Introduction
Fourteen species of Darwin’s finches (Geospizinae) occur in the Galápagos archipelago, representing 47% of the 30 resident species of land birds (Parent et al., Reference Parent, Caccone and Petren2008). Although several island populations of Darwin’s finches and mockingbirds have been lost since the arrival of humans (Steadman, Reference Steadman1986), no land bird species in the Galápagos has gone extinct in historic times (Dvorak et al., Reference Dvorak, Vargas, Fessl and Tebbich2004; Grant & Grant, Reference Grant and Grant2008b; O’Connor et al., Reference O’Connor, Sulloway and Kleindorfer2010c). However, three species, mangrove finch Camarhynchus heliobates, medium tree-finch Camarhynchus pauper and Floreana mockingbird Mimus trifasciatus, are categorized as Critically Endangered on the IUCN Red List of Threatened Species (IUCN, 2010).
Human activity over the past 500 years has dramatically altered most of the Galápagos islands. Santa Cruz Island was the last island to be colonized by humans; its first settlers arrived in the 1920s. The following decades saw the almost complete disappearance of the native humid forest on Santa Cruz and its replacement with introduced vegetation. Since the 1990s the ecological impact of human presence has accelerated because of drastic ecological and social changes (Watkins & Cruz, Reference Watkins and Cruz2007; González et al., Reference González, Montes, Rodríguez and Tapia2008). Santa Cruz now receives the highest number of tourists per year in the Galápagos and exhibits the second highest degree of degradation in several of its vegetation zones (Watson et al., Reference Watson, Trueman, Tufet, Henderson and Atkinson2010).
Here we use quantitative census data to describe the distribution and abundance of the land birds of Santa Cruz. By comparing data for 1997–1998 with data for 2008–2010 we analyse population changes and discuss the effects of drastic ecological changes on bird populations. To evaluate the impact of these changes for conservation of individual species we estimated population sizes for 2008, the year with the most complete survey coverage.
Study area
The 986 km2 Santa Cruz Island, which has a maximum altitude of 864 m, is the second largest island in the Galápagos archipelago, with 88% of its surface protected as part of the Galápagos National Park (Servicio Parque Nacional Galápagos, 2006). As a result of precipitation and temperature patterns, several vegetation zones occur along an altitudinal gradient. Besides the coastal strip the following zones are commonly distinguished (Wiggins & Porter, Reference Wiggins and Porter1971; Fig. 1): (1) Dry zone, southern slope (0–120 m, 531.7 km2), characterized by dry forest and scrub land dominated by deciduous trees, mainly palo santo Bursera graveolens, cacti Opuntia echios, Jasminocereus thouarsii and various species of scrubs. (2) Dry zone, northern slope (191.5 km2), covered by high palo santo forest. (3) Transition zone (120–300 m, 99.2 km2), a dense, mainly deciduous forest dominated by the endemics pega-pega Pisonia floribunda, guayabillo Psidium galapageiums and matazarno Piscidia carthagenensis. (4) Scalesia zone (300–650 m, 1.8 km2), an evergreen forest dominated by treelike Scalesia pedunculata; trunks and branches are densely covered with epiphytes (mosses, liverworts, ferns and others); most of this zone and smaller parts of the transition and fern zones have been converted to agriculture. (5) Agricultural zone (114.2 km2), mainly farms and grazing land, with introduced trees and shrubs (e.g. Psidium guajava, Cedrela odorata, Rubus spp.), almost completely confined to the wetter southern side of the island. (6) Fern zone (above 650 m, 17.7 km2), where vegetation consists mostly of ferns, grasses and the shrub Miconia robinsoniana; many of the introduced plant species from adjacent farmland have invaded the fern zone (Watson et al., Reference Watson, Trueman, Tufet, Henderson and Atkinson2010). (7) Cinchona zone (12 km2), areas of the fern zone that are now overgrown with forests of Cinchona pubescens (Jäger et al., Reference Jäger, Kowarik and Tye2009).
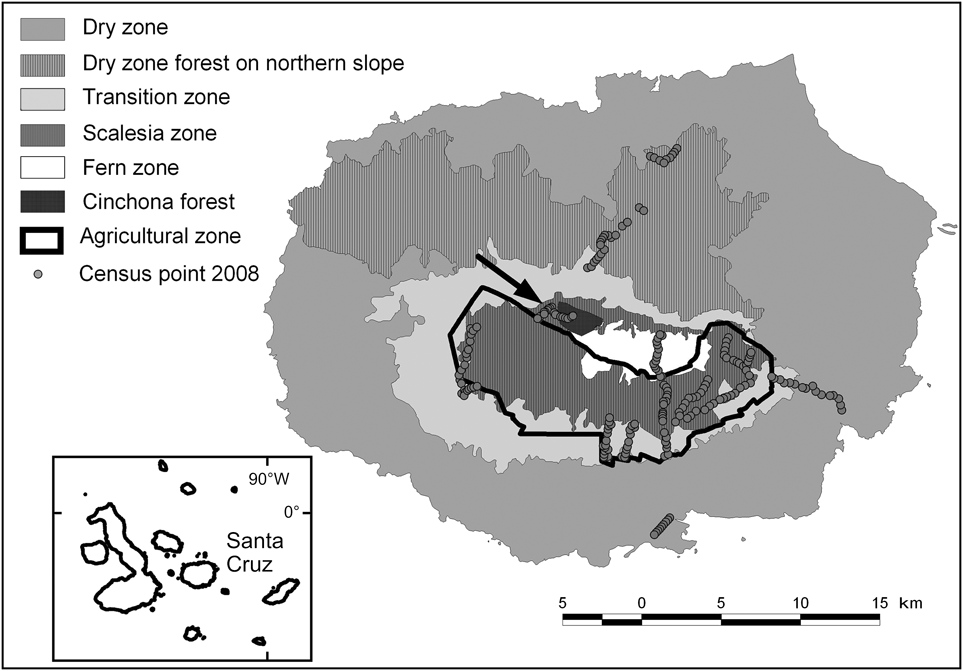
Fig. 1 The extent and location of the vegetation zones on Santa Cruz Island, based on data from Huttel (Reference Huttel, Lawesson, Hamann, Rogers, Reck and Ochoa1990), and the locations of the 233 survey points (Table 1) used in 2008. The inset indicates the location of Santa Cruz in the Galápagos archipelago.
Methods
Nomenclature of Darwin’s finches follows Petren et al. (Reference Petren, Grant and Grant1999).
Data collection
Point counts were used for all surveys. Data were collected during the early breeding season of land birds in 1997 (17 January–20 March), 1998 (15 January–17 February), 2008 (29 January–15 March) and 2010 (13 January–10 March) by MD (all years), BF (all but 2010) and EN (2010). Counts lasted for 5 minutes between 07.00 and 11.00. Darwin’s finches and other land birds on the Galápagos show high singing activity during the breeding season and mostly inhabit areas of dense vegetation, and therefore direct observations are unreliable. As our aim was to calculate relative and absolute densities in a comparable way we noted only singing birds (presumed to be territory holding males) to avoid counts of non-singing females or juveniles. For Galápagos mockingbird Mimus parvulus, Galápagos flycatcher Myiarchus magnirostris and smooth-billed ani Crotophaga ani all observations (except overflying individuals) were tallied. However, the first two species have a tendency to follow an observer and we did not therefore include these species in the between year comparison (Table 3). The dark-billed cuckoo Coccyzus melacoryphus was also excluded from this analysis because its singing activity seems to be less correlated with breeding activity and territories than other species and is dependent on other, unknown, factors. Distances of the birds to the observers were estimated to the nearest 5 m between 0 and 20 m, and to the nearest 10 m beyond 20 m. In all years we started with calibration sessions for distance estimation.
Table 1 Number of sample points for birds in each vegetation zone and year on Santa Cruz Island (see Fig. 1 for the locations of the 233 points in 2008).
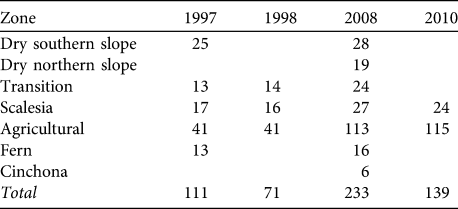
A snapshot approach was used. At the beginning we counted all singing birds as rapidly as possible; then, during the remaining minutes, we counted any additional birds in the context of the position and behaviour of the individuals already registered. Using this approach we decreased the risk of counting the same individuals twice (e.g. while moving around) and increased the probability of recognizing new birds that were silent during the initial phase. We avoided counting birds that were obviously not present at the point initially, such as individuals moving into the area during the 5-minute counting period. Points were generally counted once per year as it was assumed that the census period covered the main phase of singing activity. In 1997 and 1998 some points were counted twice; in this case the count with the highest bird numbers was used. Points covered all vegetation zones and altitudes on Santa Cruz (Table 1). Because of the difficulties of accessing some parts of the island points could not be located randomly but were placed along existing paths and small roads (we avoided counting along busy and wide roads). Points were spaced at least 500 m apart in all habitats except in the Scalesia zone, where smaller distances had to be used. Coverage of vegetation zones was uneven between years, with an emphasis on the agricultural and Scalesia zones. In 2008 we added additional survey points in the dry northern slope and Cinchona zones (Table 1). We repeated point counts in the agricultural and Scalesia zones in 2010 to test the validity of the results from 2008.
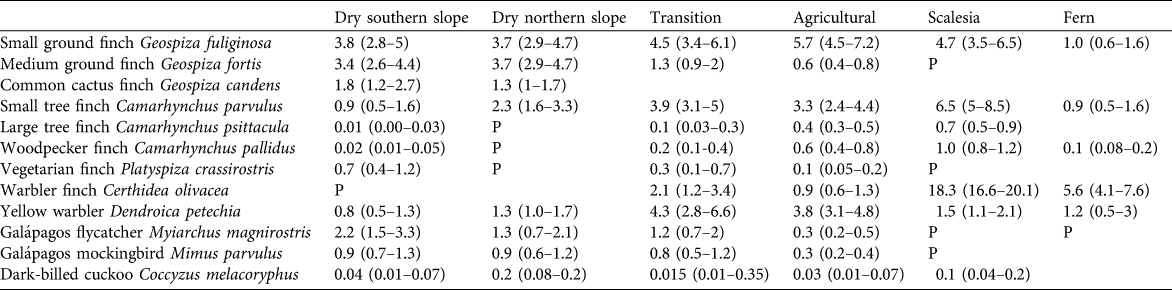
Table 2 Estimates of the density of singing males per ha (with 95% confidence intervals in parentheses) of 12 bird species in 2008, including eight species of Darwin’s finches, in the seven vegetation zones on Santa Cruz Island (Fig. 1). P (present) indicates a species was recorded in low numbers; empty fields indicate the species was not detected. Confidence intervals were calculated with Distance, using bootstrapping methods.
Data analysis
To examine temporal variation we used the numbers of birds per point to calculate relative abundance in each vegetation zone. We calculated a one-way ANOVA with year as the factor and performed pair-wise comparisons among years with a Bonferroni post-hoc test.
Bird densities for 2008 were calculated with Distance v. 5 (Thomas et al., Reference Thomas, Laake, Strindberg, Marques, Buckland and Borchers2006) using techniques recommended by Buckland et al. (Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) and Thomas et al. (Reference Thomas, Laake, Strindberg, Marques, Buckland and Borchers2006). Density estimation from point count data analysed with Distance is now widely used for surveys in tropical and insular environments (Marsden et al., Reference Marsden, Jones, Linsley, Mead and Hounsome1997; Marsden, Reference Marsden1999; Cramp et al., Reference Cramp, Pratt, Marshall, Amidon and Williams2009). Densities (singing males per km2) were calculated for each individual species in each vegetation zone; 95% confidence intervals were calculated using bootstrapping methods. All observations were entered into Distance in grouped format; intervals of either 10 or 20 m were used in the analyses. Estimated distances were inspected for outliers that could make model fit problematic, and any found were excluded. Combinations of all key models and adjustments provided by Distance were tested. The detection curve based on the model that best fitted the data was chosen automatically by Distance using Akaike’s Information Criterion. Detection curves were fitted to the data separately for each year and each vegetation zone if there were enough data (60–80 records, as recommended by Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). In the case of fewer observations, a composite detection curve using data from two or more zones was used.
To estimate populations, mean bird densities in each vegetation zone were multiplied by the estimated area of the zone. Figures for all zones were summed for each bird species; in this way we calculated an estimate for the breeding population of the entire island. For some species, such as the shy dark-billed cuckoo for which song seems to have limited territorial function, our calculations could be underestimates. The extent of each vegetation zone was derived from geographical information system data using satellite imagery (Huttel, Reference Huttel, Lawesson, Hamann, Rogers, Reck and Ochoa1990).
Results
We encountered 17 bird species during our censuses. Calculation of relative abundances and densities for four species was not possible because of small sample sizes: vermilion flycatcher Pyrocephalus rubinus (27 observations), paint-billed crake Neocrex erythrops (10), Galápagos rail Laterallus spilonotus (9), and large ground-finch Geospiza magnirostris (3). The latter may have very specific habitat requirements and thus be more abundant outside our study sites. Smooth-billed anis were encountered frequently at census points but were not included in the analysis because of their behavioural differences and consequent problems with the counting method used.
Distribution and density
Table 2 provides the density estimates for 12 species in 2008, the year with the most complete survey coverage, in the seven vegetation zones.
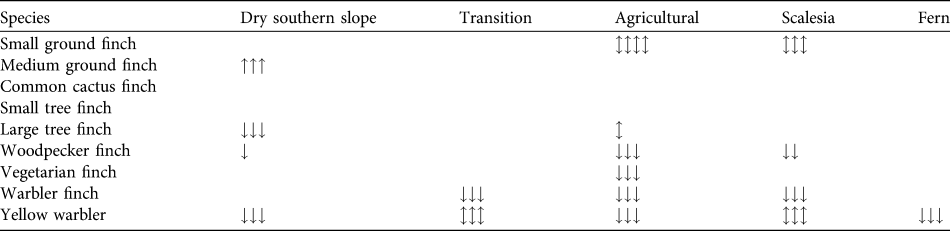
Table 3 Trends in the relative abundance of eight species of Darwin’s finches and the yellow warbler across five of the seven vegetation zones (trends cannot be examined for the dry northern and Cinchona zones as there are data for only 1 year) on Santa Cruz Island (Fig. 1). Black shading indicates that the species was not observed, grey that the species occurred irregularly (Table 2). We tested for significant differences in abundance across years and zones with an ANOVA using Bonferroni post-hoc corrections. Arrows indicate significance at P < 0.05 (one arrow), < 0.01 (two) and < 0.001 (three) and direction of change; arrows in both directions indicate fluctuating significance between years. Empty fields indicate no significant change between years.
Dry zone, southern and northern slope
The two most abundant species were the small ground finch Geospiza fuliginosa and medium ground finch Geospiza fortis; common cactus finch Geospiza scandens was common but restricted to areas with cacti (at c. 100 m altitude). The large ground finch was confined to the dry and lower transition zone but in low numbers. The dry zone is the main habitat of the vegetarian finch Platyspiza crassirostris, Galápagos mockingbird and flycatcher, although the former is rare on the northern slope. Of the tree finches only the small tree finch Camarhynchus parvulus was common, whereas woodpecker finch Camarhynchus pallidus and large tree finch Camarhynchus psittacula were both sparsely distributed and restricted to areas with tall palo santo trees. Warbler finch Certhidea olivacea occurred locally down to c. 150 m altitude. The density of the yellow warbler Dendroica petechia was low.
Transition zone
The four most common species were small ground finch, yellow warbler, small tree and warbler finches; all other species occurred at much lower densities. Vegetarian finch and Galápagos mockingbird both reached the upper limit of their distribution at 250–300 m in this zone.
Scalesia zone
The little that remains of the once expansive Scalesia forest still holds the highest densities of warbler, small tree, large tree and woodpecker finches. The small ground finch is a common breeding bird in this zone. This zone, at c. 550 m at Los Gemelos and its surroundings, is the most important remaining breeding site of the vermilion flycatcher.
Agricultural zone
The natural vegetation of the higher transition zone, most of the Scalesia zone, and parts of the fern zone have been converted to agricultural land, and changes are ongoing. These areas hold the majority of the breeding populations of several species of tree finches. Woodpecker and large tree finches were locally common in stands of higher trees, mainly Cuban cedar Cedrela odorata, avocado Persea americana, and small woodlands. Small tree and small ground finches and yellow warbler were all widespread and common in semi-open and open farmland. Vegetarian finch, Galápagos mockingbird and flycatcher, and medium ground finch were confined to lower elevations up to 300 m. Warbler finch still occurred locally in the higher elevation zones but its status has changed dramatically (see below).
Fern zone
The warbler finch was common and the small tree and small ground finches and yellow warbler were widely distributed but at low densities.
Cinchona zone
In this zone the three tree finch species and warbler finch reach densities comparable to the Scalesia forest zone.
Population changes between 1997 and 2010
We calculated changes in relative abundance for eight species of Darwin’s finches and the yellow warbler (Table 3, Appendix) in five of the vegetation zones. For the dry northern slope and Cinchona zones no comparative data are available (Table 1). The medium ground finch in the dry zone more than doubled between 1997 and 2008 (F = 35.3, P < 0.001). Small ground finch, a foraging generalist, increased significantly from 1997/1998 to 2008 in both the agricultural and Scalesia zones, followed by a significant decrease in 2010 (Scalesia: F = 17.9, P < 0.001; agricultural: F = 4.8, P < 0.01). The small tree finch, another foraging generalist, remained stable in all five vegetation zones. Large tree finch and vegetarian finch decreased in the dry zone and the agricultural zone, respectively, but these zones were not the species’ strongholds (Tables 2–3). Three insectivorous species decreased significantly in several zones. Woodpecker finch declined significantly in the dry zone (> 65%, F = 6.4, P < 0.05), the Scalesia forest (> 20%, F = 5.88, P < 0.01), and the agricultural zone (> 50%, F = 17.66, P < 0.001; Fig. 2). Warbler finch declined significantly in three of five zones (transition: > 75%, F = 30.65, P < 0.001; Scalesia: > 45%, F = 66.83, P < 0.001, agricultural zone: > 85%, F = 64.39, P < 0.001; Fig. 2); the abundance of this species did not change in the fern zone and only few data are available for the dry zone. The yellow warbler exhibited negative trends in all vegetation zones, with significant declines in the dry (> 85%, F = 85.07, P < 0.001), agricultural (> 35%, F = 38.67, P < 00.1) and fern zones (> 70%, F = 17.87, P < 0.001).
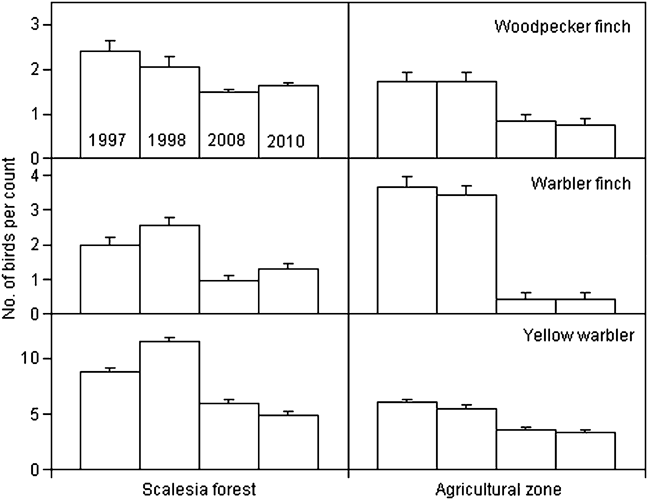
Fig. 2 Mean (± SE) number of birds per point count for woodpecker and warbler finches and yellow warbler in 1997, 1998, 2008 and 2010 in the Scalesia forest and agricultural zones of Santa Cruz Island (Fig. 1). Note the different y-axis scales. These three species showed the strongest declines in these zones.
Population estimates for 2008
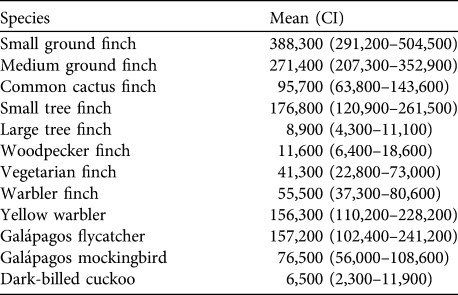
The total population of 12 of the land bird species on Santa Cruz Island in 2008 was estimated to be 1.44 million breeding pairs (assuming a 1 : 1 sex ratio); c. 1 million of these were Darwin’s finches (Table 4). The five species with > 10% of total abundance were small ground finch (26.7%), medium ground finch (18.8%), small tree finch (12.3%), Galápagos flycatcher (10.9%) and yellow warbler (10.8%). Although the warbler finch has declined dramatically during the last 10 years in the agricultural zone it still had a comparatively large breeding population with c. 55,500 singing males because of its high density in the fern zone. Vegetarian finch occurred everywhere at a low density but had a total of c. 41,300 singing males because of its wide range in the dry and transition zones. Population sizes of woodpecker finch and large tree finch, both largely confined to higher altitudes, were lower at c. 12,000 and 9,000 singing males, respectively. The breeding population of large ground finch could not be estimated but was probably in the low thousands and the vermilion flycatcher probably numbers < 200 pairs.
Table 4 Estimates of the mean total population of singing males (with confidence intervals in parentheses) of 12 bird species in 2008, including eight species of Darwin’s finches, on Santa Cruz Island (Fig. 1). Values calculated with Distance for each vegetation zone were multiplied by the respective area and then summed for the entire island. Values are rounded to the nearest 100.
Discussion
The most alarming finding of our study was the dramatic decline of at least four insectivorous bird species on Santa Cruz Island between 1997 and 2010 (warbler and woodpecker finches, yellow warbler and vermilion flycatcher, the latter already detected by Wiedenfeld, Reference Wiedenfeld2006). The population size of the formerly common vermilion flycatcher is now too small to be censused reliably with point counts. We found significant decreases, in at least one vegetation zone, of the large tree and vegetarian finches but sample sizes were low. Only three species (small ground, medium ground and small tree finches) either increased or appeared to be stable.
The majority of the populations that declined did so in the humid areas of Santa Cruz (upper transition, agricultural and Scalesia zones). These environments are the stronghold of the warbler and tree finches and have been, directly or indirectly, altered by human activity. The endemic Scalesia forest is estimated to have covered almost 100 km2 on Santa Cruz (Stewart, Reference Stewart1915) but was reduced to c. 20% of its original size by the 1980s (Huttel, Reference Huttel, Lawesson, Hamann, Rogers, Reck and Ochoa1990) and 1–2% by 2009 (Mauchamp & Atkinson, Reference Mauchamp and Atkinson2011). These remnant Scalesia forest patches, which are now of only 1–2 km2, have been invaded by introduced trees and shrubs such as C. odorata, C. pubescens and Rubus niveus (Rentería & Buddenhagen, Reference Rentería and Buddenhagen2006; Jäger et al., Reference Jäger, Tye and Kowarik2007). Invasion by R. niveus has accelerated and may have been triggered by the El Niño event of 1997–1998 (Mauchamp & Atkinson, Reference Mauchamp and Atkinson2011). Many bird species consume the fruits of R. niveus and other weed species now present in the humid zones (Kleindorfer et al., Reference Kleindorfer, Chapman, Winkler and Sulloway2006; Guerrero & Tye, Reference Guerrero and Tye2009). This change in the plant community could have resulted in an alteration of the composition of the bird community by favouring seed eaters and generalist species such as the small tree finch because they can increase seed and fruit intake and therefore counter a possible limitation in insect abundance. The intense use of herbicides to control these invasive plant species may have led to altered plant communities with reduced or altered invertebrate abundance, which could impair foraging by insectivorous bird species.
Santa Cruz has the highest human population (estimated to be 18,000; Ch. Grenier, pers. comm.) of the Galápagos and, after San Cristobal, the largest proportion of its land area modified (14%; Watson et al., Reference Watson, Trueman, Tufet, Henderson and Atkinson2010). Urban development is rapid and is closely followed by rural development (González et al., Reference González, Montes, Rodríguez and Tapia2008). The heterogeneous agricultural zone is a suitable secondary habitat for many bird species but is not within the National Park and undergoes constant changes.
A major factor affecting land birds are introduced species acting as predators, competitors or parasites, or carrying and transmitting diseases, such as rats (Fessl et al., Reference Fessl, Young, Young, Rodriguez-Matamoros, Dvorak and Tebbich2010), mosquitoes (Whiteman et al., Reference Whiteman, Goodman, Sinclair, Walsh, Cunningham and Kramer2005) and the parasitic fly Philornis downsi (Fessl & Tebbich, Reference Fessl and Tebbich2002). P. downsi larvae in finch nests were first discovered in 1997 (Fessl et al., Reference Fessl, Couri and Tebbich2001) and, since then, several studies have demonstrated their negative impact on nesting success (Dudaniec et al., Reference Dudaniec, Kleindorfer and Fessl2006; Fessl et al., Reference Fessl, Kleindorfer and Tebbich2006; Huber, Reference Huber2008; Kleindorfer & Dudaniec, Reference Kleindorfer and Dudaniec2009; O’Connor et al., Reference O’Connor, Sulloway and Kleindorfer2010c). The fly occurs at higher densities in moist habitats (Wiedenfeld et al., Reference Wiedenfeld, Jimenez, Gustavo, Fessl, Kleindorfer and Valarezo2007; O’Connor et al., Reference O’Connor, Dudaniec and Kleindorfer2010a) and birds with smaller clutch sizes (such as tree finches in general and birds in the humid zone in particular) have higher nestling mortality (Dudaniec et al., Reference Dudaniec, Fessl and Kleindorfer2007). The two species with the highest prevalence of P. downsi in their nests (woodpecker and warbler finches; Dudaniec et al., Reference Dudaniec, Fessl and Kleindorfer2007) are also the species that showed the strongest declines in our study. No data are available on the impact of P. downsi on yellow warbler nests. Exotic diseases are a significant factor in the decline of threatened species (Smith et al., Reference Smith, Sax and Lafferty2006; Barbosa & Palacios, Reference Barbosa and Palacios2009) and for the Galápagos fauna in particular (Wikelski et al., Reference Wikelski, Foufopoulos, Vargas and Snell2004; Deem et al., Reference Deem, Cruz, Jiménez-Uzcátegui, Fessl, Miller, Parker and Darwin2008; Levin et al., Reference Levin, Outlaw, Vargas and Parker2009). Avian pox (Thiel et al., Reference Thiel, Whiteman, Tirapé, Ines Baquero, Cedeño and Walsh2005; Kleindorfer & Dudaniec, Reference Kleindorfer and Dudaniec2006) and other pathogens transmitted by mosquitoes (Bataille et al., Reference Bataille, Cunningham, Cedeño, Patiño, Constantinou and Kramer2009) are of particular importance. Since 1998 the number of medium ground finches in the highlands has increased (authors, pers. obs.) and small ground finches now regularly breed in the Scalesia zone (Kleindorfer, Reference Kleindorfer2007). Small ground finch numbers fluctuate annually, indicating possible changes in movement patterns and the possibility of carrying diseases to immunologically naive highland bird populations. Highland woodpecker finches brought to captive facilities in the lowlands immediately contracted avian pox (S. Tebbich et al., unpubl. data), indicating a high susceptibility in these populations.
Climate fluctuations or climate change could cause changes in bird populations and could also magnify the impact of humans and invasive species on an ecological system. In the Galápagos in particular rainfall patterns are a driving force for breeding activity and patterns of species' interactions (Grant & Grant, Reference Grant and Grant2008a). During our survey period from 1997 to 2010 the Galápagos experienced 2 years of high rainfall (1998, 2008) and otherwise persistent drought conditions with annual rainfall < 300 mm (except for 2002 with 577 mm) in the lowlands of Santa Cruz (CDF, 2010). The strong El Niño in 1998 (1,752 mm annual rainfall at 0 m) and the moderate El Niño in 2008 (769 mm) could have led to an increase in male singing activity and an increase in population sizes as Darwin’s finches are known to produce multiple clutches in El Niño years. However, our data indicate neither outcome. The census data in 1998 were similar to the data in 1997, which was a year of normal rainfall. Also, the strong rainfall of 2008 did not lead to a measurable population increase in 2010. While these findings are surprising, most recent data suggest that high rainfall periods depress finch populations via an increase in parasite prevalence (Antoniazzi et al., Reference Antoniazzi, Manzoli, Rohrmann, Saravia, Silvestri and Beldomenico2011; J. O’Connor & S. Kleindorfer, unpubl. data; S. Tebbich, unpubl. data). The overall dry conditions since 1997 could possibly have contributed to the decline of several species.
Our findings indicate the significant decline in abundance and density of at least four passerine species on Santa Cruz. The greatest decline is that of the warbler finch, now estimated to comprise 55,000 singing males compared to > 1 million at the beginning of the 20th century (considering the original extent of forest on the island). There is evidence of changes in species’ abundances and bird community composition on other inhabited Galápagos islands. A survey on Floreana Island indicated a stable population of ground finches and small tree finches but a decline in large-bodied birds, including the medium tree finch (O’Connor et al., Reference O’Connor, Sulloway and Kleindorfer2010c), and the grey warbler finch Certhidae fusca, which is morphologically and ecologically very similar to the warbler finch (Petren et al., Reference Petren, Grant, Grant and Keller2005), is extirpated or nearly so (Grant et al., Reference Grant, Grant, Petren and Keller2005). Preliminary data for San Cristobal indicate that the population of the grey warbler finch there is in the low 10,000s (M. Dvorak & E. Nemeth, unpubl. data). On San Cristobal Island the vermilion flycatcher is considered locally extinct (Vargas, Reference Vargas1996; Wiedenfeld, Reference Wiedenfeld2006).
No bird census data exist for Isabela and Santiago Islands, the other two elevated islands on which introduced herbivores have destroyed or degraded most of the native forest (Henderson & Dawson, Reference Henderson and Dawson2009). We suspect that this has had a large negative impact on the warbler, woodpecker and large tree finches. At present these three species are considered to be at low risk of population decline (IUCN, 2010) but this may not be the case if we infer their potential population trajectory given comparable habitat loss on Santa Cruz, Isabela and Santiago.
We recommend the implementation of a full-scale monitoring programme for land birds on the inhabited islands of the Galápagos archipelago. Human impacts on these islands have been multifaceted and extensive yet there have been few bird surveys and, since 1997, there have been successful eradication efforts for alien plant species on some of the Galápagos islands and future eradication programmes are planned (Campbell & Donlan, Reference Campbell and Donlan2005; Carrión et al., Reference Carrión, Donlan, Campbell, Lavoie and Cruz2007; Donlan et al., Reference Donlan, Campbell, Cabrera, Lavoie, Carrión and Cruz2007). Bird surveys would facilitate the monitoring of the impact of disease and habitat change and assessment of the effects of island restoration projects on bird communities. We are preparing a proposal for such a survey on San Cristobal Island.
Acknowledgements
We thank A. Mauchamp and S. Deem for valuable comments. Permission for this study was granted by the Galápagos National Park; the Charles Darwin Foundation provided logistical support. Areogal and TAME provided reduced flight tickets. For funding, ST acknowledges the University of Vienna and the FWF (project number V95-B17), SK the Australian Research Council, Max Planck Institute for Ornithology, and Winifred Violet Scott Charitable Trust, and BF the Max Planck Institute for Ornithology and the UK Government’s Darwin Initiative Fund (project #15005). This publication is contribution number 2032 of the Charles Darwin Foundation for the Galápagos Islands.
Appendix
The appendix for this article is available online at http://journals.cambridge.org
Biographical sketches
Michael Dvorak is coordinating and conducting research on threatened bird species and protected areas for BirdLife Austria. Birgit Fessl is a freelance behavioural ecologist with a particular interest in conservation biology. She investigated the impact of Philornis downsi and was field manager of the mangrove finch project from 2006 to 2009. Erwin Nemeth is part of the Social Communication Group at the Max Planck Institute for Ornithology and has worked on bioacoustics and conservation of birds. Sonia Kleindorfer studies Philornis parasitism and tree finch evolution on the Galápagos Islands and avian population ecology and behaviour in Australia. Sabine Tebbich is studying the relationship between feeding ecology and cognition in Darwin’s finches and corvids and is also involved in studies of the conservation of Darwin’s finches.








