Introduction
About 370 foodborne disease outbreaks in Germany have been reported annually to the Robert Koch Institute between 2014 and 2018 through the routine surveillance system (SurvNet@rki). These outbreaks caused on average about 1700 reported gastrointestinal infections per year (range 1400–2100). However, because of under-ascertainment and under-reporting the actual number of foodborne disease outbreaks and associated illnesses in the population is assumed to be higher. A foodborne disease outbreak is defined as an ‘incidence, observed under given circumstances, of two or more human cases of the same disease and/or infection, or a situation in which the observed number of cases exceeds the expected number and where the cases are linked, or are probably linked, to the same food source’ [1]. On average, about 85% of the foodborne disease outbreaks that were reported in Germany were small (⩽5 cases), and about 50% occurred in private households. Typically, unless the disease is considered serious, e.g. botulism or haemolytic uraemic syndrome, these small outbreaks and outbreaks in single private households are not being investigated and the food item that caused the outbreak can only be guessed or suspected based on vague descriptive epidemiological evidence and often remains unknown. In contrast, larger or more serious foodborne disease outbreaks require a thorough outbreak investigation. The goal of the investigation is to identify the food item that caused the outbreak in a timely manner and to enable public health and food safety authorities to implement appropriate control measures that will stop the outbreak and prevent further illnesses.
Typically in the course of an outbreak investigation, in particular when cases are dispersed and a common place of exposure cannot be identified, case-patients are questioned extensively in exploratory interviews. The goal is to find some evidence for a common exposure, e.g. consumption of a certain food item in a defined time period before disease onset. The exploratory interviews may lead to one or more hypotheses regarding the association of certain food items with the occurrence of gastrointestinal infections in the outbreak. The hypotheses may then be tested in analytical epidemiological studies. In outbreaks where cases do not have an obvious common place of exposure, the analytical epidemiological study will often be designed as a case-control study where patients with the disease (cases) will be compared to a group of persons without the disease (controls) regarding the frequency of exposure to the suspected food items. However, it may be challenging to decide which hypotheses to test in an analytical epidemiological study. Consumed food items named frequently by case-patients in exploratory interviews may be food items that are consumed with high frequency in the general population as well and may not necessarily be causative for the infections in the outbreak. Therefore, it would be helpful to have data regarding the consumption of certain food items in the general population available for comparison with data from exploratory interviews. In contrast to other countries [2–Reference Friesema, van Gageldonk-Lafeber and van Pelt4], to our knowledge, data on the consumption of food items in the general population, which could be used for comparison and hypotheses generation, do not exist in Germany. Food consumption surveys in Germany were conducted for other purposes, e.g. to survey the population's energy and nutritional intake. Questions in these surveys were not phrased in a way that is comparable to questions posed to case-patients in an outbreak investigation. For example, the queried time periods of possible exposure differ (24-h recalls or 4-week period vs. 7-day recall period in many foodborne disease outbreaks) [Reference Heuer5–Reference Mensink7]. Also, the questionnaires were not sufficiently detailed regarding consumption of food items that may play a role in foodborne disease outbreaks [Reference Mensink7–Reference Mensink, Schienkiewitz and Lange9].
Therefore, we conducted a food consumption survey in a sample (about 1000 interviewees) of the general German speaking adult population (18 years and older) in Germany. The survey focused on the frequency of consumption of food items that caused foodborne disease outbreaks in Germany in the past (‘high risk’ food items) such as unheated sprouts and unpasteurised milk. We also queried food items that have been described in the literature as having caused foodborne disease outbreaks in other countries and may also be relevant for German consumers, e.g. certain raw vegetables. Finally, we included questions about the consumption of food items that were determined in population-based case-control studies conducted in Germany to be risk factors for certain bacterial or viral gastrointestinal infections, e.g. raw ground pork for yersiniosis [Reference Rosner10]. To analyse if this data would have been helpful in previous foodborne disease outbreak investigations, we compared data from our food consumption survey to consumption data obtained in several exemplary, published outbreak investigations in Germany [Reference Frank11–Reference Von Laer17].
Methods
Study design
A representative sample of the German speaking general adult population (about 1000 persons aged at least 18 years) with residency in private households in Germany was interviewed over the telephone using a standardised questionnaire. Random sampling was conducted as described previously using a generated telephone sampling frame provided by ‘Arbeitskreis Deutscher Markt- und Sozialforschungsinstitute (ADM) [Working group of German market and social research institutes]’ [Reference Schmich18, 19]. The sampling frame consisted of landline (60%) and cell phone numbers (40%) (dual-frame approach) in all 16 federal states in Germany. The telephone survey was designed in close collaboration between researchers at the Robert Koch Institute and experts at USUMA GmbH, a social research institute headquartered in Berlin, Germany, which was commissioned by the Robert Koch Institute for ad hoc telephone surveys [Reference Schmich18]. The questionnaire was programmed in VOXCO Command Center™ Version 1.10.5 (Voxco, Montreal, CA), a software program for telephone interviews. Following pre-tests the questionnaire was shortened to the desired interview time frame of about 20 min. Interviews were conducted from 25 September to 14 November 2017 by trained interviewers of USUMA GmbH using computer-assisted telephone interviewing (CATI). When contacting households with several potential target persons via landline phone, the interviewee was randomly selected by CATI software based on the Kish selection grid. Thus, the likelihood of being interviewed was the same for all potential interviewees in the household [Reference Schmich18, Reference Kish20]. The response rate was calculated based on the criteria of the American Association for Public Opinion Research (AAPOR) using the AAPOR outcome rate calculator for dual-frame random-digit dialling (DFRDD), version 4.0, 2016 [21]. We report ‘response rate 3’ (RR3), which is the proportion of interviews conducted in relation to all probable households in the population [21]. The following formula was used:

where I = complete interviews; P = partial interviews; R = refusal and break off with eligible case; NC = non-contact with eligible case; O = other non-interview with eligible case; UH = unknown if residential; UO = unknown other (residential, unknown if eligible) and e = the estimated proportion of cases of unknown eligibility that are eligible: e1 = the % of known-residential cases estimated to have eligible R, e2 = the % of unknown-if-residential cases that are estimated to be residential.
Data protection
The study design was approved by the data protection officer of the Robert Koch Institute. Data protection measures included adherence to EU's general data protection regulation and a voluntary commitment to the guidelines of the ADM (Arbeitskreis Deutscher Markt- und Sozialforschungsinstitute) by USUMA GmbH [Reference Schmich18, Reference Allen, Lemcke, Schmich, Häder and Häder22]. Participation in the telephone survey was voluntary. Interviews were conducted only if participants gave verbal consent to be interviewed after having been informed about the purpose of the study and data protection measures. Collected data were recorded anonymously.
Questionnaire
The questionnaire queried consumption of certain food items within the 7-day period prior to the interview. The questionnaire was developed by scientists with expertise in foodborne disease outbreak investigations at the RKI and focused on food items that have the potential to cause foodborne outbreaks (‘high risk’ food items, e.g. raw ground meat; other kinds of meat; spreadable sausages that contain raw meat; certain fast food items; lettuce; mixed salads; raw vegetables; raw (unpasteurised) milk; raw milk cheeses; ice cream; eggs and egg products; smoked and other fish; salsa and other dips; fresh fruits; frozen berries; unpasteurised fruit juices; fresh herbs; dried tomatoes; herbal teas). Where plausible, certain food item variables were also operationalised. For example, raw ground pork, raw ground beef and raw ground mixed pork/beef, were combined to ‘any raw ground meat’. The questionnaire also queried information on general food consumption habits (vegetarian/vegan; certain food restrictions); supermarkets where food items had been purchased in the 14 days before the interview; some socio-demographic data (month and year of birth; sex; federal state of residency; county of residency; number of residents in village/town/city of residence; number of individuals living in the household; level of education) and some data that were required for the determination of weighting factors in the dual-frame design (e.g. number of landline phone numbers used in the interviewee household). Interviewees were also asked if they had travelled abroad in the 7 days before the interview and if their food consumption patterns in the 7-day time period before the interview had been atypical for them, e.g. due to an illness or a diet.
Statistical analyses
Survey results were weighted to be representative of the German speaking general population of 18 years and older living in private households in Germany. An overall weighting factor was calculated that took the following aspects into account: the distribution of household sizes in the sample compared to the known distribution within the population of Germany (Micro Census; https://www-genesis.destatis.de/genesis/online); a design weighting factor reflecting the probabilities of selection as the target person for interviewing in the dual-frame study design; and an adjustment weighting factor correcting for deviations of the sample from the general population with respect to age group and sex, education and federal state of the place of residency. Data for adjustments, as of 31 December 2016, were obtained from the Federal Statistical Office (https://www-genesis.destatis.de/genesis/online).
Unweighted and weighted proportions with 95% confidence intervals (CIs) were calculated with Stata, version 15.1 (Stata Corporation, College Station, Texas, USA). Stratified analyses were conducted according to sex (male/female); age group (18–34 years, 35–64 years, 65 years and older); region of residency (North: residency in one of the following federal states: Bremen, Hamburg, Lower Saxony, Mecklenburg-West Pomerania, Schleswig-Holstein; East: Berlin, Brandenburg, Saxony, Saxony-Anhalt, Thuringia; West: Hesse, North Rhine-Westphalia, Rhineland-Palatinate, Saarland; South: Baden-Wuerttemberg, Bavaria); residency in urban vs. rural area (urban and rural as defined by the Federal Institute for Research on Building, Urban Affairs and Spatial Development) [23, Reference Schielke, Rosner and Stark24] and level of education (level one (low or medium): no graduation from school or up to 10 years of general school education (up to ‘Hauptschulabschluss’ or ‘Realschulabschluss’); level two (high): more than 10 years of general school education (‘Abitur’ or ‘Fachhochschulreife’)). For the stratified analyses we picked six exemplary food items (raw ground pork; ‘Teewurst’ (=spreadable sausage-containing raw pork); unpasteurised (raw) milk; food items prepared with raw eggs; raw sprouts or seedlings and frozen berries) because they had caused foodborne disease outbreaks in Germany in the past, or because they had been identified as risk factors for bacterial or viral foodborne infections in population-based case-control studies conducted in Germany.
Comparison with food consumption data obtained in previous outbreak investigation studies
To analyse if results from our food consumption survey would have been helpful in previous outbreak investigations we chose published examples of outbreak investigations that had been mainly conducted by the RKI, and where the food item that had caused the outbreak could be identified and was queried in our food consumption survey. Data regarding the frequency of consumption of this food item had been acquired from case-patients and a healthy comparison group (controls) in a case-control study [Reference Frank11–Reference Bayer13, 16, Reference Von Laer17], or from case-patients only [Reference Frank14, Reference Schielke15].
Results
Study population
A total of 1046 telephone interviews were completed, of which 1010 were included in the data analysis. Thirty six interviews were excluded because they were conducted as part of the pretest. In total, 50 453 telephone numbers were contacted, requiring 790 722 phone calls. The response rate (response rate 3 [21]) was 17.2% for landline telephone numbers and 16.2% for mobile telephone numbers. The overall response rate was 16.9%. On average, the telephone interview took 23 min. More women than men participated in the interviews (60% vs. 40%). The median age of participants was 59 years (range 18–95 years) (Table 1). About 5% (5.2%, 95% CI: 3.3–8.2) of study participants reported that they had travelled to another country (at least one overnight stay) in the 7 days before the interview, and about 16% (15.5%, 95% CI: 12.0–19.7) considered their food consumption pattern in the 7 days before the interview as atypical for them, when compared with a ‘normal’ week.
Table 1. Participants of the food consumption survey, Germany, 2017
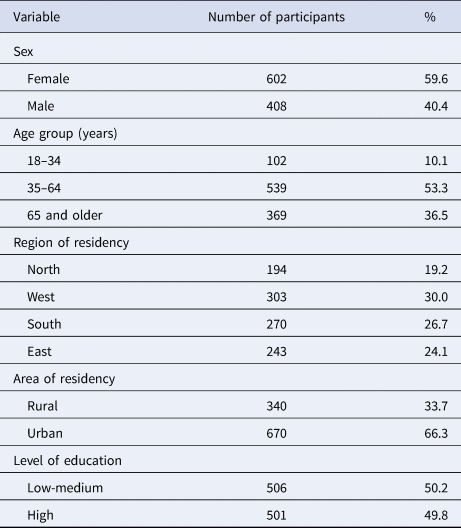
Total number of participants (completed interviews) was 1010.
Consumption of exemplary ‘high risk’ food items
The survey queried the consumption of 95 food items in the 7-day period before the interview (Table 2). Six exemplary ‘high risk’ food items were consumed by 6% to 16% of the general population. Raw ground pork was consumed by 6.5% (95% CI: 4.6–9.2) of the general population (Table 2). Raw ground pork tended to be consumed more frequently by men, persons 35–64 years of age, in eastern and northern parts of Germany and in rural regions, but 95% CIs between strata overlapped. The frequency of consumption of raw ground pork did not differ considerably between groups with high or low/medium level of school education (Table 3). ‘Teewurst’, a spreadable sausage-containing raw pork, was more frequently consumed by elderly persons (age group 65 years or older), in eastern regions of Germany, in rural areas and by persons with a low/medium level of school education. Unpasteurised milk was more frequently consumed by women, age groups up to 65 years, in the southern region, and in rural areas. Food items prepared with raw eggs, e.g. tiramisu, mayonnaise, were consumed more frequently by men, in the age group 18–34 years, in the northern region, and in rural areas. Persons of the age group 18–34 years, residents of urban regions and persons with a high level of education consumed unheated sprouts or seedlings more frequently compared with persons in the other strata of the respective category. Unheated frozen berries were consumed more frequently by women and in urban regions. Unheated frozen berries were least frequently consumed in the eastern region (Table 3).
Table 2. Consumption of food items in the general adult population (18 years and older) in the 7 days before the interview, Germany, 2017
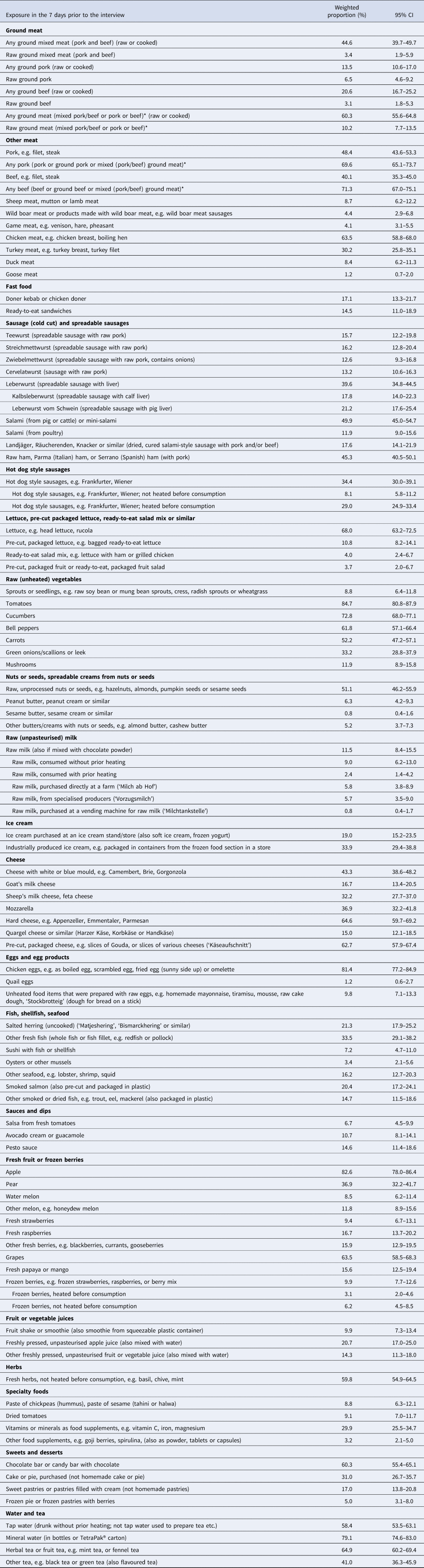
Results from weighted analyses are shown. Variables with * were operationalised.
Table 3. Consumption of six exemplary ‘high risk’ food items (proportion (%) exposed) in subgroups of the general adult population (18 years and older), Germany, 2017
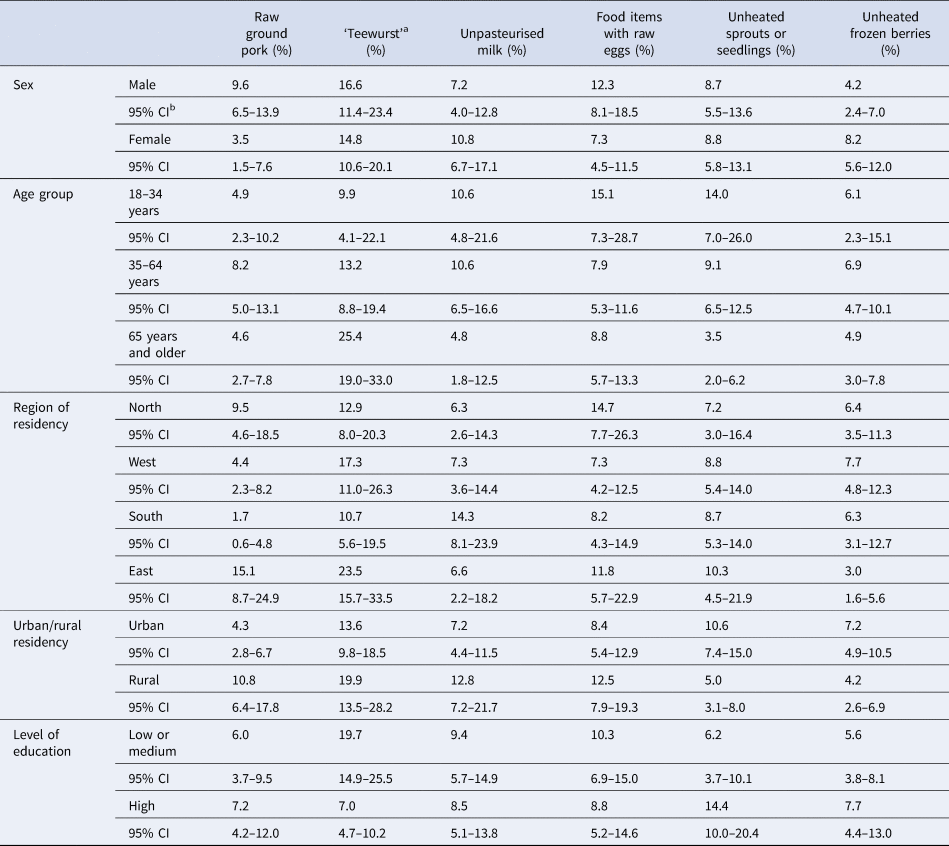
Results from weighted analyses are shown. See Table 1 for the number of participants in each subgroup.
a Teewurst = spreadable sausage-containing raw pork.
b Confidence interval.
Comparison with food consumption data obtained in previous outbreak investigations
We analysed if data from the food consumption survey would have been useful in previous outbreak investigations. The exemplary outbreaks had been caused by contaminated fenugreek sprouts (STEC O104:H4) [Reference Buchholz12], mung bean sprouts (Salmonella Newport) [Reference Bayer13], ‘Teewurst’ (Salmonella Derby) [Reference Frank14] , raw ground pork and spreadable pork sausages (Salmonella Muenchen) [Reference Schielke15] and raw ham (Salmonella Kottbus) [16, Reference Von Laer17] (Table 4). Compared with the food consumption survey the frequency of consumption of the food items that caused the respective outbreak was substantially higher among the interviewed case-patients in the exemplary outbreaks. For instance, consumption of raw sprouts or seedlings was reported by 9% of the population in our food consumption survey. This was comparable to the proportion of control persons that reported sprout consumption in case-control studies during the investigation of the STEC O104:H4-outbreak in Germany, despite slight differences regarding the time period that was considered (7 days and 14 days before the interview, respectively). In contrast, 25% of case-patients remembered having consumed sprouts before disease onset [Reference Buchholz12]. The frequency of consumption of raw tomatoes (this survey: 85%), cucumbers (73%) and leaf lettuce (68%), food items that were suspected as causative infection vehicles early on in the outbreak investigation in 2011 [Reference Frank11], did not differ substantially between the general population and the case-patients (or the control persons) in the outbreak investigation (Table 4). The frequency of consumption of food items that caused the Salmonella Newport outbreak (sprouts) or the Salmonella Kottbus outbreak (raw ham) was considerably lower in the food consumption survey when compared with case-patients and very similar, or at least in about the same range, when compared with control persons (Table 4). In a Salmonella Derby outbreak and a Salmonella Muenchen outbreak where case-control studies were not conducted as part of the outbreak investigation [Reference Frank14, Reference Schielke15], the frequency of consumption of food items that caused the outbreak (‘Teewurst’, and raw ground pork/spreadable sausage with pork, respectively) was lower in the food consumption survey than among the interviewed case-patients. When we compared the frequency of consumption of ‘Teewurst’ of case-patients of the Salmonella Derby outbreak, which had mainly affected elderly persons, with the age group 65 years and older in the food consumption survey there was still a marked difference (70% vs. 25%).
Table 4. Comparison of food consumption data obtained by questioning case-patients and control persons in past outbreak investigations in Germany with data obtained in food consumption survey of the general adult population, Germany, 2017
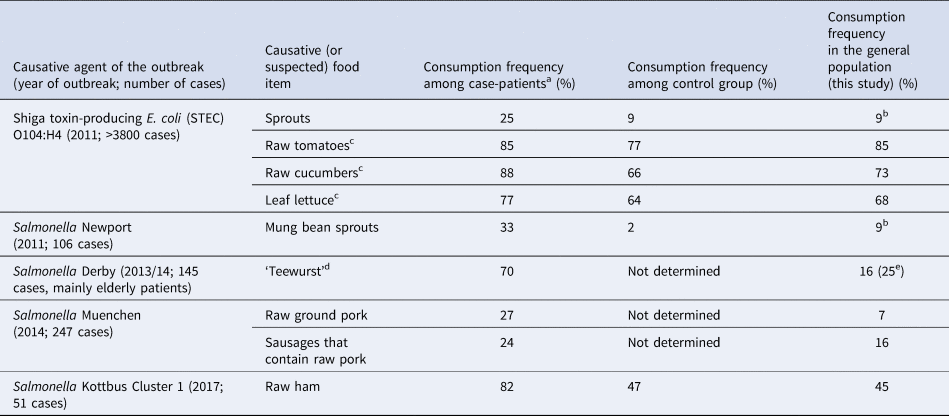
a Determined in hypothesis-generating interviews or in case-control studies.
b ‘Unheated sprouts or seedlings’.
c Food items that had been suspected early in the outbreak investigation.
d ‘Teewurst’: spreadable sausage that contains raw pork.
e Age group of 65 years and older.
Discussion
Representative data regarding the consumption of a variety of food items that are known risk factors of bacterial and viral gastrointestinal infections or that were identified as infection vehicles in past foodborne disease outbreaks were generated for the population of 18 years and older in Germany for the first time with our food consumption survey.
Food items that could be considered to put consumers at an elevated risk of obtaining a gastrointestinal infection were consumed by 6% to 16% of the population, despite publicly available consumer recommendations that state their health risks. About 10% of the population ate raw ground meat (pork, beef or a mixture) within a 7-day time period. Raw ground pork, often mixed with spices and onions (known as ‘Mett’ or ‘Hackepeter’) on bread rolls (‘Mettbrötchen’), is a popular food item in Germany, especially in certain regions (Eastern and Northern Germany). Consumption of raw ground pork has been shown to be a risk factor for infections with Yersinia enterocolitica [Reference Rosner10] and Salmonella Typhimurium [Reference Rettenbacher-Riefler25] in Germany, and was associated with an outbreak of Campylobacter coli [Reference Siffczyk26]. Consumption of raw or undercooked ground beef was associated with Shiga toxin-producing Escherichia coli (STEC) infections, albeit not statistically significantly [Reference Werber27]. Consumption of raw ground meat is discouraged by numerous consumer recommendations published by the Federal Institute for Risk Assessment (BfR) in Germany, especially with respect to vulnerable consumer groups, e.g. young children, the elderly, pregnant women and people with a compromised immune system, for example in [28–30].
Another ‘high risk’ food item, ‘Teewurst’, was consumed by 16% of the general population (over all age groups). The proportion of people in the age group ≥65 years that had consumed ‘Teewurst’ was even higher (25%). Consumer recommendations published by the BfR discourage consumption of spreadable sausages that contain raw meat. In particular, they advise against serving these food items to elderly persons and other vulnerable groups in nursing homes, hospitals or other community facilities [31]. An outbreak among mainly elderly persons, many of them in nursing homes or hospitals, caused by Salmonella Derby in Germany in 2013/2014 was associated with consumption of ‘Teewurst’, which indicated that the published recommendations may not have been known or may not have been taken seriously [Reference Frank14].
The proportion of people who reported having consumed unpasteurised milk (12%), in particular without prior heating (9%), was surprisingly high. In the United States and in Canada about 3% reported consumption of any unpasteurised milk [2, 3]. One explanation may be that some of our interviewees misclassified the type of consumed milk, even though a definition of ‘raw’ or unpasteurised milk had been provided in the interview. Unpasteurised milk that had not, or not adequately, been heated before consumption has caused numerous Campylobacter jejuni and several STEC outbreaks in Germany in the past few years [Reference Rosner and Schewe32–Reference Rosner, Mikolajetz and Schonsky34]. The BfR states in consumer information material that consumption of unheated unpasteurised milk poses a threat to human health, and advises against its consumption especially by vulnerable persons such as young children, the elderly and persons with a compromised immune system [35]. Less than 1% of our interviewees reported having consumed raw milk obtained from a milk-vending machine at a farm. In the past few years, several Campylobacter outbreaks have been reported that were associated with milk-vending machines [Reference Rosner and Schewe32, Reference Rosner, Mikolajetz and Schonsky34]. Even though a label that recommends heating of the milk before consumption must be placed on milk-vending machines, this consumer advice is not being followed by all consumers who purchase this type of milk, or the label is missing on some vending machines.
About 9% of the population reported having eaten sprouts or seedlings within a 7-day time period that were not heated before consumption. This proportion is comparable to results obtained from control persons in case-control studies conducted during the STEC O104:H4 outbreak in Germany in 2011, but higher than results from the control group in a case-control study conducted in the investigation of a Salmonella Newport outbreak caused by mung bean sprouts later in the same year (2%). Sprouts and seedlings are considered so-called ‘stealth’ food items because they may be served mixed with other food items, e.g. salads, or as toppings and, therefore, their consumption is particularly difficult to recall [Reference Dechet36]. In Canada, about 13% of the population reported consumption of any sprouts and in the United States, 4% to 8% reported sprout consumption, depending on the types of sprouts. In these food consumption surveys, it is not specified whether the sprouts were consumed cooked or uncooked [2, 3].
The main purpose of our survey was to have food consumption data available for future foodborne disease outbreak investigations. We used data from several past outbreak investigations to evaluate whether the food consumption data may have been helpful in these investigations. In all foodborne disease outbreaks listed in Table 4, results from the food consumption survey would have shown that consumption of the food item that ultimately caused the outbreak was substantially lower in the general population than in the group of case-patients. This information would have helped to direct the outbreak investigation towards the causative food vehicle earlier on in the investigations. In several unpublished outbreak investigations that have been conducted by our group since completion of our survey (Salmonella Mikawasima (2018); Salmonella Hadar (2019); hepatitis A virus (2019, still ongoing)), the food consumption data proved to be useful for hypothesis generation as well. In these outbreak investigations, initially suspected food items (cucumbers, ground meat and grapes, respectively) were considered as less likely to be the infection vehicle because they were consumed with similar frequency by case-patients and the general population. Unfortunately, the food items that caused these outbreaks could not be identified unequivocally.
The number of interviewees in our food consumption survey was much smaller than in food consumption telephone surveys that were conducted in the United States (17 372 interviewees) [2] or Canada (10 942 interviewees) [3], and our survey did not cover a 1-year period [2, 3]. There are some limitations to using data from our food consumption survey in outbreak investigations. The survey was conducted to be representative of the general German-speaking population of 18 years and older. Most likely, the data will not be very helpful in investigations of foodborne disease outbreaks among special groups of persons, for example, young children or the elderly, or if the outbreak is localised to a certain region of Germany, where food consumption patterns may differ from those of the general population. Also, we could only enquire about consumption of a limited number of food items in the survey and future outbreaks may involve food items that have not been queried in our food consumption survey. The consumption of certain food items, for example, fresh berries, likely varies by season. Our food consumption survey was deliberately conducted in the autumn (end of September to mid-November) because we considered this a time of the year, where there would be only little seasonality in food consumption, compared with, for example, the summer. We are aware that the survey should be repeated in other seasons of the year. The food consumption data may not be helpful either in the investigation of future outbreaks that will be caused by pathogens with a much longer or shorter incubation period than 7 days, the time frame we chose to question our interviewees about [Reference Morton37].
Recruitment of control persons for case-control studies in outbreak investigations is generally viewed as being time and resource intensive. Therefore, other methods are being developed in order to expand the toolbox for hypotheses generation and testing. Food consumption survey data were already used in outbreak investigations in other countries [Reference Whelan38, Reference Gambino-Shirley39]. Background exposure data from food consumption surveys can be applied for binomial probability analyses, a method that was developed at Oregon Health Authority [Reference Jervis40]. Besides food consumption survey data, market share data [Reference Russo41], shopping card or till receipt information [Reference Zenner42] and data from market-research panels [Reference Mook43–45] have been used in outbreak investigations. We consider our food consumption survey an additional helpful tool for outbreak investigations in Germany. It will allow comparison of food consumption data from case-patients obtained in exploratory, hypothesis-generating interviews with data from the general population early on in the outbreak investigation, and may support the generation of hypotheses regarding associations of illnesses with suspected food vehicles. We realise that this approach will not replace analytical epidemiological studies in future outbreak investigations, but food consumption data from the general population may allow us to limit our hypotheses to fewer suspected food items, which may facilitate and accelerate the outbreak investigation.
Acknowledgements
The authors are grateful to all study participants. We would like to thank Gert Mensink and Anna-Kristin Brettschneider for helpful comments on the questionnaire.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.






