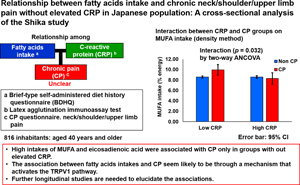
Introduction
The prevalence of chronic pain (CP) ranges between 17⋅5 and 31⋅5 %, with variations being reported among surveyed countries and age groups(Reference Croft, Lewis and Papageorgiou1–Reference Nakamura, Nishiwaki and Ushida4). A cross-sectional study by Nakamura et al. (Reference Nakamura, Nishiwaki and Ushida4) that investigated chronic musculoskeletal pain in Japanese subjects in their 30s to 50s reported that the most common pain sites were the lower back, neck, shoulders and knees. The role of inflammation in CP is considered to be greater in nociceptive pain(Reference Seifert and Baerwald5) and less in psychogenic(Reference Defrance, Foucher and Ramdani6) and neuropathic pain(Reference St. John Smith7). Regarding the relationship between inflammation and pain, high levels of C-reactive protein (CRP) were previously shown to increase cold pain sensitivity(Reference Afari, Mostoufi and Noonan8,Reference Schistad, Stubhaug and Furberg9) . Elevated CRP has also been reported in obesity, which increases pain sensitivity(Reference Eslami, Katz and White10,Reference Ray, Lipton and Zimmerman11) . On the other hand, longitudinal studies examining the relationship between CP and CRP have found no clear relationship between chronic musculoskeletal pain and high-sensitivity CRP(Reference Skarpsno, Mork and Nilsen12). Therefore, there is currently no consensus on the relationship between CP and CRP, which warrants more detailed epidemiological studies.
Non-inflammatory factors associated with CP have been identified as professional or managerial occupations, being female, BMI > 25, and a current drinking or smoking status(Reference Nakamura, Nishiwaki and Ushida13). Participants with CP also cited sleep disorders, anxiety, depression or irritable bowel as possible causes(Reference Yongjun, Tingjie and Xiaoqiu3). Therefore, non-inflammatory factors need to be considered in investigations to clarify the cause of CP.
One of the non-inflammatory factors involved in CP is nutrients(Reference Helde-Frankling and Björkhem-Bergman14,Reference Fifi and Holton15) . In our previous study(Reference Narukawa, Tsujiguchi and Hara16), we showed that the combination of an inadequate vitamin intake and depression was associated with CP. n-6 Fatty acids have also been suggested to play a role in both the induction and inhibition of pain(Reference Tokuyama and Nakamoto17–Reference Terashvili, Tseng and Wu20). Inflammatory cytokine production(Reference Tokuyama and Nakamoto17), the involvement of complex regional pain syndrome(Reference Ramsden, Gagnon and Graciosa18) and the development of mechanical allodynia(Reference Kim, Back and Davies19) have been implicated in pain induction. On the other hand, epoxyeicosatrienoic acid was found to inhibit pain(Reference Terashvili, Tseng and Wu20). However, the types of fatty acids involved in the inflammatory response, particularly CRP, have yet to be examined in detail.
Therefore, the present study investigated whether the relationship between CP and fatty acid intake differs between high and low CRP in individuals older than 40 years old in the Shika study.
Materials and methods
Participants
The present study was surveyed between October 2013 and January 2018. Twenty-one thousand and sixty-one people are living in Shika Town, Ishikawa Prefecture. Of these, 8499 are 65 years old or older; therefore, the aging rate is 42⋅2 %(21). Participants in the present study were 5013 residents aged 40 years and older living in four model districts of Shika Town (Horimatsu, Higashimasuho, Tsuchida and Togi). Previously conducted Shika studies on CP examined CP and vitamin intake(Reference Narukawa, Tsujiguchi and Hara16), CP and serum 25-hydroxyvitamin D concentrations(Reference Suzuki, Tsujiguchi and Miyagi22), and the relationship between hypertension and quality of life after adjustments for CP(Reference Kitaoka, Mitoma and Asakura23). Overall, 4724 participants completed the questionnaire survey and 1176 underwent a medical check-up. Participants who did not respond to the CP questionnaire, those without CRP quantification data, those who reported energy intake on the brief-type self-administered diet history questionnaire (BDHQ) was outside the range of 600–4000 kcal, and those receiving rheumatoid arthritis treatment were excluded. Therefore, 817 subjects (386 men and 431 women) were ultimately included in the analysis.
Evaluation items
CP was defined as persistent pain for more than 3 months(Reference Scholz, Finnerup and Attal24) or at least twice a week in the past month. Participants were asked about the presence of CP and its location on the body using a questionnaire.
A quantitative assessment of CRP was performed on blood samples taken from participants who consented to the medical check-up. CRP was measured by the latex agglutination immunoassay test (LZ test ‘Eiken’ CRP-HG; Eiken Kagaku Company Limited, Tokyo, Japan).
BDHQ was used to investigate fatty acid intake(Reference Kobayashi, Murakami and Sasaki25,Reference Kobayashi, Honda and Murakami26) . The BDHQ is a four-page questionnaire with an average response time of 15 min. It is possible to calculate the intake of approximately thirty nutrients and fifty-eight foods using a dedicated nutrient calculation programme. The validity of BDHQ has been verified by Kobayashi et al. (Reference Kobayashi, Murakami and Sasaki25,Reference Kobayashi, Honda and Murakami26) . Crude data were converted using the density method, which resulted in an intake per 1000 kcal.
Participants were asked about their sex, age, BMI, lifestyle (lack of exercise, lack of sleep, and current smoking and drinking status) and treatment of underlying diseases (diabetes and dyslipidemia) using a descriptive health questionnaire.
Statistical analysis
CP was restricted to neck/shoulder/upper limb pain and participants were classified into the non-CP and CP groups. Regarding CRP, participants were divided into low and high CRP groups based on the median of their measurements. The Student's t test was performed to examine relationships between continuous variables, while the χ 2 test was used to investigate relationships between categorical variables. A two-way analysis of covariance (two-way ANCOVA) was employed to analyse the main effects and interactions of CP and CRP on fatty acid intake. We also examined which fatty acids were associated with CP using a multiple logistic regression analysis, stratified by high and low CRP levels, with CP as the dependent variable and fatty acid intake as the independent variable. The forced imputation method was performed to select independent variables. IBM SPSS Statistics 25 (IBM, Armonk, NY, USA) was used for statistical analyses. The significance level was set at 5 %. Of the 43 fatty acids analysed, we omitted from the table those that were not significantly different in any of the analyses. Supplementary Table S1 shows the list of fatty acids analysed in the present study.
Sample size and statistical power
We used the free software G-power to calculate the sample size and statistical power. For the F tests of ANCOVA, the effect size, alpha error probability, power, number of groups and number of covariates were set to 0⋅25, 0⋅05, 0⋅95, 4 and 7, respectively. The total sample size and actual power were 210 and 0⋅950, respectively. For the Z tests for logistic regression, tails, odds ratio, mull hypothesis, alpha error probability, power, X distribution, X parm π were set to Two, 1⋅75, 0⋅25, 0⋅05, 0⋅95, Binomial and 0⋅5, respectively. The total sample size and actual power were found to be 791 and 0⋅950, respectively. Therefore, the sample size of this study was confirmed to be sufficient.
Ethical standards disclosure
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of Kanazawa University (protocol code, 1491; date of approval, 18 December 2013). Written informed consent was obtained from all subjects involved in the study.
Results
Participant characteristics
Table 1 shows participant characteristics assessed for neck/shoulder/upper limb pain, CRP and fatty acid intake. Among 817 participants, 386 males had a mean age of 63⋅69 years (sd 0⋅13) and 431 females had a mean age of 63⋅82 years (sd 10⋅68), with no significant difference between the two groups. The proportion of current smokers (P < 0⋅001), current drinkers (P < 0⋅001) and diabetics (P = 0⋅001) were significantly higher in males than in females. BMI was also significantly higher in males than in females (P < 0⋅001). The intakes of saturated fatty acids (SFA) (P < 0⋅001), monounsaturated fatty acids (MUFA) (P < 0⋅001), polyunsaturated fatty acids (PUFA) (P < 0⋅001), n-3 fatty acids (P < 0⋅001), n-6 fatty acids (P < 0⋅001), C18:1(M) (P < 0⋅001), C18:2(n6)(P(n-6)) (P < 0⋅001), C18:3(n3)(P(n-3)) (P < 0⋅001) and C20:2(n6)(P(n-6)) (P < 0⋅001) were significantly higher in females than in males.
Table 1. Participant characteristics

Abbreviations: sd, standard deviation; BMI, body mass index; CRP, C-reactive protein; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
* P-values were calculated using the Student's t test and χ 2 test for continuous and categorical variables, respectively (P-values < 0⋅05 are highlighted in bold).
Comparison of CP and non-CP
The mean ages of the 35 patients in the CP (neck/shoulder/upper limb pain) group and the 782 patients in the non-CP group were 63⋅91 and 63⋅75 years, respectively, with no significant difference (Table 2). BMI was significantly higher in the non-CP group than in the CP group (P = 0⋅007). Fatty acid intake did not significantly differ between the two groups.
Table 2. Characteristics of CP and non-CP groups for fatty acid intake

Abbreviations: CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
* P-values were calculated using the Student's t test and χ 2 test for continuous and categorical variables, respectively (P-values < 0⋅05 are highlighted in bold).
Comparison of high and low CRP
The mean age of 453 patients in the low CRP group (62⋅71 years) was significantly lower than that of 364 patients in the high CRP group (65⋅06 years, P = 0⋅001) (Table 3). The proportion of females was significantly higher in the low CRP group than in the high CRP group (P < 0⋅001). On the other hand, the percentage of current smokers (P = 0⋅012), BMI (P < 0⋅001) and CRP quantification (P < 0⋅001) were significantly lower in the high CRP group than in the low CRP group. Fatty acid intake did not significantly differ between the two groups.
Table 3. Characteristics of low and high CRP groups for fatty acid intakes

Abbreviations: CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
* P-values were calculated using the Student's t test and χ 2 test for continuous and categorical variables, respectively (P-values < 0⋅05 are highlighted in bold).
Interaction between CP and CRP on fatty acid intake
Table 4 shows the results of an analysis of the main effects and interactions of CP and CRP on fatty acid intake using a two-way ANCOVA. Covariates were adjusted for sex, age, lack of exercise, lack of sleep, current smoking or drinking status, and BMI. No fatty acids showed a main effect in the two CP groups. The fatty acids that showed a main effect in the two CRP groups were MUFA (P = 0⋅035), C18:1(M) (P = 0⋅038) and C18:3(n3)(P(n-3)) (P = 0⋅047). The fatty acids that showed an interaction between the CP and CRP groups were MUFA (P = 0⋅032), C18:1(M) (P = 0⋅037) and C20:2(n6)(P(n-6)) (P = 0⋅048). Specifically, in the high CRP group, the intakes of MUFA, C18:1(M) and C20:2(n6)(P(n-6)) were similar in the CP and non-CP groups, whereas in the low CRP group, their intakes were significantly higher in the CP group than in the non-CP group.
Table 4. Interaction between chronic neck/shoulder/upper limb pain and CRP on fatty acid intake

Abbreviations: CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Adjusted for sex, age, lack of exercise, lack of sleep, current smoker, current drinker and BMI.
* A two-way analysis of covariance (P-values < 0⋅05 are highlighted in bold).
Logistic regression analysis of CP on fatty acid intake stratified by CRP
Table 5 shows multiple logistic regression results stratified by high and low CRP groups, with CP as the dependent variable. Covariates were adjusted for sex, age, lack of exercise, lack of sleep, current smoking or drinking status, and BMI, with each fatty acid imputed individually into the independent variables. Significant independent variables in the low CRP group were SFA (OR 1⋅327; 95 % CI 1⋅014, 1⋅737; P = 0⋅040), MUFA (OR 1⋅359; 95 % CI 1⋅093, 1⋅690; P = 0⋅006), PUFA (OR 1⋅399; 95 % CI 1⋅033, 1⋅897; P = 0⋅030), n-6 fatty acids (OR 1⋅560; 95 % CI 1⋅068, 2⋅279; P = 0⋅021), C18:1(M) (OR 1⋅000; 95 % CI 1⋅000, 1⋅001; P = 0⋅006), C18:2(n6)(P(n-6)) (OR 1⋅000; 95 % CI 1⋅000, 1⋅001; P = 0⋅023), C18:3(n3)(P(n-3)) (OR 1⋅002; 95 % CI 1⋅000, 1⋅004; P = 0⋅028) and C20:2(n6)(P(n-6)) (OR 1⋅072; 95 % CI 1⋅015, 1⋅132; P = 0⋅013). In the high CRP group, no fatty acid was identified as a significant independent variable for CP. In other words, a high intake of these fatty acids significantly contributed to CP in the low CRP group only.
Table 5. Logistic regression analysis of CP and fatty acid intake stratified by CRP

Abbreviations: Exp (B), Exponentiation of the B coefficient; CI, confidence interval; CRP, C-reactive protein; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Significant estimates are in bold.
Discussion
The main result of the present study was that higher intakes of MUFA, oleic acid (C18:1) and eicosadienoic acid (C20:2) correlated with CP in the low CRP group, but not in the high CRP group.
A cross-sectional study that examined the relationship between high-sensitivity CRP and pain in daily life by Eslami et al. (Reference Eslami, Katz and White10) revealed that these two factors were related in women, but not in men; however, in a logistic regression analysis adjusted for pain-related ADL decline and BMI in women, high-sensitivity CRP was no longer associated with pain. Since the relationship between fatty acid intake and CP was influenced by CRP in the present study, this relationship appears to be complex. CRP is an indicator of inflammation(Reference Seifert and Baerwald5,Reference Sproston and Ashworth27) , which may be related to the development of CP. Therefore, high CRP is considered to be related to nociceptive pain caused by peripheral nociceptors stimulated via pain-producing substances produced through inflammation and inducing tissue damage(Reference Seifert and Baerwald5). The present results showing the absence of a relationship between fatty acid intake and CP in the high CRP group indicate the greater involvement of pain-producing substances during the inflammatory response. On the other hand, CP with low CRP may be explained by psychogenic pain(Reference Defrance, Foucher and Ramdani6,Reference Zis, Daskalaki and Bountouni28) involving depression or neuropathic pain(Reference Baron, Binder and Wasner29,Reference Finnerup, Kuner and Jensen30) affecting the somatosensory nervous system as pathophysiology. We speculate that a higher fatty acid intake may enhance the mechanisms involved in depression and nociceptive pathway sensitisation to CP.
n-6 Fatty acids, including eicosadienoic acid, are considered to play a crucial role in pain initiation and suppression(Reference Tokuyama and Nakamoto17). Taken together with the involvement of the production of inflammatory eicosanoids and inflammatory cytokines in pain initiation(Reference Tokuyama and Nakamoto17), elevated blood levels of n-6 highly unsaturated fatty acids appear to play a role in complex regional pain syndrome(Reference Ramsden, Gagnon and Graciosa18) and mechanical allodynia due to the potent activation of TRPV1 in the spinal cord(Reference Kim, Back and Davies19). On the other hand, 14- and 15-epoxyeicosatrienoic acids have been suggested to inhibit pain(Reference Terashvili, Tseng and Wu20). The present results showing that the intake of eicosadienoic acid, one of the n-6 fatty acids, was significantly higher only in the low CRP with CP group suggested that a higher intake of n-6 fatty acids is related to the development of non-inflammatory psychogenic pain(Reference Defrance, Foucher and Ramdani6,Reference Zis, Daskalaki and Bountouni28) and low-inflammatory neuropathic pain(Reference Baron, Binder and Wasner29,Reference Finnerup, Kuner and Jensen30) . These findings indicate that a higher intake of n-6 fatty acids affects non-inflammatory CP through a mechanism that activates TRPV1.
A study by Bennett and Hayes(Reference Bennett and Hayes31), in which chemesthetic subqualities elicited by oleic acid (olive oil) and capsaicin were compared in healthy adults, proposed that an unknown TRPV1 agonist was present in olive oil. Therefore, a high intake of these fatty acids may exacerbate non-inflammatory psychogenic pain related to the existing quality of life(Reference Mitoma, Kitaoka and Asakura32) as well as depression(Reference Defrance, Foucher and Ramdani6) and neuropathic pain, such as trigeminal neuralgia(Reference St. John Smith7). The present results showed that oleic acid intake was significantly higher in the low CRP with CP group only. Therefore, a possible cause of non-inflammatory CP is the higher intake of MUFA, such as oleic acid. However, several studies(Reference Sánchez-Villegas, Verberne and Irala33,Reference Lang, Beglinger and Schweinfurth34) indicated that a higher intake of MUFA, such as olive oil, was negatively associated with depression. On the other hand, very few epidemiological studies have investigated the relationship between MUFA and CP(Reference Andreeva, de Edelenyi and Druesne-Pecollo35). Based on previous findings, including epidemiology, the relationship between MUFA and CP remains unclear. Since the present study did not evaluate the relationship between psychogenic pain and depression, the relationships among MUFA, depression and CP need to be confirmed in longitudinal studies.
An overweight BMI as a CP-related factor has been examined in epidemiological studies(Reference Eslami, Katz and White10,Reference Ray, Lipton and Zimmerman11) . A cross-sectional study that investigated elderly females without dementia revealed a relationship between obesity and pain intensity in daily life(Reference Eslami, Katz and White10). Similarly, a cross-sectional study on the elderly revealed a correlation between central obesity and CP(Reference Ray, Lipton and Zimmerman11). Although these studies reported a relationship between BMI and CP, our BMI-corrected model verified that fatty acid intake correlated with CP with low CRP even after adjustments for BMI. Therefore, the present results showing a relationship between fatty acid intake and CP are not attributed to an overweight BMI.
The limitations of the present study are, first, its cross-sectional nature, due to which we were unable to examine the longitudinal causal relationship between fatty acid intake and CP. Second, since the number of participants (16 subjects) in the high CRP group with CP was small, a larger sample size analysis would be required. Third, we did not classify CRP according to reference values. Fourth, the self-administered BDHQ may lack objectivity. Fifth, we have not analysed the influence of medication treatment due to diabetes or dyslipidemia on the relationship between fatty acid intake and CP. Sixth, we have not investigated the implication of the TRPV1 pathway. Finally, since participants were volunteers, selection bias may exist.
Conclusion
We epidemiologically revealed a relationship between fatty acid intake and CP, stratified by low and high CRP. Only high intakes of MUFA and eicosadienoic acid were associated with chronic neck/shoulder/upper limb pain without elevated CRP. In psychogenic and neuropathic pain without elevated CRP, increased intakes of MUFA and eicosadienoic acid, a family member of n-6 fatty acids, appear to affect CP. Further longitudinal studies are needed to elucidate this relationship.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2022.37.
Acknowledgements
The authors thank all the participants in the Shika town study, as well as the collaborations by all of the field survey staff and experimental staff.
The present study was funded by a Grant-in-Aid for Scientific Research (B) by the Japan Society for the Promotion of Science, number 19H03882.
Conceptualisation, A. A. and H. N.; methodology, H. T., H. N.; formal analysis, H. T., M. N.; investigation, A. A., F. S., H. T., A. H., S. M., K. S., M. N., Y. S., T. T. T. N., K. O. P., T. K., S. N., K. H., A. S., T. A., H. T. and H. N.; resources, H. N.; data curation, T. K. and A. T.; writing—original draft preparation, A. A. and F. S.; writing—review and editing, H. T., A. H., S. M., T. K., K. S., M. N., Y. S., T. T. T. N., K. O. P., T. K., S. N., K. H., A. S., T. A., T. K., Y. K., H. T., A. T. and H. N.; supervision, T. K., Y. K. and H. N.; funding acquisition, H. N. All authors have read and agreed to the published version of the manuscript.
Data in the present study are available upon request from the corresponding author. Data are not publicly available due to privacy and ethical policies.