a 4-week intervention is not sufficiently long to examine the effect of cooking oils on clinical outcomes, it is very helpful to evaluate their impact on fatty acid status, a key intermediate end point. It would have been desirable to have information on safety end points, including blood lipids, and on the oils’ stability and production of peroxidation substances through heating. Finally, we could not standardize the timing of sample collection with respect to the children’s last meal due to practical difficulties. This timing was equally distributed in the intervention groups and is not expected to have introduced bias in the estimation of differences between the means of two continuous distributions. Nevertheless, it could be a source of extraneous variation that may have decreased precision in the estimation of the effects.
Conclusion
Cooking with ALA-rich oils is an effective and safe intervention to improve essential fatty acid status of children in areas where intake of these nutrients from fish is limited due to geographic or economic constrains. The long-term effect of cooking oils on children’s health indicators, such as physical growth, cognitive development and early risk factors for chronic disease, deserves examination in future intervention studies.
Acknowledgements
Financial support: The study was supported by the ASISA Research Fund at the University of Michigan. The funding source had no role on the design, conduct, analyses, interpretation or writing of this manuscript. Conflict of interest: None. Authorship: E.V. designed the study, conducted the data analysis, interpreted the results and wrote the first draft of the manuscript. C.M., M.M.-P., M.C. and L.N.V. contributed to study implementation and data collection in the field. A.B. designed the study and participated in data analysis and interpretation. All authors contributed to the manuscript write-up. Ethics of human subject participation: The research was conducted in accordance with guidelines laid down by the Declaration of Helsinki. The study protocol and procedures were approved by the Ethics Committee of Javeriana University in Bogotá, and the Health and Behavioral Sciences Institutional Review Board at the University of Michigan.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015000762







