Electrophysiological confirmation of impaired neuromuscular transmission is commonly performed in myasthenia gravis (MG) and includes single fiber electromyography (SFEMG) and repetitive nerve stimulation (RNS). While decrement reflects neuromuscular transmission failure at the neuromuscular junction, SFEMG can detect even a delay in neuromuscular transmission and is therefore more sensitive. The value of electrophysiological evaluation in MG extends beyond the ability to diagnose impaired neuromuscular transmission and is correlated with disease severity.Reference Sanders1, Reference Abraham, Breiner and Barnett2 In addition, the electrophysiological findings might assist in predicting the risk for disease exacerbations and even suggest treatment responsiveness.Reference Baruca, Leonardis and Podnar3 Similar results were not found for acetylcholine receptor antibodies.Reference Limburg, The, Hummel-Tappel and Oosterhuis4 In the current study, we aimed to explore whether higher degrees of electrophysiological abnormality at baseline are associated with more frequent exposure to a more aggressive treatment regimen. As electrophysiological findings are associated with worse clinical severityReference Abraham, Breiner and Barnett2 and a higher risk for exacerbations even with mild symptoms,Reference Baruca, Leonardis and Podnar3 we hypothesized that more severe electrophysiological findings would be associated with more frequent exposure to more aggressive treatment regimens.
We performed a retrospective chart review of 134 patients referred to the Prosserman Family Neuromuscular Clinic at the University Health Network from June 2012 to December 2015 for possible MG, who were then diagnosed as having the disorder at this initial visit. MG diagnosis was performed by a neuromuscular expert (VB) in all patients, based on a compatible clinical presentation supported by abnormal SFEMG findings. In addition, all patients underwent routine detailed clinical history taking and comprehensive neurological examination, with assessment of disease severity by the quantitative myasthenia gravis score (QMGS)Reference Nations, Wolfe, Mcintire, Herbelin, Barohn and Bryan5 at baseline visit in most patients. Eighty-seven patients who returned for follow-up in our clinic were included in the current study. The mean follow-up period was 2.6 ± 1.7 years. The remaining 47 patients who did not return for follow-up (reason unknown) did not differ from the 87 patients who did return for follow-up in terms of demographic, clinical, and electrophysiological findings (data not shown) and were excluded from further analysis. We recorded demographics, MG-related symptoms, QMGS, and electrophysiological findingsReference Abraham, Alabdali and Alsulaiman6 at the baseline (first) visit. In addition, we recorded serology results, follow-up duration, and clinical symptoms at the last follow-up visit, and MG treatments were given within the follow-up period. Only relatively common treatments given in our clinic were recorded, including pyridostigmine, prednisone, azathioprine, mycophenolate, intravenous immunoglobulins (IVIG), and plasma exchange (PLEX). QMGS was not performed at the last follow-up visit, as our practice changed in 2015 when we started to use the myasthenia gravis impairment index.Reference Barnett, Bril, Kapral, Kulkarni and Davis7
The electrophysiological assessment included SFEMG and RNS and was performed in the frontalis muscle in all patients prior to clinical evaluation or referral letter review, to maintain testing objectivity. However, as the clinic routine is to evaluate patients with suspected MG on specific days, the clinic staff was aware of the suspicion of MG. MG-related symptoms were compared between patients who had higher (≥100 µs) and lower mean jitter values, and between patients who had higher (≥20%) and lower decrement values at the baseline visit and at the last follow-up visit. These specific electrophysiological cut-off values were used due to their simplicity, and based on a previous study demonstrating the association of higher values with a more severe disease.Reference Abraham, Breiner and Barnett2 In addition, we compared treatment regimens given within the follow-up period between patients with higher (≥100 μs) and lower jitter and higher (≥10% and ≥20%) and lower decrement values (in the total cohort, in patients with QMGS < 10.5 indicating mild disease, and in patients with QMGS > 10.5, indicating moderate to severe disease).Reference Zinman, Baryshnik and Bril8 The Research Ethics Board of the University Health Network approved the study protocol and waived informed consent.
Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA). Demographic, MG-related symptoms, serologic findings, and MG treatments are presented as means and standard deviations or as numbers and percent, as appropriate, and comparison of characteristics between high and low decrement and jitter values was performed using the t-test or the χ 2-test. Fisher’s exact test was used in place of the χ 2-test when expected counts were less than five. Odds ratios (ORs) (and their 95% confidence intervals) of receiving treatment in the presence of high jitter or high decrement values (compared to those with low jitter or decrement values) were calculated using logistic regression. Significance was set at a p-value < 0.05 (two-tailed).
MG patients with more severely abnormal electrophysiological parameters (decrement ≥20% or jitter ≥100 μs recorded from the frontalis muscle) at baseline were more frequently seropositive and had generalized disease, with a higher QMGS at baseline. During the follow-up period, the most common treatment included mestinon, followed by prednisone and azathioprine (Table 1). There was no difference in treatment doses between groups, except higher dose of mestinon in patients with decrement ≥20% (324 vs. 212 mg, p = 0.01). Patients with higher decrement at baseline (≥10%) were more frequently treated with IVIG and PLEX, while patients with higher jitter were more frequently treated with IVIG (Table 1). Patients with high decrement had more frequent bulbar and respiratory symptoms at baseline, and more frequent bulbar symptoms at follow-up. In contrast, patients with high jitter had more frequent bulbar and respiratory symptoms only at the baseline visit (Table 2). MG patients with more severely abnormal electrophysiological parameters (either decrement ≥10% or jitter ≥100 μs) and patients with high QMGS (>10.5) were more frequently treated with IVIG and PLEX (Table 3). However, in a subgroup analysis including two groups with mild (QMGS < 10.5) and moderate to severe disease (QMGS > 10.5), only patients with mild disease and high decrement values (≥10%) were more frequently treated with IVIG and PLEX (ORs 6.7 and 21.5, respectively), while high jitter values were associated with a higher frequency of mycophenolate treatment (Table 3).
Table 1: Comparison of baseline characteristics and treatment during follow-up period between patients with high and low decrement and jitter values

Y=years; QMGS=quantitative myasthenia gravis score; AchRAb=elevated titer of acetylcholine receptor antibodies; F/U=follow-up; IVIG=intravenous immunoglobulins; PLEX=plasma exchange.
Data presented as n (%) or mean ± SD.
Significant p-values (<0.05) are shown in bold.
Table 2: Comparison of symptoms between MG patients with high and low decrement and jitter values at baseline and last follow-up visit
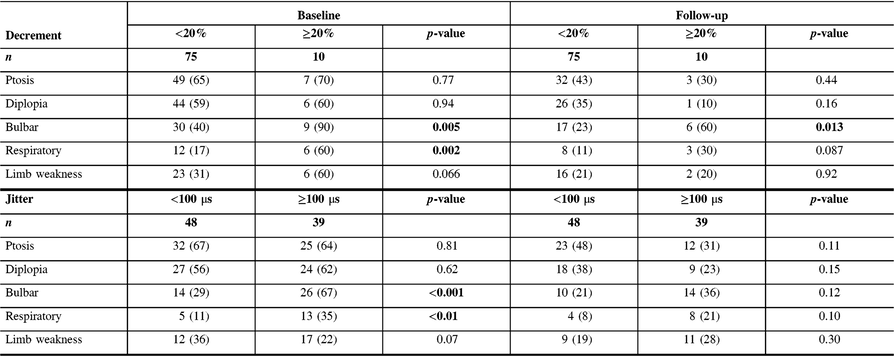
Data presented as n (%).
Significant p-values (<0.05) are shown in bold.
Table 3: Univariable odds ratio (and 95% confidence interval) of receiving indicated treatment in the presence of baseline jitter ≥100 μs or baseline decrement ≥10% and 20%, for the whole cohort, and for patients with QMGS lower and greater than 10.5
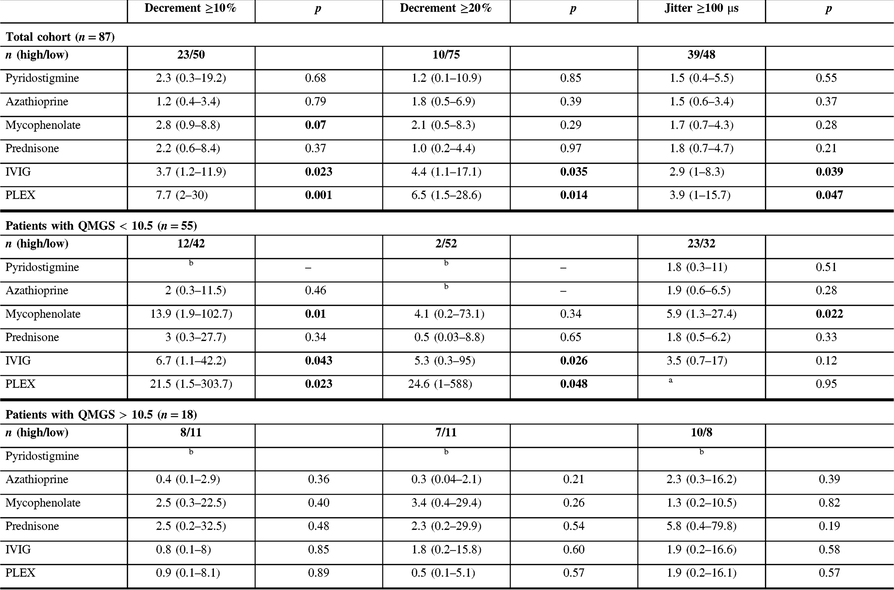
QMGS=quantitative myasthenia gravis score; IVIG=intravenous immunoglobulins; PLEX=plasma exchange.
Each odds ratio represents the odds of receiving the given treatment (dependent variable) for the following: (1) the presence of decrement ≥10% or ≥20% compared to the presence of decrement <10% or <20%, respectively or (2) the presence of jitter ≥100 μs compared to the presence of jitter <100 μs. OR is adjusted for QMGS. QMGS was not documented in 14 patients.
a All patients with jitter >100 and QMGS <10.5 were treated with PLEX.
b Could not be calculated due to low numbers.
Significant p-values (<0.05) are shown in bold.
The current study results show that MG patients with high decrement (>10%) and jitter (>100 μs) at baseline were considerably more likely to be treated during the follow-up period with immunomodulation, including IVIG and PLEX. These results are not surprising considering that more severely abnormal electrophysiology is associated with a more abnormal clinical statusReference Abraham, Breiner and Barnett2, Reference Barnett, Katzberg, Nabavi and Bril9 and a higher relapse rate.Reference Baruca, Leonardis and Podnar3 While IVIG or PLEX treatment is not infrequent in patients with a moderate to severe disease, it is rarely used in patients with mild disease, unless clinical deterioration occurs. However, there are currently no validated biomarkers allowing prediction of clinical deterioration in MG patients. Our study results show that high decrement at baseline in patients with mild disease is associated with a higher risk for future IVIG or PLEX treatment, even after adjusting for clinical severity (after splitting patients into two groups based on severity and adjusting OR for QMGS), reflecting potentially an independent prognostic role for decrement. Consequently, patients with mild disease and high decrement might benefit from more counselling regarding identifying and managing increasing symptoms earlier, and perhaps more frequent follow-up to detect clinical deterioration, although this assumption needs to be validated in prospective studies. Furthermore, although both patients with high jitter and decrement had more frequent bulbar and respiratory symptoms as baseline visit, only patients with high decrement continued to present more frequent bulbar and respiratory symptoms at the last follow-up visit, further supporting the utility of decrement as a robust prognostic tool. The absence of similar associations with high jitter might imply a need for a different cut-off value, or alternatively reflect the superiority of decrement recording for prognostic purposes.
Although patients with either high decrement or jitter had more abnormal clinical features at baseline, all showed a trend for improvement at the last follow-up visit, most likely reflecting treatment efficacy.
The current study has several limitations. It was designed as a retrospective study, and therefore the treating physician was not blinded to electrophysiological findings. However, as treatment decisions in our center are based on clinical grounds, it is unlikely that baseline electrophysiological findings had a significant impact on the decision to initiate immunomodulatory treatments such as IVIG or PLEX in later visits. Furthermore, we did not include a sufficient number of patients in order to explore differences in less frequent treatments, such as rituximab or cyclophosphamide, and had 12 patients with mild MG and decrement ≥10%, and only 2 with decrement ≥20%. In addition, we used electrophysiological data from a single cranial muscle (frontalis), and therefore cannot address the generalizability of the study results for other muscles. We also used previously defined cut-off values for jitter (>100 μs) and decrement (>10%),Reference Abraham, Breiner and Barnett2 which may not be optimal for predicting response to different treatment regimens. Finally, our follow-up period was limited (less than 3 years), and therefore we cannot predict whether a similar trend would be demonstrated also in the longer term. In conclusion, in patients with mild MG, more severely abnormal electrophysiological findings may be associated with more frequent exposure to more aggressive treatment regimens.
Conflict of Interest
Vera Bril has been a consultant for Bionevia, CSL, Dainnipon Sumitomo, Eisai, Grifols, Lilly, and Pfizer, and has received research support from Bionevia, CSL, Grifols, Takeda (Shire), Octapharma, Alexion, Argenx, Alynlam, UCB, Akcea, Pfizer, and Powell-Mansfield, outside the submitted work. Alon Abraham and Leif E. Lovblom have no conflicts of interest to declare.
Statement of Authorship
AA: study concept and design, analysis and interpretation of data.
LEL: study concept and design, analysis and interpretation of data.
VB: study concept and design, analysis and interpretation of data, study supervision, critical revision of manuscript for intellectual content.





