Frequent egg consumption has been linked to increased risk of CVD and mortality in diabetes( Reference Hu, Stampfer and Rimm 1 , Reference Djoussé and Gaziano 2 ). Recent observational evidence has also suggested that eating eggs regularly is associated with a higher risk of developing type 2 diabetes( Reference Djoussé, Gaziano and Buring 3 – Reference Radzevičienė and Ostrauskas 5 ) and gestational diabetes( Reference Qiu, Frederick and Zhang 6 ). However, this association has not been consistently demonstrated across different populations. For example, egg consumption was not associated with incident diabetes in older individuals with limited egg intake( Reference Djoussé, Kamineni and Nelson 7 ). To date, little is known regarding the potential mechanisms that may explain these reported associations. Since egg consumption is common, it is important to explore how this modifiable exposure is associated with the metabolic traits underlying type 2 diabetes. To our knowledge, only one previous study has investigated the association of egg consumption with insulin sensitivity( Reference Djoussé, Kamineni and Nelson 7 ). In a cross-sectional analysis of the Cardiovascular Health Study, higher egg consumption was significantly associated with higher fasting blood glucose, fasting insulin and homeostasis model assessment–insulin resistance( Reference Djoussé, Kamineni and Nelson 7 ). The association of egg consumption with β-cell function or insulin clearance has not been examined.
Because of the inconsistent associations of egg consumption with incident diabetes and the limited available evidence on potential mechanisms implicating eggs in diabetes pathogenesis, we aimed to examine the association between egg consumption and detailed measures of insulin sensitivity (SI), acute insulin response (AIR) and metabolic clearance rate of insulin (MCRI) from a frequently sampled intravenous glucose tolerance test (FSIGTT) in the multi-ethnic cohort of the Insulin Resistance Atherosclerosis Study (IRAS).
Participants and methods
Study population
The present cross-sectional study used data from the IRAS, which recruited 1625 participants from four clinical centres located in San Antonio, TX, San Luis Valley, CO, Oakland, CA and Los Angeles, CA between October 1992 and April 1994. The study design and research methods have been published in detail( Reference Wagenknecht, Mayer and Rewers 8 ). The institutional review boards of the clinical centres approved the study protocol and all participants provided written informed consent. The sample size of the present analysis was 949 participants, after excluding those with prevalent diabetes at baseline (n 537), as well as those with missing values of egg consumption, SI, AIR or MCRI (n 139).
Data collection
Participants attended two visits at the baseline examination and again at 5-year follow-up examination. The two visits at each examination occurred approximately one week apart. They were asked to fast for 12 h, to abstain from alcohol and heavy exercise for 24 h before each visit, as well as to abstain from smoking on the morning of the examination. Height and weight were measured to the nearest 0·5 cm and 0·1 kg, respectively. BMI was calculated as weight in kilograms divided by the square of height in metres. Waist and hip circumferences were measured to the nearest 0·5 cm using a steel tape. All measurements were taken in duplicate following standardized procedures and we used the averages of these measurements in the analyses. Blood pressure was measured using a standard mercury sphygmomanometer after participants were rested for 5 min. We used the average of the second and the third readings for analysis. Participants reported demographic and socio-economic information (e.g. age, sex, ethnicity, education, income) as well as lifestyle factors (e.g. smoking, alcohol consumption, physical activity) in standardized questionnaires( Reference Wagenknecht, Mayer and Rewers 8 ).
Measurement of egg consumption
At baseline, centrally trained interviewers conducted interviews to complete a semi-quantitative FFQ. The FFQ was designed to assess usual dietary intake in the past year. The 114-item FFQ was adapted from the National Cancer Institute Health Habits and History Questionnaire (NCI-HHHQ) and the food items were expanded to reflect the dietary intake of the diverse IRAS populations. The FFQ was validated against eight 24 h dietary recalls. The IRAS investigators used the HHHQ-DIETSYS analysis software (version 3·0, 1993) to analyse nutrient intake from the FFQ( Reference Mayer-Davis, Vitolins and Carmichael 9 ). Each item of the FFQ provided nine frequency options, from ‘never or less than once a month’ to ‘six or more times per day’. Participants were asked to indicate how often they consumed each item and to specify whether they consumed a small, medium or large portion compared with individuals in similar age and sex groups. To calculate the intake of each item, the frequency of intake was weighted by the portion size indicated, using a factor of 0·5, 1 and 1·5 for small, medium and large, respectively. The FFQ included one question regarding egg consumption, including omelettes and frittata. We divided egg consumption into four categories: less than one medium egg per week, one to less than three medium eggs per week, three to less than five medium eggs per week and five or more medium eggs per week.
Measurement of insulin sensitivity, β-cell function and insulin clearance
A modified FSIGTT was used to measure insulin sensitivity, β-cell function and insulin clearance( Reference Bergman, Finegood and Ader 10 ). Modifications to the original protocol included: (i) insulin, instead of tolbutamide, to ensure adequate levels of plasma insulin to calculate insulin sensitivity accurately across a broad range of glucose tolerance( Reference Welch, Gebhart and Bergman 11 ); and (ii) a reduced sampling protocol, using twelve instead of thirty samples, because of the large number of participants( Reference Steil, Volund and Kahn 12 ). Insulin resistance, expressed as SI, was calculated using minimal model analysis (MINMOD version 3·0)( Reference Pacini and Bergman 13 ). A higher value of SI indicates an increase in insulin sensitivity. β-Cell function was measured by AIR defined as the average increase in plasma insulin at time points 2 and 4 min after infusing glucose( Reference Lorenzo, Wagenknecht and D'Agostino 14 ). A higher value of AIR indicates an increase in insulin secretion. MCRI was calculated as the ratio of the insulin dose over the incremental area under the curve of insulin from 20 min to infinity( Reference Polonsky, Pugh and Jaspan 15 ) using the following equation:
where Dose represents the amount of insulin injected at 20 min, Ins(t) the plasma insulin concentration in standard units at each FSIGTT sampling point and Ins(0) the fasting plasma insulin concentration determined before injecting glucose in the FSIGTT. A higher value of MCRI indicates an increase in insulin clearance.
Biochemical analysis
Plasma glucose was measured using the glucose oxidase technique on an auto-analyser. Plasma insulin level was determined with the dextran–charcoal RIA( Reference Wagenknecht, Mayer and Rewers 8 ). Measurements of plasma lipids and lipoproteins were determined at the central IRAS laboratory using the Lipid Research Clinics methods( Reference Howard, Mayer-Davis and Goff 16 ).
Statistical analysis
We described the characteristics of participants at baseline, stratified by categories of egg consumption, using means and standard deviations for normally distributed continuous variables, medians and interquartile ranges for skewed continuous variables and percentages for categorical variables. We used ANOVA, Kruskal–Wallis tests and χ 2 tests to determine whether continuous and categorical variables differed across the distribution of egg consumption. We described the association of egg consumption with potential mediators (e.g. dietary cholesterol, dietary saturated fat, energy intake and BMI) and food choices which may cluster with egg consumption using Spearman's rank correlation coefficient.
We modelled measures of insulin sensitivity, β-cell function and insulin clearance, including SI, fasting insulin, AIR and MCRI, as continuous outcome variables. The distributions of these outcome variables were skewed; therefore, we natural log-transformed them for normality. For SI, we added a constant of one to all values before the log-transformation because of the presence of zero values in the data. We used unadjusted and multivariable-adjusted linear regression to explore the association between egg consumption and each outcome measure. We reported the regression coefficients and their 95 % confidence intervals in each model, and presented the P value for linear trend across categories of egg consumption.
We included covariates in multivariable models if they were risk factors for diabetes based on previous literature, if they were significantly correlated with egg consumption in the IRAS data or if they were of a priori clinical relevance. Potential confounders included age, sex, ethnicity, education, income, smoking, alcohol consumption, energy expenditure, family history of diabetes, and consumption of processed meats, fast foods, red meats, dairy products, vegetables, whole grains, coffee and tea.
We used a staged approach to enter covariates into the regression model. We first adjusted for demographics (i.e. age, sex, ethnicity, study centre), followed by socio-economic status (i.e. education, income), lifestyle factors (i.e. smoking, alcohol consumption, energy expenditure), family history of diabetes and food items (processed meats, fast foods, red meats, dairy products, vegetables, whole grains, coffee and tea). In this multivariable-adjusted model, we then entered dietary factors (i.e. energy, saturated fat and cholesterol), BMI and waist:hip ratio (WHR) one variable at a time to determine if any of these variables had an impact on the associations observed. We used Sobel–Goodman tests, which bootstrap the standard errors of the mediated effects, to determine whether dietary cholesterol and BMI mediated the association of egg consumption with insulin sensitivity and clearance( Reference Mackinnon, Warsi and Dwyer 17 ). Regression models were based on 949 participants with data on egg consumption SI, AIR and MCRI; sample sizes in full multivariable-adjusted models ranged from 937 to 942 due to occasional missing values on covariates. We examined the interaction between egg consumption and age, sex, ethnicity or BMI status on all outcome variables. Statistical analyses were performed using the statistical software package STATA 12·0.
Results
Characteristics of the study population, stratified by categories of egg consumption, are shown in Table 1. The median age of the participants was 54 years (range 40–69 years) and 45 % were men. Among these participants, 40 % were Caucasians, 26 % were African Americans and 34 % were Hispanics. Approximately 50 % of the study population reported consuming less than one egg per week. More Hispanics reported eating five or more eggs per week. Participants with higher income and higher educational level reported eating eggs less frequently. Participants who ate eggs regularly were more likely to be male and to smoke. In addition, they had higher values of BMI and WHR, as well as higher dietary intakes of energy, saturated fat and cholesterol. Frequent consumption of eggs was associated with lower values of SI and MCRI and higher values of AIR and fasting insulin. There was no significant difference in fasting blood glucose and 2-h post-load blood glucose related to the number of eggs consumed per week. Egg consumption was positively and significantly correlated with dietary cholesterol, dietary saturated fat, energy intake, BMI and WHR, as well as consumption of processed meats, fast foods, red meats, dairy products, vegetables, whole grains, coffee and tea (Table 2).
Table 1 Baseline characteristics of non-diabetic participants (n 949) in the Insulin Resistance Atherosclerosis Study (IRAS) by distribution of egg consumption
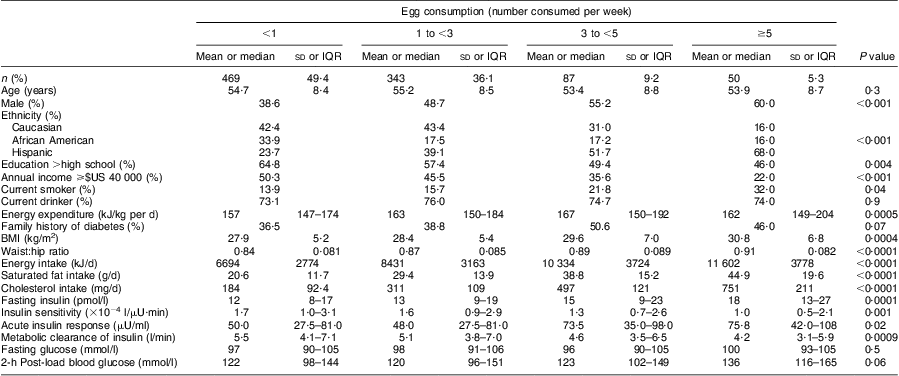
Data are presented as mean and standard deviation, percentage, or median and interquartile range (IQR).
ANOVA, Kruskal–Wallis tests and χ 2 tests were used to determine whether continuous and categorical variables differed across the distribution of egg consumption.
Table 2 Spearman correlation analysis between egg consumption and indices of obesity and food items among non-diabetic participants (n 949) in the Insulin Resistance Atherosclerosis Study (IRAS)
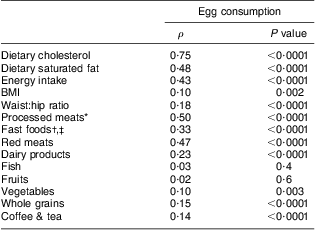
Spearman's rank correlation coefficient was used to describe the correlation of egg consumption with indices of obesity and food items.
*Processed meats refer to ham, sausage, hot dogs, bacon and bologna.
†Fast foods refer to burgers, French fries, fried chicken, fried fish and pizza.
‡Dairy products refer to cheese, milk and yoghurt.
Egg consumption was inversely associated with SI and MCRI, and positively associated with fasting insulin and 2-h post-load blood glucose, in unadjusted regression models and in models adjusted for potential confounders, including demographic, socio-economic, lifestyle and dietary factors and family history of diabetes. Egg consumption was not associated with SI-adjusted AIR and fasting blood glucose in unadjusted and multivariable-adjusted regression models. There was no interaction between egg consumption and age, sex, ethnicity and BMI status on any of the outcomes (Table 3).
Table 3 Estimated regression coefficients (and 95% confidence intervals) on the association between egg consumption and insulin metabolism and blood glucose among non-diabetic participants (n 949) in the Insulin Resistance Atherosclerosis Study (IRAS)

SI, insulin sensitivity; MCRI, metabolic clearance of insulin; AIR, acute insulin response.
Model 1: adjusted for age, sex, ethnicity, study centre.
Model 2: Model 1 + education, income, smoking, alcohol consumption, energy expenditure, family history of diabetes.
Model 3: Model 2 + dietary factors (processed meats, fast foods, red meats, dairy products, fruits, whole grains, coffee and tea).
Unadjusted and multivariable-adjusted linear regression analyses were used to explore the association between egg consumption and each outcome measure.
In a series of regression models to explore the potential mediating/confounding effects, we observed that the associations of egg consumption with SI, fasting insulin, MCRI and 2-h post-load blood glucose remained statistically significant after adjusting for energy intake, dietary saturated fat or WHR. However, all of these associations were attenuated to non-significance by dietary cholesterol and BMI (Table 4). The Sobel–Goodman mediation tests showed that dietary cholesterol and BMI are intermediates in the association of egg consumption with insulin sensitivity and clearance (Table 5).
Table 4 Estimated regression coefficients (and 95% confidence intervals) on the association between egg consumption and insulin metabolism and blood glucose, adjusted for potential mediators, among non-diabetic participants (n 937 to 942) in the Insulin Resistance Atherosclerosis Study (IRAS)
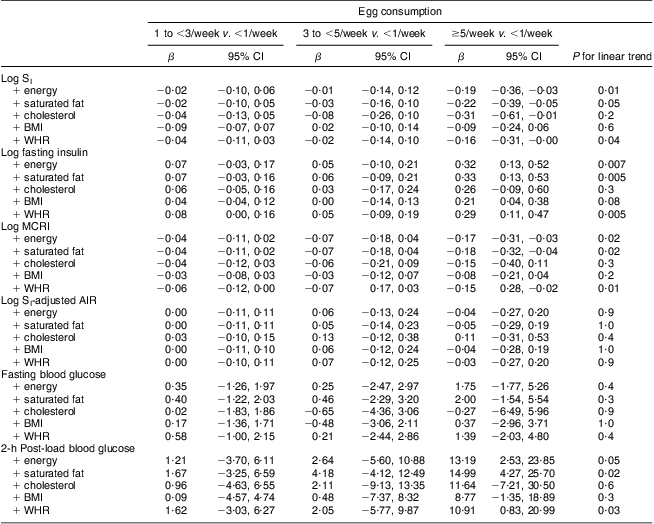
SI, insulin sensitivity; HR, waist:hip ratio; MCRI, metabolic clearance of insulin; AIR, acute insulin response.
Base model adjusted age, sex, ethnicity, study centre, education, income, smoking, alcohol consumption, energy expenditure, family history of diabetes and dietary factors; each row represents the addition to this model of the individual variable indicated.
Unadjusted and multivariable-adjusted linear regression analyses were used to determine the association between egg consumption and each outcome measure.
Table 5 Sobel–Goodman mediation analysis for the association of egg consumption with insulin sensitivity and clearance among non-diabetic participants (n 937 to 942) in the Insulin Resistance Atherosclerosis Study (IRAS)

SI, insulin sensitivity; MCRI, metabolic clearance of insulin.
Sobel–Goodman mediation tests were used to determine whether dietary cholesterol or BMI mediates the association of egg consumption with insulin sensitivity and clearance.
Discussion
The present cross-sectional study extends the scientific literature on the association of egg consumption with the risk of diabetes. Specifically, while egg consumption was inversely associated with direct measures of insulin sensitivity and clearance, these associations were mediated by dietary cholesterol or BMI in the non-diabetic multi-ethnic population of the IRAS.
Limited evidence is available to describe the association between egg consumption and the metabolic traits underlying type 2 diabetes. The Cardiovascular Health Study reported a significant positive association of egg consumption with fasting blood glucose and surrogate measures of insulin resistance (i.e. fasting insulin and homeostasis model assessment–insulin resistance); however, as suggested by the authors of that paper, the small absolute differences they reported were unlikely of any clinical significance( Reference Djoussé, Kamineni and Nelson 7 ). No previous studies have examined these associations using detailed measures of insulin sensitivity, secretion and clearance.
Our findings suggest a strong mediating role of dietary cholesterol in the association of egg consumption with insulin sensitivity and clearance. Dietary cholesterol has been shown to be associated with an increased risk of incident type 2 diabetes( Reference Djoussé, Gaziano and Buring 3 , Reference Meyer, Kushi and Jacob 18 ) and gestational diabetes( Reference Qiu, Frederick and Zhang 6 ). Egg consumption has traditionally been accompanied by food choices that are high in fat and cholesterol, excessive dietary cholesterol could result in the accumulation of hepatic cholesterol esters and TAG, which in turn activate liver X receptors (LXR), nuclear receptors that regulate hepatic lipid metabolism( Reference Basciano, Miller and Naples 19 ). LXR activation decreases insulin receptor β-subunit mass, reduces insulin receptor substrate-1 tyrosine phosphorylation and increases protein tyrosine phosphatase IB protein mass; all of these metabolic derangements interrupt hepatic insulin signalling and aggravate insulin resistance( Reference Basciano, Miller and Baker 20 ). Experimental evidence has shown that treating insulin-resistant mice with synthetic LXR ligands improves glucose tolerance and increases peripheral glucose uptake( Reference Commerford, Vargas and Dorfman 21 ).
A number of previous prospective studies have shown an independent association between egg consumption and incident diabetes( Reference Djoussé, Gaziano and Buring 3 – Reference Qiu, Frederick and Zhang 6 ). While these studies have adjusted for a wide range of potential confounders in the regression analyses, only one of them has accounted for dietary cholesterol( Reference Djoussé, Gaziano and Buring 3 ). In the Women's Health Study, the association between egg consumption and type 2 diabetes was attenuated after additional adjustment for dietary cholesterol( Reference Djoussé, Gaziano and Buring 3 ).
BMI also mediates the association of egg consumption with insulin sensitivity and clearance. As discussed earlier, egg consumption is often accompanied by food choices that are high in fat and cholesterol in Western countries; thus, it is plausible that excessive intakes of these high-fat and energy-dense foods increase the risk of diabetes through obesity which leads to insulin resistance, inflammation and other disorders in the pathogenesis of diabetes. We have previously reported in the IRAS cohort that higher egg consumption was clustered in a dietary pattern with red meat, low-fibre bread and cereals, tomato vegetables, fried potatoes and cheese, which in turn increased the risk of incident diabetes( Reference Liese, Weis and Schulz 22 ). To support this notion, a recent study demonstrated that intake of restaurant meals was associated with risk of incident diabetes and this association was mediated by weight gain and obesity( Reference Krishnan, Coogan and Boggs 23 ). In addition, further adjustment for the consumption of processed meats and fast foods attenuated the associations of egg consumption with insulin sensitivity and clearance, which provides further support that these associations were mediated by food choices which are characterized by high dietary intake of cholesterol. Lastly, egg consumption may cluster with other lifestyle factors that impact on insulin sensitivity and clearance. We have adjusted for alcohol consumption, smoking, physical activity and a wide range of food items that were correlated with egg consumption in IRAS. However, we could not rule out the possibility of residual confounding by factors that we did not measure or measured imprecisely.
The strengths of the present study included the well-characterized multi-ethnic population of the IRAS cohort and the detailed measurements of insulin sensitivity, secretion and clearance. Our study has potential limitations. Egg consumption was measured by self-report which increases the chance of measurement error and bias. If the measurement errors were random, then the inverse association of egg consumption with insulin sensitivity and clearance would be stronger than we observed. However, any systematic difference in reporting egg consumption based on obesity status or social desirability would bias the association in either direction. We did not have information on cooking methods. Individuals who habitually consume poached or boiled eggs may be characterized by different lifestyle patterns which may modify their risk of diabetes. Finally, our results may not be generalizable to populations with other ethnic backgrounds.
Conclusion
The associations that we observed suggest that egg consumption is associated with lower insulin sensitivity and clearance, but that these associations are mediated by dietary cholesterol or BMI. Our findings reinforce public health recommendations to limit dietary intake of cholesterol and to reduce weight as preventive strategies for type 2 diabetes.
Acknowledgements
Sources of funding: C.C.L. is supported by a postdoctoral research fellowship from the Banting & Best Diabetes Centre, University of Toronto, Canada. A.J.H. holds a Tier II Canada Research Chair in Diabetes Epidemiology. IRAS was supported by grants U01-HL47892, U01-HL47902, DK-29867 and R01-58329 from the National Heart, Lung, and Blood Institute, and by grant M01-RR-43 from the National Institutes of Health. Conflicts of interest: The authors declare that there is no conflict of interest associated with this manuscript. Authors’ contributions: L.E.W., S.M.H. and M.J.R. designed the study. C.C.L. and A.J.H. analysed the data and wrote the manuscript. All authors contributed to the discussion and approved the contents of the manuscript.







