The importance of nutrition for physical health is well established; however, its role in mental health is less clear. Growing evidence suggests that B-vitamins such as folate( Reference Gilbody, Lightfoot and Sheldon 1 ) and vitamin B12 Reference Robinson, O’Luanaigh and Tehee (2 ) are implicated in mental health. Both folate and vitamin B12 are necessary for the methylation of homocysteine to methionine( Reference Bottiglieri 3 ), the precursor of S-adenosylmethionine (SAMe)( Reference Crellin, Bottiglieri and Reynolds 4 ). Homocysteine is an amino acid that, at high levels, is associated with adverse health outcomes such as CVD( Reference Zakai, Katz and Jenny 5 ) and depression( Reference Tiemeier, van Tuijl and Hofman 6 ); SAMe is a methyl donor with potential antidepressant properties due to its involvement in the metabolism of neurotransmitters such as norepinephrine, dopamine, melatonin and serotonin( Reference Moretti, Torre and Antonello 7 ). Functional deficiencies of folate or vitamin B12 may, therefore, potentially result in disturbed mood either directly or indirectly via elevated homocysteine( Reference Bottiglieri 3 ) and reduced SAMe concentrations( Reference Penninx, Guralnik and Ferrucci 8 ).
Cross-sectional studies have found an association between depression and concentrations of folate( Reference Gilbody, Lightfoot and Sheldon 1 , Reference Jacka, Maes and Pasco 9 ), vitamin B12 Reference Moorthy, Peter and Scott (10 , Reference Seppälä, Koponen and Kautiainen 11 ) and homocysteine( Reference Dimopoulos, Piperi and Salonicioti 12 , Reference Nabi, Bochud and Glaus 13 ). However, reduced appetite due to depression( Reference Engel, Siewerdt and Jackson 14 ) may account for such relationships. Several studies have supported a longitudinal relationship between folate and depression( Reference Kim, Stewart and Kim 15 ), variability in negative affect (NA)( Reference Williams, Stewart-Knox and McConville 16 ) and diagnosis of depression up to 11–16 years of follow-up( Reference Tolmunen, Hintikka and Ruusunen 17 ). Vitamin B12 has also been associated with later depression( Reference Kim, Stewart and Kim 15 , Reference Hintikka, Tolmunen and Tanskanen 18 ) and depressive symptoms over an average of 7·2 years( Reference Skarupski, Tangney and Li 19 ), and homocysteine has been longitudinally associated with depression( Reference Kim, Stewart and Kim 15 , Reference Forti, Rietti and Pisacane 20 ).
Intervention trials provide further support: initial folate levels have been shown to predict differential response to treatment of depressive symptoms( Reference Alpert, Silva and Pouget 21 – Reference Papakostas, Petersen and Lebowitz 24 ), although initial levels of vitamin B12 and homocysteine did not( Reference Papakostas, Petersen and Mischoulon 22 – Reference Papakostas, Petersen and Lebowitz 24 ). Treatment with methyltetrahydrofolate (MTHR) has also been found to improve depressive symptoms( Reference Guaraldi, Fava and Mazzi 25 , Reference Passen, Cucinotta and Abate 26 ), and Almeida et al.( Reference Almeida, Marsh and Alfonso 27 ) have reported that folic acid+vitamin B6+vitamin B12 supplementation for 1–10·5 years was associated with a reduced risk of onset of major depression. However, others have reported no benefit of folic acid alone( Reference Williams, Stewart-Knox and Bradbury 28 ), folic acid and vitamin B12 Reference Walker, Mackinnon and Batterham (29 , Reference Christensen, Aiken and Batterham 30 ) or of folic acid, vitamin B6 and vitamin B12 ( Reference Ford, Flicker and Thomas 31 ). Others have found evidence that folate potentiates the effects of standard antidepressant treatment( Reference Alpert, Mischoulon and Rubenstein 32 – Reference Godfrey, Toone and Bottiglien 34 ).
Cross-sectional relationships have been assessed between positive mood and homocysteine( Reference Jensen, Dehlin and Erfurth 35 ) and folate and vitamin B12 Reference Cassidy, Kotynia-English and Acres (36 ). Jensen et al.( Reference Jensen, Dehlin and Erfurth 35 ) reported an inverse association between high homocysteine concentrations and life satisfaction (LS), zest for life and subjective health in older people (>80 years), and Cassidy et al.( Reference Cassidy, Kotynia-English and Acres 36 ) reported no association between folate and vitamin B12 deficiencies and mood in community-dwelling older women (>70 years), although participants had low levels of folate and vitamin B12 deficiencies.
Several longitudinal trials have compared various multi-combinations of vitamins, minerals, amino acids, antioxidants and essential fatty acids with placebo and have reported some benefit on mood in children( Reference Kaplan, Fisher and Crawford 37 ) and anti-social behaviour in prisoners( Reference Gesch, Hammond and Hampson 38 ). Several of them have assessed Berocca® (Bayer Australia Ltd) and reported positive effects after approximately 1 month on anxiety and perceived stress( Reference Schlebusch, Bosch and Polglase 39 ), stress-related symptoms( Reference Schlebusch, Bosch and Polglase 40 ) and on stress, mental health and vigour( Reference Kennedy, Veasey and Watson 41 ). Two other studies have reported positive effects on stress with supplementation for 1 month with Centrum® (Pfizer) in healthy male adults( Reference Long and Benton 42 ), and on alertness, concentration and mental and physical stamina with a 3-month supplementation with Swisse Men’s Ultivite® (Swisse Wellness Inc.) in healthy males( Reference Harris, Kirk and Rowsell 43 ). These trials suggest that nutrients have the potential to influence positive mood in a range of population types; however, their interpretation is confounded by the use of large omnibus inclusion of vitamins. One placebo-controlled trial that has assessed the sole use of folic acid for mood over 12 weeks in healthy males found no difference on the measures of positive affect (PA) or NA, despite increased serum and erythrocyte folate and decreased plasma homocysteine levels in response to treatment( Reference Williams, Stewart-Knox and Bradbury 28 ).
The exact nature of a relationship between folate, vitamin B12, homocysteine and depression is confounded by different initial levels of nutrients and mental health, measurement of mental health and whether covariates are considered. We assessed the relationships between folate, vitamin B12, homocysteine and positive mental health. Positive mental health is operationalised here as subjective well-being (SWB), a multi-dimensional construct( Reference Davern and Cummins 44 ) that includes affect – the presence of PA and the absence of NA – and cognitive evaluations of life – LS – as three distinct components. The aims of this study were to assess (1) the direct effects of folate, vitamin B12 and their interaction on SWB; and (2) the indirect effects of these nutrients and their interaction on SWB as mediated via homocysteine with consideration of potential covariates including age, sex, BMI, socioeconomic status, smoking status, education, use of cardiovascular medications, alcohol intake, energy intake, energy expenditure, physical health and levels of n-3 PUFA.
Methods
Participants
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects were approved by the Human Experimentation Ethics Committee of CSIRO Health Sciences and Nutrition. Written informed consent was obtained from all the subjects. The trial can be found in the Australia and New Zealand Clinical Trials Register: ACTRN12607000278437.
Participants (n 391; 53·7 % female) were community-living older adults aged between 64 and 91 years (mean72·3 (sd 5·54) years) who were enrolled in the Older People, Omega-3 and Cognitive Health (EPOCH) trial – an 18-month randomised controlled trial assessing the effects of n-3 fish oil on cognitive functioning in healthy, community-dwelling older adults( Reference Danthiir, Burns and Nettelbeck 45 ). Those with a current clinical diagnosis of major depression, dementia or a history of drug or alcohol abuse were excluded from the study; for further exclusion criteria, see Danthiir et al. ( Reference Danthiir, Burns and Nettelbeck 45 ).
Measures
Positive and negative affect: Positive and Negative Affect Schedule
The Positive and Negative Affect Schedule (PANAS)( Reference Watson, Clark and Tellegen 46 ) contains twenty mood descriptors (ten positive; ten negative) and requires respondents to rate ‘to what extent have you felt this way during the past week?’ from 1 (very slightly, or not at all) to 5 (extremely); higher scores indicate greater PA or NA. The PANAS has demonstrated acceptable internal consistency reliability for both PA (range α=0·86–0·90) and NA (range α=0·84–0·87) in undergraduate students( Reference Watson, Clark and Tellegen 46 ), and has been validated in a sample of older adults (PA range: α=0·84–0·96 and NA range: α=0·64–0·91).
Life satisfaction: Satisfaction with Life Scale
The Satisfaction with Life Scale (SWLS)( Reference Diener, Emmons and Larsen 47 ) is a five-item questionnaire designed to measure global LS, the cognitive component of SWB. Respondents indicated their level of agreement with each item (e.g. item 1; ‘In most ways, my life is close to my ideal’) on a seven-point Likert scale (1=strongly disagree to 7=strongly agree), with higher scores indicating greater satisfaction. The SWLS has demonstrated adequate internal consistency reliability (average α=0·78) and has been validated for use in aged populations (α=0·83)( Reference Pavot, Diener and Colvin 48 ).
Biochemical assays: folate, vitamin B12 and homocysteine
Overnight (approximately 12 h) fasted blood samples were forwarded to an accredited clinical pathology laboratory (IMVS) for analysis. Serum folate (nmol/l), serum vitamin B12 (pmol/l) and plasma homocysteine (µmol/l) concentrations were tested according to the methods previously outlined( Reference Danthiir, Burns and Nettelbeck 45 ), and reference ranges (serum folate: range 5·0–45·0 nmol/l fasted; serum vitamin B12: range 100–700 pmol/l; and plasma homocysteine: range 4·0–140·0 µmol/l fasted) were established in the clinical pathology laboratory in accordance with the Australian National Guidelines.
Covariates
Additional variables included for consideration as potential covariates included age, sex, education, socio-economic status, BMI, smoking status, Self-Reported Physical Health (SF-36v2)( Reference Ware, Kosinski and Dewey 49 ), total daily energy intake (CCVFFQ)( Reference Giles and Ireland 50 ), alcohol intake (CCVFFQ)( Reference Giles and Ireland 50 ), omega-3 index (EPA+DPA/total fatty acids), physical activity (YPAS)( Reference Dipietro, Caspersen and Ostfeld 51 ) and use of cardiovascular medications. A complete description of these methods can be found in the study protocol( Reference Danthiir, Burns and Nettelbeck 45 ).
Procedure
Data presented here are from the EPOCH trial, a more detailed methodology of the study protocol can be found in the study by Danthiir et al.( Reference Danthiir, Burns and Nettelbeck 45 ). Expressions of interest were sought from potential participants recruited via local advertisements, media releases and organisations for older people. Participants who met the initial inclusion criteria were screened for dementia using a modified telephone version of the Mini–Mental State Examination( Reference Newkirk, Kim and Thompson 52 ) and written informed consent was obtained. Four assessment sessions (baseline, 6, 12 and 18 months) were conducted, during which fasted blood samples were collected for determining the fatty acid profile (each assessment) as well as plasma homocysteine, serum folate and serum vitamin B12 levels (first and final assessments). Paper questionnaires were mailed to participants before each assessment, the CCVFFQ was completed at baseline and at study completion; all the other questionnaires were completed at each of the four assessments. Height was assessed at baseline only and weight at each assessment.
Statistical analyses
Cross-lagged path analysis, a class of structural equation modelling for longitudinal data, was used to assess mediation because it allows causal inferences to be drawn from non-experimental, longitudinal data. Cross-lagged path analyses were used to assess the direct effects of vitamin B12, folate and their interaction on PA, NA and LS as well as the indirect effects of vitamin B12, folate and their interaction on PA, NA and LS via homocysteine as the mediator. Two cross-lagged regression models were thus specified to assess the following: (1) the direct effects of vitamin B12, folate and their interaction, measured at baseline, on PA, NA and LS 18 months later, controlling for their baseline levels; and (2) the indirect effects of vitamin B12, folate and their interaction on PA, NA and LS via the mediator homocysteine, controlling for their baseline levels and baseline levels of homocysteine. These models were estimated for all the participants from the EPOCH trial( Reference Danthiir, Burns and Nettelbeck 45 ), including those in the n-3 fish oil group and those in the placebo group. Measurement invariance between these two participant groups, for both cross-lagged regression models, was first established to ensure that there were no significant differences between groups on any of the estimated paths in both specified models.
Results
Preliminary analyses
List-wise deletion was applied to <1 % of the cases that were unable to be estimated (missing >50 % of a scale); remaining missing values (<5 % with responses <50 % and missing at random) were estimated with the Expectation–Maximisation algorithm( Reference Dempster, Laird and Rubin 53 ). Study attrition was minimal; 90 % of the participants completed the baseline and the 18-month assessments (n 391 at baseline, n 355 at 18 months). Independent samples t tests confirmed that there were no significant differences at baseline between those who completed the 18-month assessment compared with those who dropped out subsequent to baseline assessment for vitamin B12, folate, homocysteine and SWB (PA, NA and LS) measures.
Descriptive statistics
Means, standard deviations and zero-order correlations for measures of SWB, vitamin B12, folate and homocysteine are presented in Table 1. Mean levels of vitamin B12, folate and homocysteine were all within the normal range; 6·8 % were marginally folate deficient (6·8–11 nmol/l), 7·5 % were hyperhomocysteinaemic (homocysteine >15 µmol/l) and 8·4 % were vitamin B12 deficient (vitamin B12 <148 pmol/l) and 24·3 % were marginally vitamin B12 deficient (148–222 pmol/l). Females had significantly lower mean plasma homocysteine concentrations (mean10·0 (sd 3·1) µmol/l) and significantly higher serum vitamin B12 concentrations (mean322·3 (sd 216·5) pmol/l) compared with males (mean11·2 (sd 3·1) pmol/l; mean268·6 (sd 136·4) pmol/l, respectively); no sex differences were observed for serum folate. Average LS scores, reported in Table 1, were only slightly above the neutral point of 20 on the scale. This is considerably lower than that previously reported in older adults( Reference Pavot, Diener and Colvin 48 ) and Australian adults( Reference Gannon and Ranzijn 54 ). Correlations between folate, vitamin B12 and homocysteine levels were as expected. Homocysteine was negatively correlated with folate and vitamin B12, and folate and vitamin B12 were positively correlated. PA, NA and LS were highly correlated with each other from baseline to 18 months. PA and homocysteine were negatively correlated both at baseline and at 18 months; vitamin B12 at baseline was negatively correlated with LS at 18 months.
Table 1 Associations between folate, vitamin B12 and homocysteine with positive affect (PA), negative affect (NA) and life satisfaction (LS) at baseline and 18 months (Pearson’s correlations, mean values and standard deviations)

* P<0·05.
Main analyses
Mediation was assessed using a cross-lagged path analysis to allow causal inferences to be drawn from the non-experimental, longitudinal data. The autoregressive component accounts for dependence between repeated measurements and baseline inter-correlations between all the variables account for time point dependence. The residuals associated with each of the latent constructs were also allowed to co-vary across time. With only two waves of data, mediation is inferred from the product of the paths from the baseline independent variables (vitamin B12, folate or their interaction) to the mediator (homocysteine) measured at 18 months, and from baseline mediator (homocysteine) to the dependent variables (PA, NA or LS) measured at 18 months via the indirect effects model. The combination of three independent and three dependent variables resulted in a total of nine potential mediation relationships being assessed. Nutrient variables were scaled( Reference Gelman 55 ) to reduce residual variances and to aid model convergence, and centred to aid parameter interpretation. Homocysteine and vitamin B12 were both positively skewed; however, natural log transformations did not alter the pattern of the results, and thus untransformed variables are reported here.
Confirmatory factor analysis model estimation
Models were estimated in Mplus version 5.21( Reference Muthén and Muthén 56 ) using the weighted least squares mean- and variance-adjusted (WLSMV) estimator due to the use of categorical (item-level) data. Models were assessed based on absolute and comparative fit statistics; models indicate acceptable fit when there is little discrepancy between the estimated and the actual variance–covariance matrix. A root mean square error of approximation (RMSEA) <0·06 and a non-significant χ 2 distribution indicate acceptable absolute fit, and a comparative fit index (CFI) and Tucker Lewis index (TLI) >0·95 indicate good comparative fit. Emphasis will be placed on the RMSEA, CFI and TLI, given that the χ 2 distribution is sensitive to sample size( Reference Bentler and Bonnet 57 ).
Covariates
Bivariate correlations were used to determine which of the twelve potential demographic and health covariates were significantly (P<0·05) related to either folate and/or vitamin B12 and SWB outcomes. Sex, age, use of cardiovascular medications and physical health (all r<0·3) satisfied these criteria at baseline, and thus were included in the final model. These variables were specified as covariates by regressing all baseline nutrition and SWB variables onto these four variables.
Direct effects from vitamin B12, folate and their interaction on subjective well-being
The direct effects model assesses whether vitamin B12, folate or the interaction between the two are causally related to PA, NA or LS at 18 months, controlling for both the prediction of these by PA, NA and LS at baseline and for covariates. Note that homocysteine is also included in this model but only related to itself, via auto-regression. The significant χ 2 distribution indicated poor fit (χ 2(173)=337·1 P<0·05); however, χ 2 is sensitive to larger sample sizes( Reference Bentler and Bonnet 57 ). (WLSMV estimates df and χ 2 value to best approximate the correct P value, therefore these cannot be interpreted in the standard way( Reference Muthén 58 ).) Acceptable absolute and comparative fit indices (RMSEA=0·05, CFI=0·96, TLI=0·98) suggest that the direct effects model provided a good fit to the data. All stability coefficients were large and significant (PA β=0·78; NA β=0·76; LS β=0·81; vitamin B12 β=0·76; folate β=0·67; interaction β=0·56; and homocysteine β=0·71; all P<0·001), suggesting that baseline levels of SWB components and nutrients are strong predictors of their subsequent 18-month measurements. Table 2 shows that vitamin B12, folate and their interaction weakly but significantly predicted PA 18 months later, beyond the prediction afforded by baseline PA. Vitamin B12, folate and the interaction between the two did not contribute to the prediction of NA or LS beyond baseline measures of these constructs.
Table 2 Standardised parameter estimates for cross-lagged regression models: direct and indirect effects from respective modelsFootnote * (Regression coefficients with their standard errors)
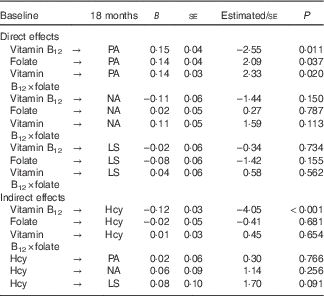
PA, positive affect; NA, negative affect; LS, life satisfaction; Hcy, homocysteine.
* Both models fit using the weighted least squares mean- and variance-adjusted estimator.
Indirect effects from vitamin B12, folate and their interaction on Subjective Well-Being via homocysteine
The indirect effects model (Fig. 1) assesses whether vitamin B12, folate or their interaction at baseline predict homocysteine at 18 months and whether homocysteine at baseline predicts PA, NA or LS at 18 months. The significant χ 2 distribution again indicated poor fit (χ 2(170)=327·5; P<0·05); however, acceptable absolute and comparative fit indices (RMSEA=0·05, CFI=0·97, TLI=0·98) suggest that the indirect effects model provided a good fit to the data. Again, all stability coefficients were large and significant (PA β=0·62; NA β=0·73; LS β=0·83; vitamin B12 β=0·77; folate β=0·62; interaction β=0·73; and homocysteine β=1·23; all P<0·001). Parameter estimates for baseline homocysteine to PA, NA and LS at 18 months were all weak and non-significant. Table 2 shows that folate and the interaction with vitamin B12 were not significant predictors of homocysteine at 18 months, but vitamin B12 at baseline was a significant predictor of homocysteine at 18 months. Mediation was assessed within two-wave, cross-lagged analyses as the product of the path from the independent variable (baseline) to the mediator (18 months) and the path from the mediator (baseline) to the dependent variable (18 months); if the product of these two paths is significantly different from zero, then we can infer partial mediation. With three predictors (vitamin B12, folate and their interaction), three outcome variables (PA, NA and LS) and one mediator, a total of nine potential mediations were assessed. Indirect effects for each of the nine models ranged from 0·0001 to 0·010, and Sobel’s Z test( Reference Sobel 59 ) confirmed that none were significantly different from zero.

Fig. 1 Indirect effects cross-lagged regression model including covariates. The direct effects cross-lagged regression model is specified separately and differs in that it excludes the regression of positive affect (PA), negative affect (NA) and life satisfaction (LS) at 18 months onto baseline homocysteine and the regression of homocysteine at 18 months onto baseline vitamin B12, folate and their interaction and instead estimates the direct parameters from baseline vitamin B12, folate and their interaction on PA, NA and LS at 18 months. *Covariates include sex, age, use of cardiovascular medications and physical health; indicators of PA, NA and LS, and co-varying residuals for these indicators between baseline and 18 months, are not included for simplicity.
Discussion
The results suggest a direct effect of vitamin B12, folate and their interaction on PA, but not on NA or LS. Only vitamin B12 significantly predicted homocysteine 18 months later and homocysteine did not predict PA, NA or LS, thus providing no support for any indirect effects of vitamin B12, folate or their interaction on PA, NA or LS through homocysteine as the mediator.
Negative associations between folate and vitamin B12 at baseline and homocysteine 18 months later reflect the causal relationship of folate and vitamin B12 with levels of homocysteine( Reference Bottiglieri 3 ). Our cross-sectional analyses suggest that folate contributes more to homocysteine than vitamin B12, consistent with previous reports( Reference Bjelland, Tell and Vollset 60 ). The relationship between baseline vitamin B12 and 18-month homocysteine was of a similar magnitude; however, the relationship was considerably attenuated between folate at baseline and 18-month homocysteine. Accounting for autoregressive effects of homocysteine further attenuated both relationships reducing that between folate and homocysteine to non-significance. This suggests that vitamin B12 influences homocysteine in this sample, but that folate does not. There are two potential explanations: first, previous reports have been made based on either cross-sectional( Reference Bjelland, Tell and Vollset 60 ) or longitudinal relationships without accounting for previous levels of homocysteine, which provided a considerably attenuated estimate in our own sample. Second, this could be explained by the low rates of folate deficiency present in this sample (6·8 %), whereas previous studies have recorded much higher rates of deficiencies( Reference Morris, Fava and Jacques 61 ), particularly in clinical populations( Reference Bottiglieri, Laundy and Crellin 62 ).
A similar pattern of results emerged when assessing the effect of homocysteine on the three components of SWB. Despite the presence of cross-sectional relationships between homocysteine and PA, there was no longitudinal relationship, suggesting that homocysteine may be only a marker for levels of PA. There were no observed relationships between homocysteine and either NA or LS. If homocysteine is only a marker for low levels of PA, then what is the cause? One possibility is that folate and/or vitamin B12 levels influence PA, and therefore elevated homocysteine is associated with PA due to its dependence on these B-vitamins. There were no cross-sectional relationships between folate or vitamin B12 with PA; however, cross-lagged analyses accounting for both autoregressive effects and the inter-correlation between PA, NA and LS demonstrated a causal relationship between folate (β=0·14), vitamin B12 (β=0·15) and their interaction (β=0·14) and subsequent levels of PA. There were no direct effects of folate, vitamin B12 or their interaction on subsequent levels of NA or LS. The results suggest that homocysteine does not mediate the relationship between folate, vitamin B12 or their interaction and PA, NA or LS. One previous study with older people found a relationship between elevated homocysteine and lower LS( Reference Jensen, Dehlin and Erfurth 35 ), although another found no relationship between folate and vitamin B12 with mood in a similar sample( Reference Cassidy, Kotynia-English and Acres 36 ). Both these studies are consistent with our own cross-sectional results, therefore highlighting the importance of longitudinal data to accurately reflect the proposed biological pathways.
The explanation that homocysteine is cross-sectionally related to PA due to its dependence on folate and vitamin B12, rather than exerting a causal influence on PA, is supported by CVD research. Evidence suggests that folate deficiency alone may account for the risk of cardiovascular events, with homocysteine being just a marker for low folate( Reference Verhaar, Stroes and Rabelink 63 ). This conclusion is supported by Verhaar et al.( Reference Verhaar, Wever and Kastelein 64 ), who reported that folate was beneficial for hypercholesterolaemia, independent of its effect on lowering homocysteine, and by Lewis et al.( Reference Lewis, Lawlor and Davey Smith 65 ) who reported an association between genotype MTHFR C677T and depression. This genotype influences functioning of the folate metabolic pathway, therefore further supporting a causal relationship of folate with depression. It has been established that CVD and depression are bi-directionally associated( Reference Van der Kooy, van Hout and Marwijk 66 ), suggesting that folate and/or vitamin B12 deficiencies, rather than elevated homocysteine, could be the common pathogenesis underlying the link between CVD and mental health.
Limitations
Generalisability was limited to those with normal levels of vitamin B12, folate and homocysteine, those without diagnosed depression and to older adults, because nutrient absorption and therefore dietary requirements are known to differ across the lifespan( 67 ). Serum measurements of vitamin B12 and folate have been criticised because they reflect recent dietary intake, whereas RBC measurements provide a more accurate reflection of body stores and are not affected by recent diet. However, serum vitamin B12 and folate demonstrated stability between the two time points 18 months apart; based on Table 1, serum vitamin B12 at baseline explained 58·4 % of the variance in serum vitamin B12 18 months later, and serum folate at baseline explained 36·8 % of the variance in serum folate 18 months later. Furthermore, only 16·6 % changed categories between the two time points for vitamin B12 (i.e. from deficient to non-deficient), and only 8·4 % for folate, thus providing further evidence for the broad stability of serum vitamin B12 and serum folate between the two time points, 18 months apart.
Future directions
Future research in this area should include measures of positive mental functioning and extend methods beyond the use of single, isolated nutrients to more accurately reflect nutritional intake. Future research could test additional potential nutritional pathways by considering the use of dietary patterns. Oxidative stress is another potential mechanism that could be explored to explain the link between folate and vitamin B12 with mental and cardiovascular health, given its implication in the pathogenesis of CVD( Reference Griendling and Alexander 68 ), and the fact that folate possesses antioxidant potential( 67 ). Analyses of nutritional pathways and dietary patterns will also be enhanced with the use of longitudinal data to correctly reflect the temporal nature of these hypotheses. As we have shown here, cross-sectional correlations can conceal the true nature of data patterns. Where limited resources impose constraints on longitudinal data collection, the use of retrospective data-collection methods can be useful, such as the Lifetime Diet Questionnaire( Reference Hosking, Danthiir and Nettelbeck 69 ) – a recently developed tool designed to access dietary intake across the lifespan.
Conclusion
This is the first study to assess the role of vitamin B12, folate and homocysteine in SWB in a representative sample of older community-living adults. The results suggest that higher levels of vitamin B12 and folate are beneficial for aspects of positive mental health in non-clinical, aged populations. Recommendations for optimal levels of vitamin B12 and folate for positive mental health are based on the observation of a direct effect of these B-vitamins on PA, rather than on their ability to lower levels of homocysteine.
Acknowledgements
The authors thank the following organisations for assistance in recruiting participants: Council on the Ageing (SA), Active Ageing Australia (SA), University of the Third Age (Adelaide, Port Adelaide, Tea Tree Gully, Elizabeth, Campbelltown and Noarlunga branches), Life Care Churches of Christ and Lifestyles SA and their retirement villages.
Supported by the University of Adelaide and the Commonwealth Scientific and Industrial Research Organisation Animal, Food and Health Sciences Brailsford Robertson Award (V. D., N. R. B.), a National Health and Medical Research Project (grant no. 578800; V. D., N. R. B.), and an Australian Postgraduate Award (L. C. E.).
V. D. and N. R. B. initiated and obtained funding for the project, and V. D. conceptualised the study design. The data were collected by L. C. E. and V. D. L. C. E. devised the idea for the manuscript, conducted the data analysis and prepared the manuscript. V. D. and N. R. B. both provided conceptual input for the analyses, commented on drafts, made suggestions regarding the presentation of material in the paper and provided editorial input. All the authors read and approved the final version of the manuscript.
There are no conflicts of interest.






