INTRODUCTION
Hookworm causes iron deficiency and anaemia (Roche and Layrisse, Reference Roche and Layrisse1966); and maternal iron deficiency anaemia is associated with adverse pregnancy outcomes including still birth, prematurity, low birth weight and possibly maternal mortality (Allen, Reference Allen2000). Because of this, in 1994, an informal consultation of the World Health Organisation (WHO) recommended that hookworm control using levamisole or pyrantel, or the benzimidazoles albendazole or mebendazole, should be included in strategies for the improvement of health in girls and women in areas where hookworm is endemic and anaemia prevalent (WHO, 1995). In particular it was suggested that a single dose of anthelminthic treatment could be given in pregnancy, during the second to third trimester; routine anthelminthic treatment was already being implemented during antenatal care in Sri Lanka (Atukorala et al. Reference Atukorala, de Silva, Dechering, Dassenaeike and Perera1994; de Silva et al. Reference de Silva, Sirisena, Gunasekera, Ismail and de Silva1999). However, at that time no randomised trials to assess the risks and benefits of this intervention had been undertaken.
A further consultation in 2002 considered the use of praziquantel during pregnancy and lactation (WHO, 2002); previous recommendations had been against this. The consultation recognised that the former recommendations could result in exclusion of women in endemic areas from treatment of schistosomiasis for a large proportion of their reproductive lives. It was proposed that as well as specific end-organ damage, schistosomiasis in pregnancy might contribute to important subtle maternal morbidity including anaemia and decreased work capacity, and perhaps to impaired fetal growth. There was no evidence of fetal toxicity for praziquantel in animals, and its use during human pregnancy in a small number of documented cases had shown no severe adverse effects. Therefore the consultation recommended the use of praziquantel during pregnancy. Again, no randomised trials of praziquantel treatment during pregnancy had been undertaken and in 2006 a WHO working group on schistosomiasis listed among its priorities to “conduct without delay randomised, placebo-controlled trials of praziquantel treatment in pregnancy for all species of human schistosome in areas of both high and low transmission” (WHO, 2006).
Since these meetings, two randomised trials on the use of benzimidazoles during pregnancy have been reported (Haider et al. Reference Haider, Humayun and Bhutta2009), one from Peru (Larocque et al. Reference Larocque, Casapia, Gotuzzo and Gyorkos2005, Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006) and one from Sierra Leone (Torlesse and Hodges, Reference Torlesse and Hodges2001), and in Uganda we have conducted a randomised trial of both albendazole and praziquantel during pregnancy, using a 2×2 factorial design: the Entebbe Mother and Baby Study (Elliott et al. Reference Elliott, Kizza, Quigley, Ndibazza, Nampijja, Muhangi, Morison, Namujju, Muwanga, Kabatereine and Whitworth2007). We here summarise the results of our trial, with follow-up of infants to one year of age, and discuss the implications of our findings, and of the findings of other recent studies, for recommendations on the routine use of anthelminthic drugs during pregnancy, and for further research priorities.
The Entebbe Mother and Baby Study [ISRCTN32849447]
Although the WHO consultations focused mainly on potential benefits for anaemia and helminth-specific pathology in the mother and for growth in the fetus, there are other important potential effects of helminths and anthelminthic treatment during pregnancy. Helminths have a major impact on the host immune system, modulating the response both to themselves and to unrelated antigens (van Riet et al. Reference van Riet, Hartgers and Yazdanbakhsh2007). Before the start of our trial, it had been observed that exposure to maternal helminths in utero could result in sensitisation of the fetus to helminth antigens (Novato-Silva et al. Reference Novato-Silva, Gazzinelli and Colley1992; Malhotra et al. Reference Malhotra, Ouma, Wamachi, Kioko, Mungai, Omollo, Elson, Koech, Kazura and King1997), and in modulation of the infant immune response to neonatal BCG immunisation (Malhotra et al. Reference Malhotra, Mungai, Wamachi, Kioko, Ouma, Kazura and King1999). The Entebbe Mother and Baby Study was therefore designed to address the hypothesis that helminth infections can influence the immune response to non-worm antigens (including immunogens and pathogens), that these effects can be established in utero and that they can be modified by anthelminthic treatment during pregnancy.
The study commenced in 2002 as a trial of albendazole versus placebo during pregnancy, but was suspended following publication of the WHO recommendations on use of praziquantel during pregnancy (Allen et al. Reference Allen, Crompton, de Silva, LoVerde and Olds2002) and modified to allow us to investigate effects of praziquantel treatment during pregnancy, as well as of albendazole treatment. Data collected before this modification created a preliminary study of the effects of albendazole treatment during pregnancy among 104 mothers and their infants. In this preliminary study we observed a protective effect of maternal helminth infection, and a possible detrimental effect of albendazole treatment during pregnancy, on the incidence of infantile eczema (Elliott et al. Reference Elliott, Mpairwe, Quigley, Nampijja, Muhangi, Oweka-Onyee, Muwanga, Ndibazza and Whitworth2005). Therefore we also modified the trial design to allow us to investigate effects of anthelminthics during pregnancy on allergic disease outcomes.
The main Entebbe Mother and Baby Study thus compared single-dose albendazole (400 mg) versus placebo and praziquantel (40 mg/kg) versus placebo, given during the second or third trimester of pregnancy, in a 2×2 factorial design. All women were treated with both albendazole and praziquantel after delivery. The study included a further randomisation of the children, from age 15 months to five years, to quarterly albendazole versus placebo; this part of the study will be completed in 2011 and will not be discussed in this review.
The study is based in Entebbe and the neighbouring sub-county of Katabi, a peninsula in Lake Victoria, Uganda. We recruited 2507 women at the government district hospital between 2003 and 2005. Most women were poor: 82% reported a monthly cash income of less than 30,000 Uganda shillings (then approximately US$20) per month. They came from the relatively urban setting of Entebbe Municipality and from the surrounding fishing communities and rural farming communities. The prevalence of helminth infection at enrolment was high: 68% had at least one helminth infection, 45% had hookworm, 21% M. perstans and 18% had Schistosoma mansoni; other species were less common (Woodburn et al. Reference Woodburn, Muhangi, Hillier, Ndibazza, Namujju, Kizza, Ameke, Omoding, Booth and Elliott2009). For intestinal helminths these results were based on examination of two slides from a single stool sample, implying that the true prevalence of helminths was higher (Hall, Reference Hall1981; Utzinger et al. Reference Utzinger, Booth, N'Goran, Muller, Tanner and Lengeler2001). Although the prevalence of infection was high, intensity was generally low, according to WHO classifications (WHO, 1995, 1999): 85% of those with hookworm had egg counts below 1000 eggs per gram (epg) of stool (91% below 2000 epg), and 65% of those with S. mansoni had counts below 100 epg. At age one year, only 44 of 1358 infants examined had helminth infections, the commonest being Ascaris (15 infants), Trichuris (12 infants) and Mansonella (eight infants); because these numbers were so small the effects of infants’ helminth infections are not considered further in this review.
EFFECTS OF ANTHELMINTHIC TREATMENT IN PREGNANCY
On helminth prevalence
Anthelminthic treatment during pregnancy was effective in our study (Ndibazza et al. Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka, Kizindo, Duong, Kleinschmidt, Muwanga and Elliott2010) (Fig. 1). The prevalence of hookworm declined in the albendazole treatment group from 45% to 5%. This was similar to the effect of albendazole reported by Torlesse and Hodges, (Reference Torlesse and Hodges2001) but greater than the effect reported by Larocque et al. (Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006) for single-dose mebendazole, in accord with the greater efficacy of albendazole against hookworm (Keiser and Utzinger, Reference Keiser and Utzinger2008). There was no change in hookworm prevalence in the albendazole placebo group. Single-dose albendazole was also effective for Ascaris, but had little effect on Stronglyloides or Trichuris. Praziquantel was effective against S. mansoni: prevalence declined from 18% to 5%. This is important because pregnancy is associated with immunosuppression and the efficacy of praziquantel may depend partly on immunologically-mediated killing following disruption of the tegument and exposure of antigens on the surface of the parasite (Doenhoff et al. Reference Doenhoff, Cioli and Utzinger2008). We found that boosts in cytokine and antibody response to schistosome antigens after treatment tended to be lower when praziquantel was given during pregnancy than when it was given after delivery, but the cure rate for S. mansoni was similar (Tweyongyere et al. Reference Tweyongyere, Mawa, Ngom-Wegi, Ndibazza, Duong, Vennervald, Dunne, Katunguka-Rwakishaya and Elliott2008, Reference Tweyongyere, Mawa, Emojong, Mpairwe, Jones, Duong, Dunne, Vennervald, Katunguka-Rwakishaya and Elliott2009).
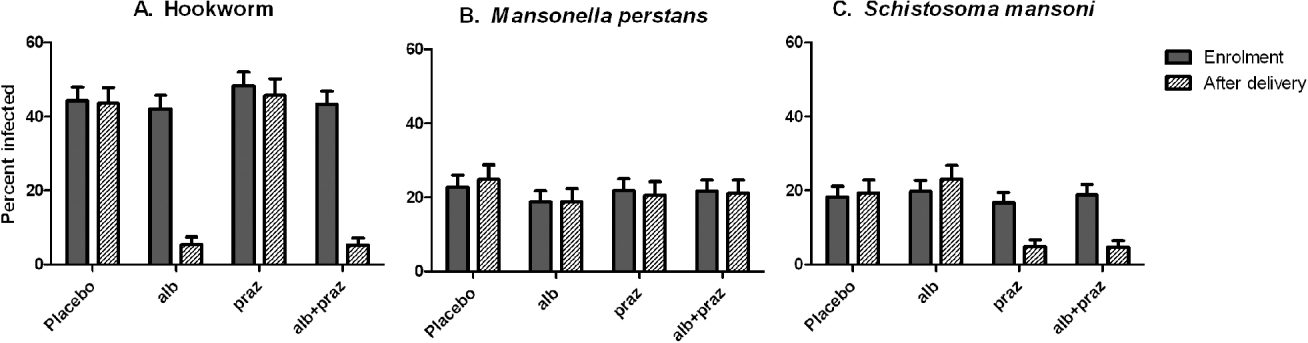
Fig. 1. Prevalence of the three commonest helminth infections at enrolment and after delivery, according to treatment allocation during pregnancy, among 2507 women participating in the Entebbe Mother and Baby Study. Placebo: double placebo group; alb: single dose albendazole (400 mg) plus placebo for praziquantel; praz: single dose praziquantel (40 mg/kg) plus placebo for albendazole; alb+praz: single dose albendazole (400 mg) plus praziquantel (40 mg/kg). Error bars show the upper 95% confidence interval for the percentage prevalence of infection.
On maternal anaemia
The WHO consultations discussed above anticipated that reductions in anaemia would be an important benefit of intervention against hookworm during pregnancy, and also a benefit of treating schistosomiasis during pregnancy. In our study, the prevalence of anaemia at baseline was 40%. No individual helminth species was associated with the presence of anaemia, but increasing intensity of hookworm was associated with lower haemoglobin level (Muhangi et al. Reference Muhangi, Woodburn, Omara, Omoding, Kizito, Mpairwe, Nabulime, Ameke, Morison and Elliott2007). These findings were similar to those of Larocque et al. (Reference Larocque, Casapia, Gotuzzo and Gyorkos2005) in Peru, where the prevalence and intensity of hookworm infection were similar. By contrast to a study in Tanzania (Ajanga et al. Reference Ajanga, Lwambo, Blair, Nyandindi, Fenwick and Brooker2006), we found no association at baseline between maternal anaemia and intensity of S. mansoni.
We provided haematinics routinely to all women, as well as two doses of intermittent presumptive treatment for malaria with sulphadoxine-pyrimethamine. We found no overall benefit of anthelminthic treatment during pregnancy on maternal anaemia after delivery and no benefit of albendazole in the subgroup of mothers shown to have hookworm at baseline, or of praziquantel among mothers shown to have S. mansoni infection (Ndibazza et al. Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka, Kizindo, Duong, Kleinschmidt, Muwanga and Elliott2010). An exploratory analysis stratified for hookworm intensity suggested a possible benefit of albendazole among women with moderate to heavy hookworm, but this difference in effect was not statistically significant. Again, the findings of Larocque et al. (Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006) were similar: no benefit for maternal anaemia in the context of the provision of haematinics. The Sierra Leone trial (Torlesse and Hodges, Reference Torlesse and Hodges2001) compared single-dose albendazole with haematinics in a factorial design and found some benefit of albendazole but a greater benefit of haematinics for both anaemia and iron status. Together, these results suggest that, given adequate provision of haematinics, the benefit of routine anthelminthics during pregnancy for maternal anaemia may be less than had been anticipated.
On birth weight
The possibility that birth weight would be improved by routine treatment with benzimidazoles during pregnancy in areas of high hookworm prevalence was suggested by the cross-sectional study in Sri Lanka (de Silva et al. Reference de Silva, Sirisena, Gunasekera, Ismail and de Silva1999). Very low birth weight (below 1·5 kg) was less common among women who reported taking mebendazole during pregnancy than among those that did not. Similarly, a non-randomised study of albendazole treatment in Nepal suggested a benefit for birth weight (Christian et al. Reference Christian, Khatry and West2004). The principal limitation of these studies was the possibility that taking anthelminthics was associated with better overall care-seeking behaviour and hence better outcomes mediated by a variety of factors. More recently, studies in animals suggested possible adverse effects of schistosomiasis on birth weight and other perinatal outcomes, but no adequate studies have been conducted to explore similar effects in humans (Friedman et al. Reference Friedman, Mital, Kanzaria, Olds and Kurtis2007).
Neither we nor Larocque et al. (Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006) found any benefit of anthelminthic treatment during pregnancy for mean birth weight or low birth weight (below 2·5 kg) (Ndibazza et al. Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka, Kizindo, Duong, Kleinschmidt, Muwanga and Elliott2010). Larocque and colleagues found a possible benefit of mebendazole for very low birth weight (below 1·5 kg), but only seven infants fell into this category in their study. In our study, 11 infants were very low birth weight and there was no association between this outcome and the treatment the mother had received. Again, the provision of adequate haematinics could be a factor in preventing an adverse effect of hookworm mediated by iron deficiency, and a consequent benefit of albendazole, from becoming evident, but the role of iron and folic acid supplementation in determining pregnancy outcomes other than anaemia remains uncertain (Pena-Rosas and Viteri, Reference Pena-Rosas and Viteri2006).
On congenital anomalies
The principal mode of action of benzimidazoles is to bind tubulin and inhibit the synthesis of microtubules which are ubiquitous components of the eukaryotic cytoskeleton. Their specificity for helminths is relative and selectivity seems to be related to the stability of binding between the drug and the tubulin molecules (Lacey, Reference Lacey1990; MacDonald et al. Reference MacDonald, Armson, Thompson and Reynoldson2004). Thus interference with processes such as mitosis provide a plausible mechanism of fetal toxicity for benzimidazoles, and there is evidence of toxicity in animal models (Dayan, Reference Dayan2003). Although this was recognised by the WHO committee in 1995, differences in dose, metabolism and pharmacokinetics between animal models and human usage led them to suggest that benzimidazoles were probably safe for pregnant and lactating women and their offspring (WHO, 1995). In the cross-sectional study in Sri Lanka, the number of congenital anomalies was higher among infants of women who reported taking mebendazole during the first trimester than among those that did not, but this difference was not statistically significant (de Silva et al. Reference de Silva, Sirisena, Gunasekera, Ismail and de Silva1999) and, overall, observational studies have not reported a significant excess of congenital anomalies following use of benzimidazoles in human pregnancy (Diav-Citrin et al. Reference Diav-Citrin, Shechtman, Arnon, Lubart and Ornoy2003).
The mode of action of praziquantel is less certain (Doenhoff et al. Reference Doenhoff, Cioli and Utzinger2008), but for this drug there is no evidence of genotoxicity or fetal toxicity (Dayan, Reference Dayan2003; Adam et al. Reference Adam, Elwasila and Homeida2005).
We avoided anthelminthic treatment during the first trimester, and found no evidence of association between either albendazole or praziquantel and congenital anomalies in our study (Ndibazza et al. Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka, Kizindo, Duong, Kleinschmidt, Muwanga and Elliott2010). Similarly, the trials in Peru and Sierra Leone found no evidence of fetal toxicity for benzimidazoles given in the second trimester (Torlesse and Hodges, Reference Torlesse and Hodges2001; Larocque et al. Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006). These findings are encouraging, but pharmacovigilance remains appropriate, especially if anthelminthics are given in the first trimester.
On perinatal and infant mortality
The observational studies in Sri Lanka and Nepal suggested a benefit of benzimidazoles during pregnancy for perinatal mortality and infant survival to 6 months of age (de Silva et al. Reference de Silva, Sirisena, Gunasekera, Ismail and de Silva1999; Christian et al. Reference Christian, Khatry and West2004). However, we found no benefit of either albendazole or praziquantel during pregnancy for rates of still birth, neonatal mortality or infant mortality (Ndibazza et al. Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka, Kizindo, Duong, Kleinschmidt, Muwanga and Elliott2010; Webb et al. Reference Webb, Mawa, Ndibazza, Kizito, Namatovu, Kyosiimire-Lugemwa, Nanteza, Nampijja, Muhangi, Woodburn, Akurut, Mpairwe, Akello, Lyadda, Bukusuba, Kihembo, Kizza, Kizindo, Nabulime, Ameke, Namujju, Tweyongyere, Muwanga, Whitworth and Elliott2011). Similarly, Larocque et al. (Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006) found no effect of mebendazole on perinatal mortality.
On infant response to immunisation
The evidence that led to the hypothesis that antenatal exposure to helminth infections has an important influence on the infant response to immunisation in low-income and tropical settings has been reviewed elsewhere (Labeaud et al. Reference Labeaud, Malhotra, King, King and King2009). As discussed above, this hypothesis was the principal motivation for our study. We used a six-day whole blood culture assay at age one year to assess the cellular response to BCG and tetanus immunisation, stimulated using crude culture filtrate proteins of Mycobacterium tuberculosis and tetanus toxoid (TT), respectively; we examined production of type 1 (interferon (IFN)-γ) type 2 (interleukin (IL)-5 and IL-3) and regulatory (IL-10) cytokines in supernatant using enzyme-linked immunosorbent assays (ELISAs). ELISAs were also used to assess antibody responses to TT and measles immunisation.
We found no overall effect of either treatment on the response to immunisation (Webb et al. Reference Webb, Mawa, Ndibazza, Kizito, Namatovu, Kyosiimire-Lugemwa, Nanteza, Nampijja, Muhangi, Woodburn, Akurut, Mpairwe, Akello, Lyadda, Bukusuba, Kihembo, Kizza, Kizindo, Nabulime, Ameke, Namujju, Tweyongyere, Muwanga, Whitworth and Elliott2011). In sub-group analyses we found a reduction in type 2 cytokine responses to tetanus toxoid among infants of mothers with hookworm who received albendazole, compared to those who received placebo, but there was no effect on total immunoglobulin (Ig)G production, the key parameter for protection following tetanus immunisation (Plotkin, Reference Plotkin2010). No other effects were observed for the response to BCG, tetanus or measles immunisation in the planned sub-group analyses for effects of albendazole in mothers with hookworm, or of praziquantel in mothers with S. mansoni. Such results could occur if effects of helminth exposure were established prior to the trial intervention, say during the first trimester, and could not be reversed by subsequent treatment. This explanation is unlikely since, in observational analyses, infant response to vaccines showed no consistent associations with the presence of maternal hookworm or S. mansoni (Elliott et al. Reference Elliott, Mawa, Webb, Nampijja, Lyadda, Bukusuba, Kizza, Namujju, Nabulime, Ndibazza, Muwanga and Whitworth2010).
In observational analyses, maternal infection with the filarial helminth, Mansonella perstans, was associated with increased IL-10 responses to the vaccine antigens. Curiously, this effect was evident principally in the albendazole placebo group, although Mansonella prevalence was not affected by the single dose of albendazole given. Maternal Mansonella showed no effect on type 1 or type 2 responses (Elliott et al. Reference Elliott, Mawa, Webb, Nampijja, Lyadda, Bukusuba, Kizza, Namujju, Nabulime, Ndibazza, Muwanga and Whitworth2010).
Taken together, our results suggest that maternal helminth infection is unlikely to be a major contributor to poor efficacy of immunisation in infancy in the tropics, and routine anthelminthic treatment during pregnancy is unlikely to lead to important improvements in this outcome.
On infectious disease incidence in infancy
There is considerable evidence, both from animal models and from studies in humans, that exposure to helminths in utero programmes the infant response to subsequent challenge, influencing susceptibility to infection, and immunologically-mediated pathology (Lammie et al. Reference Lammie, Hitch, Walker Allen, Hightower and Eberhard1991; Steel et al. Reference Steel, Guinea, McCarthy and Ottesen1994; Malhotra et al. Reference Malhotra, Mungai, Wamachi, Tisch, Kioko, Ouma, Muchiri, Kazura and King2006). Pursuing the idea that some of the immunoregulatory effects of helminths may influence responses to bystander antigens, we investigated whether anthelminthic treatment during pregnancy influenced infant susceptibility to infectious diseases. We found no effect on incidence of malaria or on malaria infection at age one year; and no effect on incidence of diarrhoea or lower respiratory tract infection (Webb et al. Reference Webb, Mawa, Ndibazza, Kizito, Namatovu, Kyosiimire-Lugemwa, Nanteza, Nampijja, Muhangi, Woodburn, Akurut, Mpairwe, Akello, Lyadda, Bukusuba, Kihembo, Kizza, Kizindo, Nabulime, Ameke, Namujju, Tweyongyere, Muwanga, Whitworth and Elliott2011).
In two previous studies concerning helminths and HIV infection, we had noted a temporary increase in HIV load following treatment of S. mansoni (Elliott et al. Reference Elliott, Mawa, Joseph, Namujju, Kizza, Nakiyingi, Watera, Dunne and Whitworth2003; Brown et al. Reference Brown, Mawa, Joseph, Bukusuba, Watera, Whitworth, Dunne and Elliott2005), so a particular concern was that treatment with praziquantel during pregnancy might result in increased intrauterine HIV transmission. Our study had little power to assess this outcome and was inconclusive, but vertical HIV transmission was, if anything, lower among HIV-positive mothers that received praziquantel (OR 0·60 (95% CI 0·29–1·23)) (Webb et al. Reference Webb, Mawa, Ndibazza, Kizito, Namatovu, Kyosiimire-Lugemwa, Nanteza, Nampijja, Muhangi, Woodburn, Akurut, Mpairwe, Akello, Lyadda, Bukusuba, Kihembo, Kizza, Kizindo, Nabulime, Ameke, Namujju, Tweyongyere, Muwanga, Whitworth and Elliott2011).
On allergy
There is considerable evidence from animal models, and some from studies in humans, that helminth infection can protect against allergic disease through active immunoregulatory pathways (Smits et al. Reference Smits, Everts, Hartgers and Yazdanbakhsh2010). The results of epidemiological studies to date have been less clear-cut. In cross-sectional studies atopy (represented by skin-prick test positivity) has shown a fairly consistent inverse association with helminth infection, and hookworm has shown a consistent inverse association with asthma, but associations with asthma for other helminths, and associations between helminths and eczema, have been variable (Leonardi-Bee et al. Reference Leonardi-Bee, Pritchard and Britton2006; Flohr et al. Reference Flohr, Quinnell and Britton2009). Some, but not all, intervention studies have shown an effect of anthelminthic treatment on skin-prick test responses to allergens but, prior to our study, none had shown an effect on an allergic disease outcome (Lynch et al. Reference Lynch, Hagel, Perez, Di Prisco, Lopez and Alvarez1993; van den Biggelaar et al. Reference van den Biggelaar, Rodrigues, van Ree, van der Zee, Hoeksma-Kruize, Souverijn, Missinou, Borrmann, Kremsner and Yazdanbakhsh2004; Cooper et al. Reference Cooper, Chico, Vaca, Moncayo, Bland, Mafla, Sanchez, Rodrigues, Strachan and Griffin2006; Endara et al. Reference Endara, Vaca, Chico, Erazo, Oviedo, Quinzo, Rodriguez, Lovato, Moncayo, Barreto, Rodrigues and Cooper2010; Flohr et al. Reference Flohr, Tuyen, Quinnell, Lewis, Minh, Campbell, Simmons, Telford, Brown, Hien, Farrar, Williams, Pritchard and Britton2010).
In the Entebbe Mother and Baby Study, eczema was the commonest allergic condition observed during infancy, as expected for the age group, with an incidence of 10 per 100 person years of follow-up. In a sub-group of infants for whom skin-prick testing was conducted, eczema was strongly associated with skin prick test positivity, indicating that it was atopic. We found that albendazole treatment during pregnancy was associated with an increased risk of eczema in the whole study population (Cox proportional Hazard Ratio (HR) 1·82 (95% CI 1·26–2·64)), while praziquantel treatment was associated with increased risk of eczema among infants of mothers with S. mansoni (HR 2·65 (95% CI 1·16–6·08), interaction P value 0·02) (Mpairwe et al. Reference Mpairwe, Webb, Muhangi, Ndibazza, Akishule, Nampijja, Ngom-Wegi, Tumusiime, Muwanga, Rodrigues and Elliott2011) (Fig. 2).
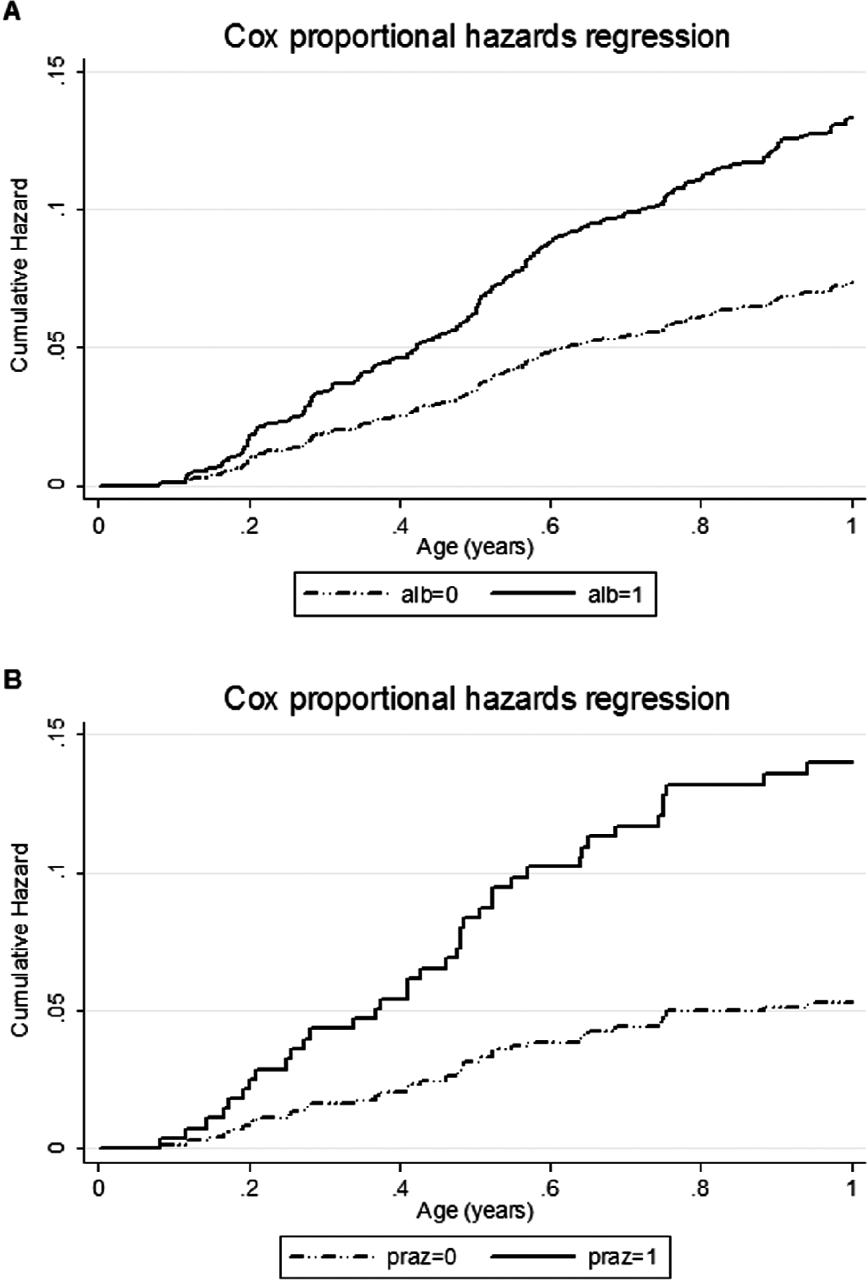
Fig. 2. The effect of treatment with albendazole or praziquantel during pregnancy on the incidence of eczema during infancy in the Entebbe Mother and Baby Study. (A) The effect of albendazole among 2345 live-born infants of all mothers, with or without detected helminth infection. (B) The effect of praziquantel among 421 infants of mothers with detected Schistosoma mansoni infection. Shown are the cumulative hazard functions based on multiple eczema events. Alb, albendazole; praz, praziquantel.
For albendazole, we expected that the effects would be strongest among infants of women with hookworm – the commonest species, and most susceptible to albendazole treatment – but, surprisingly, stratification by maternal hookworm status showed no difference in effect (interaction P value 0·52). Indeed, an effect of albendazole was seen even among infants of mothers with no detected helminth infection. This may mean that the effect of albendazole was mediated (a) by the drug itself, (b) by an effect on low intensity hookworm, or other albendazole-susceptible helminth species, not detected by the single stool sample examined or (c) by an effect on another organism. Albendazole has a broad spectrum of action, sometimes overlooked in relation to anthelminthic treatment programmes, and candidates for option (c) include malaria, intestinal protozoa, microsporidia and commensal yeasts (Cruz and Edlind, Reference Cruz and Edlind1997; Skinner-Adams et al. Reference Skinner-Adams, Davis, Manning and Johnston1997; MacDonald et al. Reference MacDonald, Armson, Thompson and Reynoldson2004; Solaymani-Mohammadi et al. Reference Solaymani-Mohammadi, Genkinger, Loffredo and Singer2010).
By contrast, our findings for schistosomiasis are entirely consistent with a protective effect of exposure to maternal S. mansoni infection that is removed by praziquantel treatment.
Our study is thus the first to demonstrate an effect of anthelminthic treatment on an allergic disease outcome and provides support for the hypothesis that helminths protect against allergic disease. Why did we observe an effect, while previous studies in school-age children did not? Given the low prevalence of allergic disease in rural tropical environments, several of the previous studies had limited power to show an effect on disease outcomes. However, an important possibility is that our study showed an effect because susceptibility to allergic disease is programmed in utero or very early in post-natal life, with relatively little effect of subsequent intervention (von Mutius and Le Souef, Reference von Mutius and Le Souef2007). Thus, from the perspective of allergology, our results provide important evidence that pre-natal or very early post-natal interventions may allow the primary prevention of allergic disease in children at risk.
CONCLUSIONS AND FURTHER RESEARCH NEEDS
The Entebbe Mother and Baby Study is the first randomised, double-blind, placebo-controlled trial of praziquantel treatment during pregnancy. It is also the first trial to examine the effects of praziquantel or of benzimidazole treatment during pregnancy on the broad range of maternal and infant outcomes that might be influenced by pre-natal exposure to helminths. Some of the long-term effects of anthelminthic treatment during pregnancy, including effects on the offspring's own susceptibility to infection and morbidity when exposed to the same species, have yet to be examined. However, the results to date, taken together with the findings of other recent studies, suggest that the risks and benefits of anthelminthic treatment during pregnancy, and policy regarding routine provision of anthelminthics during antenatal care, may need to be reviewed.
In Entebbe, where good, general antenatal care was provided, there was a possible benefit of albendazole for anaemia in mothers with moderate to heavy hookworm infection, but otherwise none of the expected benefits of anthelminthic treatment on anaemia, birth weight, perinatal mortality, infant mortality or infant response to immunisation were realised. These findings were in keeping with results from the two other benzimidazole trials (Torlesse and Hodges, Reference Torlesse and Hodges2001; Larocque et al. Reference Larocque, Casapia, Gotuzzo, MacLean, Soto, Rahme and Gyorkos2006). Mothers with possible symptomatic helminth infection were excluded from our trial, but such women were unusual in our setting: of 11,783 screened for the study only 17 were excluded for haemoglobin below 8 g/dl, 15 for diarrhoea with blood in stools and none for clinically apparent severe liver disease (Webb et al. Reference Webb, Mawa, Ndibazza, Kizito, Namatovu, Kyosiimire-Lugemwa, Nanteza, Nampijja, Muhangi, Woodburn, Akurut, Mpairwe, Akello, Lyadda, Bukusuba, Kihembo, Kizza, Kizindo, Nabulime, Ameke, Namujju, Tweyongyere, Muwanga, Whitworth and Elliott2011). It other settings, or with other helminth species (for example S. japonicum), the balance of benefits to mother and infant may differ. However, the minimal effects associated with helminths and anthelminthic treatment in our setting are put further into perspective by the strong adverse effects of malaria and HIV infection observed; the prevalence of malaria was 13% and of HIV 12% at baseline, during pregnancy, in our study. We found that maternal malaria and HIV infection were strongly associated with maternal anaemia (Muhangi et al. Reference Muhangi, Woodburn, Omara, Omoding, Kizito, Mpairwe, Nabulime, Ameke, Morison and Elliott2007), and were also associated with reduced birth weight and increased perinatal mortality (unpublished data). Maternal and infant HIV infection, and infant malaria, were associated with deleterious effects on the infant response to immunisation (Elliott et al. Reference Elliott, Mawa, Webb, Nampijja, Lyadda, Bukusuba, Kizza, Namujju, Nabulime, Ndibazza, Muwanga and Whitworth2010).
The three trials have been conducted to date in areas with high hookworm prevalence but low intensity, and this is the common pattern of helminth infection. However, a trial in a setting with heavy hookworm infection might have a different result, especially if background levels of nutrition and iron status are poor. Trial results might also be expected to differ according to helminth species: Necator americanus and Ancylostoma duodenale may differ in their pathogencity for anaemia (Albonico et al. Reference Albonico, Stoltzfus, Savioli, Tielsch, Chwaya, Ercole and Cancrini1998) – and we have not yet determined the prevalent species in Entebbe; schistosome species clearly differ in the immunopathology that they induce (Burke et al. Reference Burke, Jones, Gobert, Li, Ellis and McManus2009). The three reported studies demonstrate that placebo-controlled trials can be conducted safely, and additional trials are still needed in different settings.
The observed adverse effect on infantile eczema is a concern, and contributes to the current equipoise in evidence regarding the potential risks and benefits of anthelminthic therapy during pregnancy. Whether this finding can be generalised to other environments is uncertain: Entebbe is unusual among lake-shore communities in Uganda in being relatively urbanised and traversed by a major highway, and hosting the international airport. Pollutants may contribute to the induction of allergy (Venn et al. Reference Venn, Yemaneberhan, Bekele, Lewis, Parry and Britton2001, Reference Venn, Yemaneberhan, Lewis, Parry and Britton2005), and the results of anthelminthic therapy in such a setting may differ from the results in a purely rural context.
A key question is whether effects on infantile eczema will this translate into an impact on asthma in later life? Studies in affluent countries suggest that while infantile eczema per se may not predict later asthma, early development of atopy, and eczema associated with atopy and wheeze in early childhood, may be associated with atopic asthma at school-age (Illi et al. Reference Illi, von Mutius, Lau, Nickel, Gruber, Niggemann and Wahn2004, Reference Illi, von Mutius, Lau, Niggemann, Gruber and Wahn2006; Williams and Flohr, Reference Williams and Flohr2006). Asthma mortality depends not just on prevalence, but also on quality of care; already 80% of asthma deaths occur in low- and middle-income countries (WHO, 2010). It is possible that, as low-income countries develop, anthelminthic treatment programmes will contribute to an epidemic increase in allergic disease similar to that experienced in affluent countries during the 20th century (Bach, Reference Bach2002). Further research is needed to determine whether this is likely to be the case; if so, measures to equip such countries to manage such an epidemic need to be planned.
Conversely, studies of the underlying mechanisms of helminth-allergy interactions in endemic countries have the potential to provide insights for the development of new tools for the prevention and management of allergic disease, with global health benefits.
ACKNOWLEDGEMENTS
This review is presented on behalf of all members of the Entebbe Mother and Baby Study team. We thank our colleagues at Entebbe Hospital, particularly the midwives; the study nurses and field team, the Entebbe and Katabi local council field-workers; and our colleagues at the Medical Research Council and Uganda Virus Research Institute. We thank the mothers and babies for their participation in the study.
FINANCIAL SUPPORT
This work was supported by the Wellcome Trust (A.M.E, grant numbers 064693, 079110), (H.M., grant number 074791).




