The incidence and prevalence of chronic kidney disease are increasing worldwide(Reference Eknoyan, Lameire and Barsoum1). The dietary advice for patients with chronic kidney failure is to consume a diet with modest protein restriction to limit the development of toxic nitrogenous metabolites, uraemic symptoms and other metabolic complications(Reference Kopple2, 3). Still, little is known about whether various types of dietary proteins may affect kidney function and metabolism of amino acids and proteins in individuals with developed or high risk of developing impaired kidney function, and in the healthy population. Intake of fish, which is an excellent protein source, has been shown to be associated with reduced risk of developing kidney disease both in the general population(Reference Gopinath, Harris and Flood4) and in patients with type 1 diabetes(Reference Mollsten, Dahlquist and Stattin5). Thus, it is important to obtain more information on whether protein metabolism is influenced by fish intake in persons with healthy kidneys and those with reduced kidney function, and studies in animal models may be valuable tools to obtain such information.
Obesity is associated with proteinuria and focal segmental glomerulosclerosis both in humans and rats, and increases the risk of developing and adversely affects the progression of kidney disease(6–Reference Wang, Chen and Song11). The kidneys are important regulators of amino acid and protein metabolism, and have a major role in amino acid homeostasis through the synthesis, degradation, filtration, reabsorption and urinary excretion of amino acids and peptides(Reference Garibotto, Sofia and Saffioti12). Normally, nitrogen excreted in urine is mainly in the form of carbamide and ammonia, with <1 % of renal nitrogen in the form of other compounds such as uric acid, proteins, amino acids, nitric oxide metabolites and nitrates(Reference Garibotto, Sofia and Saffioti12, Reference Woolf and Giles13). The obese Zucker fa/fa rats spontaneously develop proteinuria and focal segmental glomerulosclerosis as they grow older, with indications of decreased renal function at about 12 weeks of age(Reference Laping, Olson and Day14, Reference Kasiske, O’Donnell and Keane15). Dietary interventions using obese rat models that spontaneously develop renal disease, such as the Zucker fa/fa rats, are relevant to understanding the pathophysiology of obesity-associated morbidity in humans(Reference Stevenson, Wheeldon and Gades9, Reference Kasiske, Cleary and O’Donnell16).
The possibility of identifying biomarkers of fish intake is intriguing, and such biomarkers will be of great value in assessing compliance in clinical trials. Intervention studies with fatty fish may consider measuring long-chain n-3 PUFA in isolated phospholipids from serum or plasma or in isolated erythrocytes, which are methods that are very time-consuming, expensive and require a relatively large sample volume. Promising candidate markers for fish intake include 1-methylhistidine (1-MeHis), 3-methylhistidine (3-MeHis), trimethylamineoxide (TMAO) and creatine, and we have recently shown that urine concentrations of these four compounds were markedly higher in Zucker fa/fa rats fed cod protein compared with rats fed milk proteins(Reference Drotningsvik, Midttun and McCann17).
The main aim of the present study was to investigate amino acid metabolism and vitamin status in rats with impaired renal function and signs of podocyte damage (Zucker fa/fa rats)(Reference Vikoren, Drotningsvik and Mwakimonga18) compared with rats with normal kidney function (Long-Evans rats), when all rats were fed a normal rat diet (control diet; American Institute of Nutrition (AIN)-97G(Reference Reeves, Nielsen and Fahey19)). We also investigated the effects of salmon intake on concentrations of amino acids and their metabolites in plasma and urine, and searched for possible biomarkers of salmon intake in the two rat strains. Since kidney function is related to vitamin status and vitamin deficiencies are not uncommon in patients with impaired renal function(Reference Gonzalez, Sachdeva and Oliver20, Reference Steiber and Kopple21), lipid-soluble and water-soluble vitamins were measured in plasma from both rat strains.
We hypothesised that the differences in kidney function between the two rat strains would be reflected by higher concentrations of nitrogen-containing compounds including amino acids in urine and lower plasma concentrations of most amino acids and vitamins in Zucker fa/fa rats compared with Long-Evans rats. In addition, since Zucker fa/fa rats fed baked salmon had a better renal function compared with those fed a control diet(Reference Vikoren, Drotningsvik and Mwakimonga18), we expected that baked salmon-fed Zucker fa/fa rats would have lower concentrations of nitrogen-containing compounds including amino acids in urine and higher concentrations of amino acids in plasma when compared with their control group. We expected no differences for nitrogen-containing compounds in urine and plasma between Long-Evans rats fed a baked salmon diet or control diet since no change in renal function was expected. Regarding 1-MeHis, 3-MeHis, TMAO and creatine, higher urine concentrations of all these four compounds were expected in both Zucker fa/fa rats and Long-Evans rats fed a baked salmon diet.
Materials and methods
Ethical statement
The study protocol was approved by the National Animal Research Authority of Norway, in accordance with the Animal Welfare Act and the regulation of animal experiments (approval no. 2014-6979). The animal care and use program at the Faculty of Medicine at the University of Bergen is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The present study conformed to the ‘ARRIVE Guidelines for Reporting Animal Research’(Reference Kilkenny, Browne and Cuthill22).
Animals
Two experiments were conducted. In the first experiment, twelve male Zucker fa/fa rats (HsdHlr:ZUCKER-Leprfa; Harlan Laboratories) were randomly assigned to two experimental groups of six rats each with comparable mean body weight. In the second experiment, twelve male Long-Evans rats (Crl:LE; Charles River Laboratories) were assigned to two experimental groups of six rats each with comparable mean body weight. The rats in the two experiments were treated in exactly the same manner, and therefore, the designs of the two experiments are presented together. Rats were housed in pairs in cages with wood chip bedding and plastic housing in a room maintained at a 12-h light–dark cycle (light from 07.00 to 19.00 hours) with a constant temperature of 20–23°C and relative humidity of 65 (sd 15) %. The rats were acclimatised for at least 1 week under these conditions before the start of the experiments.
To ascertain that any differences in metabolites between the strains were not a consequence of differences in body weights, we wanted the rats to have a similar body weight at euthanasia in the two experiments. The intervention period in the Zucker fa/fa rat experiment started when the rats weighed 350 (sd 20) g (i.e. 8–9 weeks old), and after the 4-week intervention, the rats weighed 554 (sd 26) g. Based on the growth chart from Charles River Laboratories, the starting weight of Long-Evans rats should be about 440 g to achieve the same body weight as the Zucker fa/fa rats after 4-week intervention. Thus, the intervention started when the Long-Evans rats weighed 430 (sd 30) g (i.e. 13–15 weeks old), and at endpoint, the mean body weight for all Long-Evans rats was 572 (sd 38) g. Growth curves for both experiments were published in Vikøren et al. (Reference Vikøren, Drotningsvik and Bergseth23).
Design
Rats were fed modified, semi-purified experimental diets prepared according to the instructions for AIN-93G(Reference Reeves, Nielsen and Fahey19) with an addition of 1·6 g methionine per kg of diet as recommended by Reeves(Reference Reeves and Watson24) for 4 weeks. The diets are described in online Supplementary Table S1. The control diet contained 20 wt% proteins from casein (Sigma-Aldrich). Skin-free salmon fillets from Atlantic Salmon with low EPA and DHA content (farmed Salmo salar fed 6 % EPA + DHA of fatty acids until 1200 g body weight and 4·5 % EPA + DHA in following feeds(Reference Sissener, Waagbø and Rosenlund25)) were provided by Leroy Seafood Group. Baked salmon fillets were prepared in an oven at 180°C for 20 min (no oil or fat were added), and were minced, freeze-dried and grinded before it was mixed with the other ingredients in the diets and frozen. All other ingredients were purchased from Dyets Inc. Freshly thawed feed was refilled every day. Rats had free access to tap water, feed and wood chewing sticks. At 1 week before euthanasia, rats were housed individually in cages with grids for 24 h without fasting in advance for urine collection.
The rats were euthanised while under anaesthesia with isoflurane (Isoba vet, Intervet; Schering-Plough Animal Health) mixed with nitrous oxide and oxygen after a 12-h fast with free access to tap water. Blood was drawn directly from the heart and collected in Vacuette Z Serum Clot Activator Tubes (Greiner Bio-One) for serum isolation, and in Vacuette K2EDTA tubes (Greiner Bio-One) for plasma isolation. Serum and plasma samples were stored at −80°C until analysis.
The personnel handling the rats and conducting the analyses were blinded to the rats’ group allocation, and rats were handled and euthanised in random order.
Analyses in serum, plasma and urine
Serum concentrations of total protein, albumin, creatinine, uric acid and carbamide were analysed by accredited methods at the Laboratory of Clinical Biochemistry at Haukeland University Hospital. Plasma concentrations of ammonium and bicarbonate, and urine concentrations of total protein, albumin, carbamide, ammonium, uric acid and creatinine, were analysed using the Cobas c111 system (Roche Diagnostics GmbH) using the appropriate kits from Roche Diagnostics. Urine concentrations of total protein and albumin, and serum concentrations of total protein, albumin and creatinine, from the Zucker fa/fa rat experiment have been published previously(Reference Vikoren, Drotningsvik and Mwakimonga18).
Urine concentrations of cystatin C and T-cell immunoglobulin mucin-1 (TIM-1) were quantified using the Mouse/Rat Cystatin C Quantikine® ELISA (MSCTC0) and the Rat TIM-1/KIM-1/HAVCR Quantikine® ELISA (RKM100) from R&D Systems (Bio-Techne). Serum vitamin D was measured by ELISA, using the 25-OH Vitamin D Total (Rat) kit (EIA-553; DRG Instruments GmbH). Serum concentrations of 25-OH vitamin D in the Zucker fa/fa rat experiment have been published previously(Reference Vikoren, Drotningsvik and Bergseth26). Plasma for glutathione measurements was added four volumes of ice-cold 5 % metaphosphoric acid, mixed and stored on ice for 15 min before centrifugation, and the supernatant was stored at −80°C until analysis. Total and oxidised glutathione were analysed using the glutathione (GSSG/GSH) detection kit (ADI-900-160) from Enzo Life Sciences AG. Reduced glutathione was calculated as the difference between total and oxidised glutathione.
Amino acids, selected metabolites of amino acids and potential biomarkers of fish intake, that is, TMAO, 1-MeHis (π-methylhistidine), 3-MeHis (τ-methylhistidine) and creatinine, were measured in plasma and urine by Bevital AS (http://www.bevital.no) using GC-MS/MS and LC-MS/MS, as previously described(Reference Midttun, Kvalheim and Ueland27, Reference Midttun, McCann and Aarseth28). We analysed 3-hydroxyisobyturate (3-HIB)(Reference Midttun, McCann and Aarseth28), TMAO, 1-MeHis and 3-MeHis(Reference Midttun, Kvalheim and Ueland27) by adding ion-pairs for the analytes and isotope-labelled internal standards to the existing assays. Arginine, aspartic acid, glutamine, α-ketoglutaric acid and 3-HIB were measured in plasma but could not be quantified in urine. Sarcosine was measured in urine but could not be measured in EDTA-plasma since sarcosine has been found in the Vacuette K2EDTA tubes. Otherwise, the same compounds were analysed in plasma and urine. Thiamine (vitamin B1), riboflavin (vitamin B2), nicotinamide (vitamin B3), pyridoxal 5′-phosphate (vitamin B6) and other vitamers of these vitamins as well as kynurenine(Reference Midttun, McCann and Aarseth28) and other metabolites in the kynurenine pathway(Reference Midttun, Hustad and Ueland29) were analysed in plasma by Bevital AS. Thiamine, thiamine monophosphate, nicotinic acid, nicotinamide, N1-methylnicotinamide, picolinic acid and quinolinic acid with corresponding isotope-labelled internal standards were added to the previously published assay(Reference Midttun, Hustad and Ueland29).
Analyses of diets
Dietary indispensable amino acids and arginine, which have been published previously(Reference Vikoren, Drotningsvik and Bergseth26), and citrulline were analysed by Nofima BioLab. Powder diets were extracted three times using boiling water as described by Christman(Reference Christman30), and 1-MeHis, 3-MeHis, creatine and TMAO were quantified as described above(Reference Midttun, Kvalheim and Ueland27, Reference Midttun, McCann and Aarseth28).
Outcomes
The primary outcome was to investigate amino acid metabolism and vitamin status in rats with impaired renal function (Zucker fa/fa rats) compared with rats with normal kidney function (Long-Evans rats). The secondary outcomes were to investigate the effects of salmon intake on the concentrations of amino acids and their metabolites in plasma and urine, and to search for possible biomarkers of salmon intake in the two rat strains.
Sample size
Two experiments are presented in the present paper, that is, a Zucker fa/fa rat experiment and a Long-Evans rat experiment. The experiments were initially designed to investigate the metabolic differences between the strains and to investigate the effects of intake of baked salmon on lipid metabolism in a rat model with reduced renal function (Zucker fa/fa rats) and in a rat model with normal renal function (Long-Evans rats). Results showing that the strains were significantly different in regard to lipid metabolism and that baked salmon intake affected lipid metabolism differently in these two rat models with six rats per group have been published(Reference Vikoren, Drotningsvik and Mjos31). In the present study, we wanted to study additional biomarkers in the same setting. In line with the Three Rs (reduction, refinement and replacement) of animal experiments, we chose to undertake more analyses using the biological samples harvested from these experiments that were already conducted. Therefore, no power analysis has been conducted for the present study. We have shown that a group size of six rats was sufficient to distinguish between Zucker fa/fa rats and Long-Evans rats in regard to lipid metabolism(Reference Vikoren, Drotningsvik and Mjos31), and in a previous study in Zucker fa/fa rats we have shown that six rats per group was sufficient to detect differences in amino acid composition between a group fed cod protein and a control group(Reference Drotningsvik, Midttun and McCann17). Therefore, we found it worth looking into differences in amino acid compositions in plasma and urine as well as vitamin status between Zucker fa/fa rats and Long-Evans rats after salmon feeding.
Statistical analysis
Data were tested for normality using the Shapiro–Wilks test, and since most data were normally distributed, they were evaluated by Student’s t test or one-way ANOVA with Tukey's honestly significant difference (HSD) post hoc test for multiple comparisons when appropriate, and the cut-off level for statistical significance was set to 0·05. Rats fed a casein-based diet served as controls in both experiments. One Zucker fa/fa rat in the control group of the first experiment was euthanised due to a serious lesion and poor health in the first week of the study and was not included in the results, therefore n 5 in the control group and n 6 in the baked salmon group. In the second experiment (Long-Evans rats), we had n 6 in both the baked salmon group and control group. Data are presented as means with standard deviations. Patterns of biomarker concentrations were investigated separately in urine and plasma by performing principal component analysis (PCA) on matrixes containing centred and standardised concentrations. Statistical analyses were performed using SPSS version 25 for Windows (SPSS Inc.) and R version 3.2.3 (http://www.r-project.org) with the prcomp package.
Results
Markers of renal function in two rat strains fed control diet
Urine concentrations (relative to creatinine) of the nitrogen-containing compounds including total protein, albumin, carbamide, uric acid, ammonium and total amount of amino acids as well as the kidney function markers cystatin C and TIM-1 were significantly higher in Zucker fa/fa rats fed the control diet compared with Long-Evans rats fed the same diet (Table 1). Circulating concentrations of total protein and albumin were similar in the two rat strains fed the control diet. Circulating total amount of amino acids, creatinine and bicarbonate concentrations were lower, whereas carbamide, ammonium and uric acid concentrations were higher in Zucker fa/fa rats compared with Long-Evans rats (Table 2).
Table 1. Nitrogen-containing compounds, cystatin C and T-cell immunoglobulin mucin-1 (TIM-1) measured in urineFootnote *
(Mean values and standard deviations)
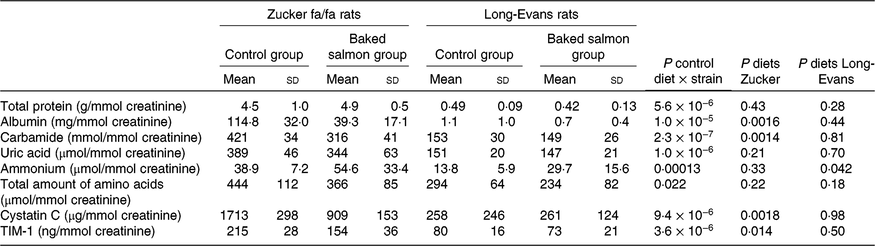
* Urine values are shown for n 5 in the control group and n 6 rats in the baked salmon group in the Zucker fa/fa rat experiment, and for n 6 in the control group and n 6 rats in the baked salmon group in the Long-Evans rat experiment. P < 0·05 was considered significant. Groups are compared within each experiment using the independent-samples t test assuming equal variances. Control groups in each rat experiment are compared using the independent-samples t test assuming equal variances.
Table 2. Circulating analytes relevant to kidney functionFootnote *
(Mean values and standard deviations)

* Plasma and serum values are shown for n 5 in the control group and n 6 rats in the baked salmon group in the Zucker fa/fa rat experiment, and for n 6 in the control group and n 6 rats in the baked salmon group in the Long-Evans rat experiment. P < 0·05 was considered significant. Groups are compared within each experiment using the independent-samples t test assuming equal variances. Control groups in each rat experiment are compared using the independent-samples t test assuming equal variances.
Amino acids, amino acid metabolites and vitamins in two rat strains fed control diet
To gain more insight into how amino acid homeostasis differs in Zucker fa/fa rats with reduced renal function compared with rats with normal kidney function such as the Long-Evans rats, we measured amino acids and their metabolites in urine and plasma, and tryptophan metabolites were measured in plasma. Since kidney function is related to vitamin status, we also measured lipid-soluble and water-soluble vitamins in plasma from both strains.
Differences between the strains were evident when rats fed the control diet were compared using PCA for analytes in urine and circulation. For urine, the first two principal components (PC) explained 79·6 % of the variance in the dataset, and showed a distinct separation between the two rat strains along PC1. Higher urine concentrations of most amino acids and their amino acids were observed, in addition to higher concentrations of nitrogen-containing compounds and markers of kidney function in Zucker fa/fa rats compared with Long-Evans rats fed the control diet (Fig. 1).

Fig. 1. Principal component analysis (PCA) of urine biomarkers from Zucker fa/fa rats (□, n 5) and Long-Evans rats (○, n 6) fed the control diet. Scores (a) and loadings (b) from the first two principal components (PC1 and PC2) are obtained from PCA using centred and standardised biomarker concentrations (relative to creatinine) in urine. The score plot (a) shows the rat groups by strain, while the loading plot (b) shows the biomarkers. ADMA, asymmetric dimethylarginine; Ala, alanine; Alb, albumin; Asn, asparagine; Bet, betaine; Carb, carbamide; Crn, creatine; CysC, cystatin C; DMG, dimethylglycine; Gln, glutamine; Gly, glycine; His, histidine; Ile, isoleucine; TIM1, T-cell immunoglobulin mucin-1; Kyn, kynurenine; Leu, leucine; Lys, lysine; 1-MeHis, 1-methylhistidine; 3-MeHis, 3-methylhistidine; Met, methionine; MetS, methionine sulfoxide; NH3, ammonium; Orn, ornithine; Phe, phenylalanine; Pro, proline; Sarc, sarcosine; SDMA, symmetric dimethylarginine; Ser, serine; tCys, total cysteine; tHcy, total homocysteine; Thr, threonine; TMAO, trimethylamine N-oxide; TML, trimethyllysine; tProt, total protein; Trp, tryptophan; Tyr, tyrosine; UA, uric acid; Val, valine.
For circulating concentrations of amino acids and their metabolites as well as vitamins from both Zucker fa/fa rats and Long-Evans rats, the PCA demonstrated a clear separation between rat strains where the two first PC explained 68·2 % of the variation in the dataset with higher concentrations of vitamins and lower concentrations of several amino acids (especially arginine), total glutathione and most tryptophan metabolites in Zucker fa/fa rats compared with Long-Evans rats (Fig. 2). Concentrations of individual biomarkers in urine and in circulation are presented in online Supplementary Tables S2 and S3.
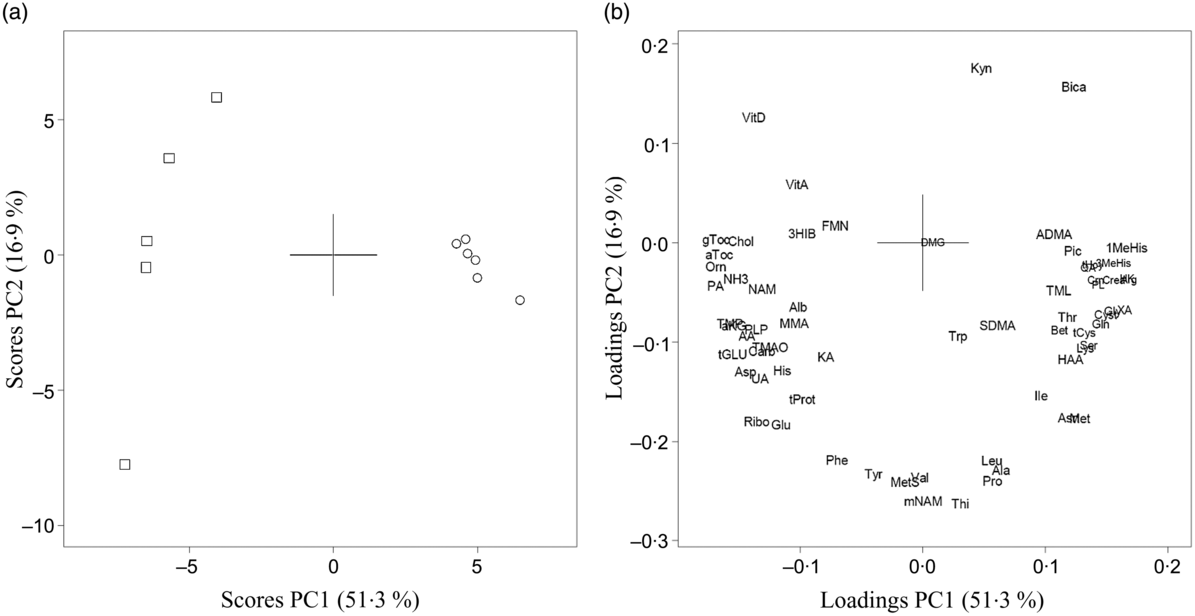
Fig. 2. Principal component analysis (PCA) of circulating biomarkers from Zucker fa/fa rats (□, n 5) and Long-Evans rats (○, n 6) fed the control diet. Scores (a) and loadings (b) from the first two principal components (PC1 and PC2) are obtained from PCA using centred and standardised biomarker concentrations. The score plot (a) shows the rat groups by strain, while the loading plot (b) shows the biomarkers. AA, anthranilic acid; ADMA, asymmetric dimethylarginine; aKG, α-ketoglutaric acid; Ala, alanine; Alb, albumin; Arg, arginine; Asn, asparagine; Asp, aspartic acid; aTOC, α-tocopherol; Bet, betaine; Bica, bicarbonate; Carb, carbamide; Chol, choline; Crea, creatinine; Crn, creatine; Cyst, cystathionine; DMG, dimethylglycine; FMN, flavin mononucleotide; Gln, glutamine; Glu, glutamic acid, Gly, glycine; gTOC, γ-tocopherol; HAA, 3-hydroxyanthranilic acid; 3HIB, 3-hydroxyisobutyrate; His, histidine; HK, 3-hydroxykynurenine; Ile, isoleucine; KA, kynurenic acid; Kyn, kynurenine; Leu, leucine; Lys, lysine; 1-MeHis, 1-methylhistidine; 3-MeHis, 3-methylhistidine; Met, methionine; MetS, methionine sulfoxide; MMA, methyl malonic acid; mNAM, N1-methylnicotinamide; NAM, nicotine amide; NH3, ammonium; Orn, ornithine; PA, 4-pyridoxic acid; Phe, phenylalanine; Pic, picolinic acid; PL, pyridoxal; PLP, pyridoxal 5′-phosphate; Pro, proline; QA, quinolinic acid; Ribo, riboflavin; SDMA, symmetric dimethylarginine; Ser, serine; tCys, total cysteine; tGLU, total glutathione; tHcy, total homocysteine; Thi, thiamine; Thr, threonine; TMAO, trimethylamine N-oxide; TML, trimethyllysine; TMP, thiamine monophosphate; tProt, total protein; Trp, tryptophan; Tyr, tyrosine; UA, uric acid; Val, valine; VitA, vitamin A; VitD, vitamin D; XA, xanthurenic acid.
Diets and growth in two rat strains fed control diet or baked salmon diet
The baked salmon diet contained more (i.e. >1 g/kg diet difference) of the indispensable amino acids lysine and methionine when compared with the control diet; otherwise, the contents of indispensable amino acids were similar between the diets, with a slightly higher content of the conditionally essential amino acid arginine in the baked salmon diet (online Supplementary Table S1).
1-MeHis, 3-MeHis, creatine and TMAO were found in the baked salmon diet (77, 2·3, 7132 and 473 µmol per kg of diet, respectively) but not in the control diet. Citrulline was not detected in either of the diets.
The growth and daily energy intake were similar in both dietary groups within the Zucker fa/fa rat experiment and within the Long-Evans rat experiment, as we have previously published(Reference Vikøren, Drotningsvik and Bergseth23), indicating that protein utilisation in the dietary groups within each rat strain was comparable. The mean daily energy intake was similar between the two groups (P ANOVA = 0·24), with a significantly larger body weight gain in Zucker fa/fa rats compared to Long-Evans rats (P ANOVA 1·5 × 10−8, using Tukey’s HSD post hoc test)(Reference Vikøren, Drotningsvik and Bergseth23).
Effects of salmon intake on markers of renal function, amino acids and their metabolites in urine
Urine concentrations (relative to creatinine) of albumin, carbamide, cystatin C and TIM-1 were significantly lower in Zucker fa/fa rats fed the baked salmon diet compared with Zucker fa/fa rats fed the control diet, whereas no differences were seen between these groups for total protein, total amount of amino acids, uric acid and ammonium concentrations (Table 1). In the Long-Evans rat experiment, rats fed the baked salmon diet had higher urine ammonium concentration compared with those fed the control diet; otherwise, no differences were seen in urine concentrations of nitrogen-containing compounds, cystatin C or TIM-1.
The results of PCA for amino acids, amino acid metabolites and markers of kidney function in urine from Zucker fa/fa rats (Fig. 3(a) and (b)) and Long-Evans rats (Fig. 3(c) and (d)) demonstrated a separation along PC2 between the two dietary groups. 1-MeHis, 3-MeHis, TMAO and creatine concentrations were higher, with a trend towards lower concentrations of amino acids in both rat strains fed the baked salmon diet. PCA analysis of all rats on both diets combined showed that the two rat strains were separated with a trend towards generally higher concentrations of all biomarkers except kynurenine in Zucker fa/fa rats (Fig. 4). The concentrations of individual compounds in urine are presented in online Supplementary Table S2. The results show that in the Long-Evans rat experiment, urine concentrations of cysteine, methionine, methionine sulfoxide, homocysteine and proline were significantly lower in rats fed the baked salmon diet compared with the control group, whereas urine concentrations of amino acids generally did not differ by diet in the Zucker fa/fa rat experiment.
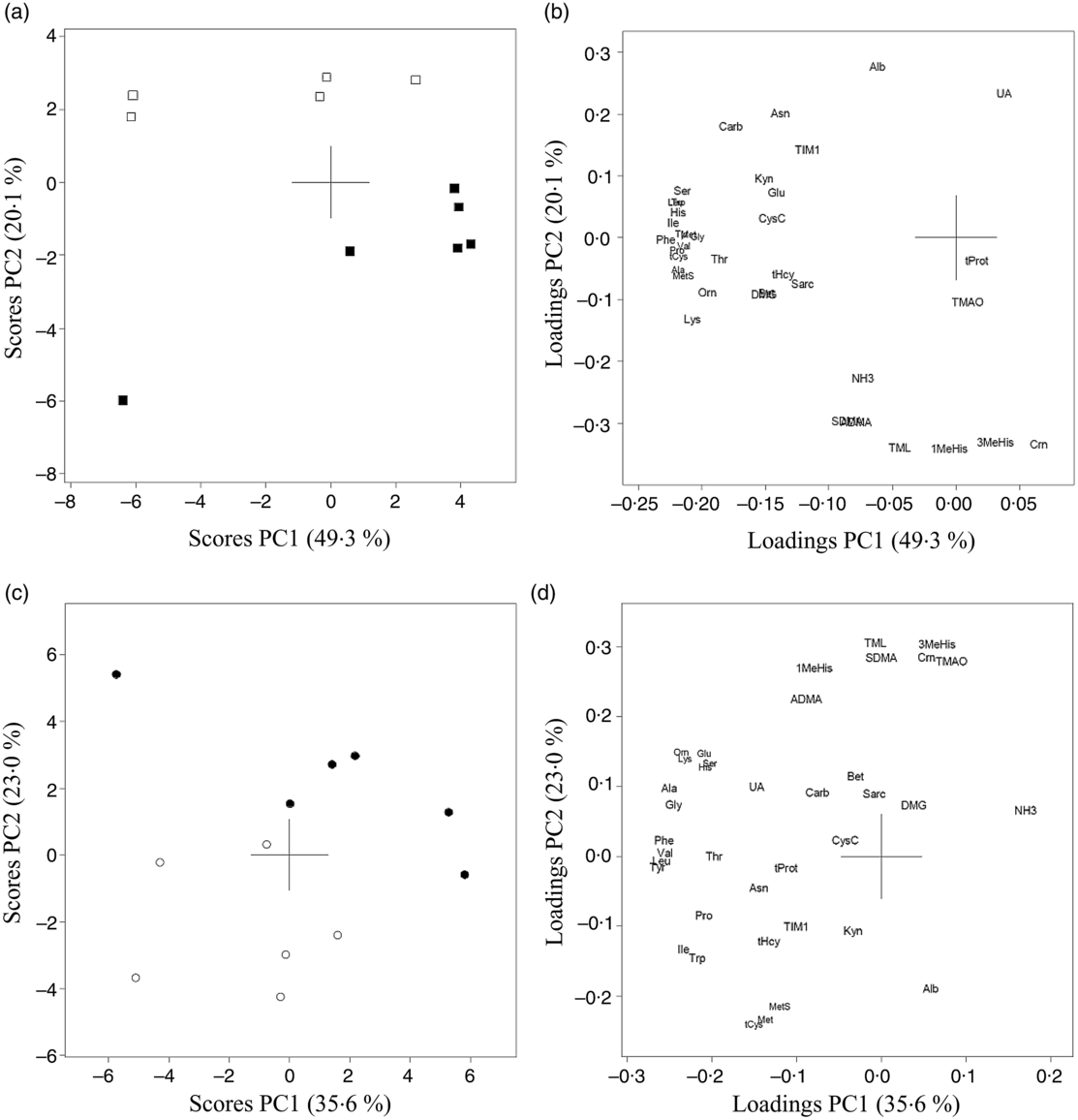
Fig. 3. Principal component analysis (PCA) of urine biomarkers from Zucker fa/fa rats fed the control diet (□, n 5) or baked salmon diet (▪, n 6) (a and b), and Long-Evans rats fed the control diet (○, n 6) or baked salmon diet (•, n 6) (c and d). Scores (a and c) and loadings (b and d) from the first two principal components (PC1 and PC2) are obtained from PCA using centred and standardised biomarker concentrations (relative to creatinine) in urine. The score plot (a) shows the rat groups by strain, while the loading plot (b) shows the biomarkers. ADMA, asymmetric dimethylarginine; Ala, alanine; Alb, albumin; Asn, asparagine; Bet, betaine; Carb, carbamide; Crn, creatine; CysC, cystatin C; DMG, dimethylglycine; Gln, glutamine; Gly, glycine; His, histidine; Ile, isoleucine; TIM1, T-cell immunoglobulin mucin-1; Kyn, kynurenine; Leu, leucine; Lys, lysine; 1-MeHis, 1-methylhistidine; 3-MeHis, 3-methylhistidine; Met, methionine; MetS, methionine sulfoxide; NH3, ammonium; Orn, ornithine; Phe, phenylalanine; Pro, proline; Sarc, sarcosine; SDMA, symmetric dimethylarginine; Ser, serine; tCys, total cysteine; tHcy, total homocysteine; Thr, threonine; TMAO, trimethylamine N-oxide; TML, trimethyllysine; tProt, total protein; Trp, tryptophan; Tyr, tyrosine; UA, uric acid; Val, valine.
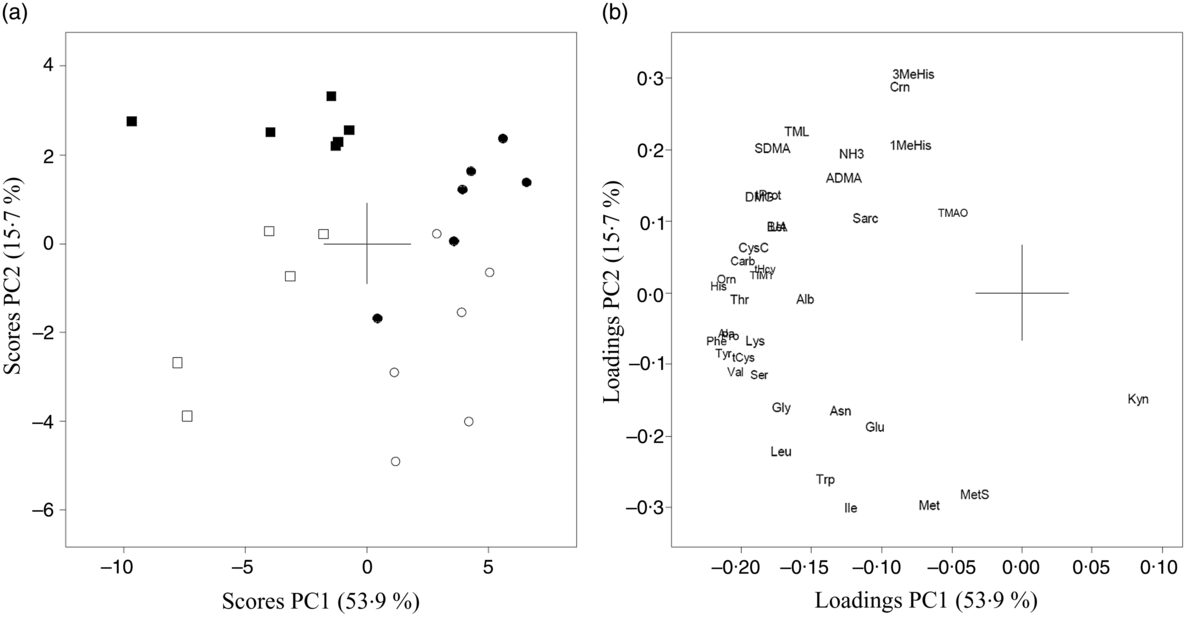
Fig. 4. Principal component analysis (PCA) of urine biomarkers from Zucker fa/fa rats fed the control diet (□, n 5) or baked salmon diet (▪, n 6), and Long-Evans rats fed the control diet (○, n 6) or baked salmon diet (•, n 6). Scores (a) and loadings (b) from the first two principal components (PC1 and PC2) are obtained from PCA using centred and standardised biomarker concentrations (relative to creatinine) in urine. The score plot (a) shows the rat groups by strain, while the loading plot (b) shows the biomarkers. ADMA, asymmetric dimethylarginine; Ala, alanine; Alb, albumin; Asn, asparagine; Bet, betaine; Carb, carbamide; Crn, creatine; CysC, cystatin C; DMG, dimethylglycine; Gln, glutamine; Gly, glycine; His, histidine; Ile, isoleucine; TIM1, T-cell immunoglobulin mucin-1; Kyn, kynurenine; Leu, leucine; Lys, lysine; 1-MeHis, 1-methylhistidine; 3-MeHis, 3-methylhistidine; Met, methionine; MetS, methionine sulfoxide; NH3, ammonium; Orn, ornithine; Phe, phenylalanine; Pro, proline; Sarc, sarcosine; SDMA, symmetric dimethylarginine; Ser, serine; tCys, total cysteine; tHcy, total homocysteine; Thr, threonine; TMAO, trimethylamine N-oxide; TML, trimethyllysine; tProt, total protein; Trp, tryptophan; Tyr, tyrosine; UA, uric acid; Val, valine.
Effects of salmon intake on circulating markers of renal function, amino acids and their metabolites, vitamins and potential biomarkers of salmon intake
Intake of the baked salmon diet did not affect circulating concentrations of total protein, albumin, total amount of amino acids, creatinine, carbamide, bicarbonate and uric acid within either of the two rat strains (Table 2). Plasma ammonium was lower in Zucker fa/fa rats fed the baked salmon diet when compared with their controls, but no difference was seen in plasma ammonium between Long-Evans rats fed the baked salmon diet and control diet.
Individual PCA for Zucker fa/fa rats (online Supplementary Fig. S1(a) and (b)) and Long-Evans rats (online Supplementary Fig. S1(c) and (d)), including all amino acids, their metabolites and nitrogen-containing compounds in circulation, displayed tendencies towards separation between the dietary groups. A detailed analysis (online Supplementary Tables S3 and S4) showed that in Zucker fa/fa rats, plasma concentrations of arginine, creatine, total cysteine, asymmetric dimethylarginine, 1-MeHis, 3-MeHis and quinolinic acid were higher, while histidine, α-tocopherol, riboflavin and flavin mononucleotide concentrations were lower in the baked salmon diet group compared with the control group, with no difference between the dietary groups for the kynurenine:tryptophan ratio. In the Long-Evans rat experiment, rats in the baked salmon group had higher plasma concentrations of arginine, creatine, 1-MeHis and γ-tocopherol, lower kynurenine:tryptophan ratio, and lower concentrations of several tryptophan pathway metabolites when compared with the control group. When rats from both strains on both diets were included in the same PCA, Zucker fa/fa rats fed the baked salmon diet were located closer to Long-Evans rats, suggesting that Zucker fa/fa rats were more similar to lean rats after salmon intake (online Supplementary Fig. S2).
Discussion
In the present study we compared biomarker concentrations in two rat strains: Zucker fa/fa rats, which are prone to developing impaired kidney function, and Long-Evans rats, which have normal kidney function. We observed that the concentrations of markers of renal function and most amino acids in urine, as well as plasma concentrations of most vitamins, were higher in Zucker fa/fa rats compared with Long-Evans rats fed the control diet. Dietary consumption of salmon led to lower urine concentrations of several established renal function markers in Zucker fa/fa rats, indicating a better kidney function, whereas salmon intake did not affect renal function markers in Long-Evans rats. We also found that urinary concentrations of 1-MeHis, 3-MeHis, TMAO and creatine, as well as plasma concentrations of 1-MeHis and creatine were higher in rats fed salmon, and may thus be useful as biomarkers of salmon intake in rats. To the best of our knowledge, this is the first study that compared these parameters between a rat model with impaired kidney function and a rat model with normal kidney function, and investigated the effects of fish intake in both rat models.
We have recently shown that the Zucker fa/fa rats in this study showed signs of podocyte damage, based on both histological findings and biochemical analyses(Reference Vikoren, Drotningsvik and Mwakimonga18). Analysis of kidney function markers in rats fed the control diet showed that urine concentrations (relative to creatinine) of total protein, albumin and total amount of amino acids were higher in Zucker fa/fa rats compared with Long-Evans rats. In addition, urine concentrations of cystatin C and TIM-1, both indicative of tubular injury(Reference Han, Waikar and Johnson32–Reference Conti, Moutereau and Zater34), were higher in Zucker fa/fa rats, thus confirming that tubular reabsorption in Zucker fa/fa rats was impaired compared with Long-Evans rats. This is supported by a lower serum carbamide concentration in Long-Evans rats. Serum creatinine is no longer regarded a sensitive marker of kidney function(Reference Star35) since its concentration may remain normal until about 50 % of kidney function is lost and is mainly contingent upon muscle mass, muscle function, diet and health status(Reference Stevens, Coresh and Greene36). Thus, a higher serum creatinine concentration in Long-Evans rats compared with Zucker fa/fa rats is probably linked to a greater relative muscle mass in the former, and not a consequence of impaired filtration by the kidneys. The kidneys play a central role in protein metabolism and urine secretion of amino acids and proteins. Amino acids filtered by the glomeruli are normally reabsorbed by the tubules and are therefore not excreted in urine(Reference Garibotto, Sofia and Saffioti12, Reference Woolf and Giles13), and aminoaciduria is an early sign of renal tubular damage with reduced tubular reabsorption of amino acids occurring before proteinuria(Reference Betts and Green37, Reference Macpherson, Moscarello and Goldberg38). Therefore, our observation that Zucker fa/fa rats fed the control diet had higher urine concentrations of most amino acids and their metabolites when compared with Long-Evans rats fed the same diet is in line with the impaired renal function in Zucker fa/fa rats. The amino acid composition in urine was not reflected in plasma, where PCA showed a clear separation between strains due to lower concentrations of several amino acids, total glutathione and most tryptophan metabolites in Zucker fa/fa rats compared with Long-Evans rats. Especially the difference between plasma arginine concentrations between the strains is of interest, since the proximal tubule is the major site of arginine synthesis, mainly from citrulline produced in the intestine from glutamine or from diet(Reference Featherston, Rogers and Freedland39, Reference Dhanakoti, Brosnan and Herzberg40). Thus, the markedly lower plasma arginine concentration in Zucker fa/fa rats is probably a consequence of lower capacity for endogenous arginine synthesis due to pronounced tubular damage(Reference Vikoren, Drotningsvik and Mwakimonga18).
Glutathione is a tripeptide consisting of cysteine (produced from methionine and homocysteine via cystathionine in the transsulfuration pathway), glycine and glutamate. We found that Zucker fa/fa rats had a higher concentration of glutathione, both in its oxidised and reduced forms, and lower concentrations of its precursors methionine, homocysteine, cystathionine, cysteine and glycine in plasma compared with Long-Evans rats. This suggests that in Zucker fa/fa rats, the endogenous synthesis of the powerful antioxidant, glutathione, is upregulated, possibly to counteract and prevent lipid peroxidation in these hyperlipidaemic rats(Reference Vikoren, Drotningsvik and Mjos31, Reference Araujo, Barbosa and Hsin41) and/or as a consequence of disturbances in antioxidant defence systems as kidney function deteriorates(Reference Ceballos-Picot, Witko-Sarsat and Merad-Boudia42).
More than 95 % of orally ingested TMAO is excreted unchanged by the kidneys in humans(Reference Al-Waiz, Mitchell and Idle43), and circulating TMAO concentration has been found to increase when glomerular filtration and plasma clearance decrease in patients with renal dysfunction(Reference Zeisel and Warrier44). The higher concentration of TMAO in plasma in Zucker fa/fa rats compared with Long-Evans rats may be a consequence of impaired kidney function in the former rats; however, the higher urine concentration of TMAO in Zucker fa/fa rats also suggests that TMAO was successfully filtrated by the kidneys. It is possible that the higher TMAO concentration in both plasma and urine from Zucker fa/fa rats compared with Long-Evans rats may be caused by differences in gut microbiota between these strains, as TMAO is produced by certain gut microbes from phosphatidylcholine and l-carnitine(Reference Zeisel and Warrier44). In this context, it is of interest that obese Zucker fa/fa rats have different intestinal microbiota populations compared with lean Zucker rats, with obese rats having more Akkermansia mucinophila (Reference Hakkak, Korourian and Foley45), which has been shown to be associated with intestinal production of TMAO in mice(Reference Koeth, Levison and Culley46).
The higher plasma concentration of all fat-soluble vitamins and the majority of water-soluble vitamins in Zucker fa/fa rats compared to Long-Evans rats was clearly demonstrated in the PCA, despite similar energy intake between the two groups. This was an unexpected finding since the loss of both fat-soluble and water-soluble vitamins in urine has been demonstrated to be higher in rats with tubular injury(Reference Imai, Sano and Fukuwatari47, Reference Smazal, Borcherding and Anderegg48) and since vitamin D insufficiency is highly prevalent in patients with chronic kidney disease(Reference Gonzalez, Sachdeva and Oliver20). Transport proteins for fat-soluble vitamins, including retinol-binding protein and vitamin D-binding protein, and water-soluble vitamins are filtered in the glomeruli and reabsorbed in the renal tubule or secreted in urine(Reference Birn49, Reference Christensen, Birn and Storm50). Thus, lower vitamin concentrations could be expected in Zucker fa/fa rats compared with Long-Evans rats. Circulating concentrations of many compounds, including vitamin A(Reference Yatzidis, Digenis and Fountas51) and 4-pyridoxic acid(Reference Coburn, Reynolds and Mahuren52), have been shown to be markedly elevated in patients with uraemic syndrome caused by compromised renal function, which agrees with the higher serum carbamide concentration observed in Zucker fa/fa rats compared with Long-Evans rats in the present study. Additionally, slower clearance of chylomicrons in Zucker fa/fa rats(Reference Redgrave53) leads to a longer circulation time for vitamins obtained from diets, and this is in accordance with the higher circulating concentrations of lipids in Zucker fa/fa rats compared with Long-Evans rats(Reference Vikoren, Drotningsvik and Mjos31) and may, at least partially, explain the higher plasma vitamin concentrations in Zucker fa/fa rats.
In the present study we found that urine concentrations (relative to creatinine) of total amino acids as well as of total protein were not different between Zucker fa/fa rats fed the baked salmon diet or the control diet, despite less pronounced tubular damage(Reference Vikoren, Drotningsvik and Mwakimonga18) and lower urine concentrations of albumin, cystatin C and TIM-1 in the baked salmon group. We have previously shown that when Zucker fa/fa rats were fed cod protein, the total amount of free amino acids in urine was markedly lower compared to controls, accompanied by lower urine cystatin C and TIM-1 concentrations, indicating better kidney function in cod protein-fed rats(Reference Drotningsvik, Midttun and McCann17). Differences in urine concentrations of amino acids between Zucker fa/fa rats fed the baked salmon diet or control diet were less pronounced, but PCA showed a trend towards lower amino acid concentrations in rats fed the baked salmon diet. The markedly higher urine concentrations of both cystatin C and TIM-1 in the present study suggest a more advanced stage of renal damage compared with the cod protein study. This is supported by a markedly lower plasma arginine concentration in Zucker fa/fa rats in the present study compared with the cod protein study(Reference Drotningsvik, Midttun and McCann17), which is suggestive of a reduced capacity for arginine synthesis due to tubular injury(Reference Featherston, Rogers and Freedland39, Reference Dhanakoti, Brosnan and Herzberg40). In Long-Evans rats, no differences were seen between dietary groups in urine concentrations of albumin, total amino acids, cystatin C and TIM-1. The kidney function is normal in Long-Evans rats; therefore, no differences were expected in these parameters, but also in these rats, the PCA showed a trend towards lower urine concentrations of amino acids in rats fed the baked salmon diet, which was more pronounced than for Zucker fa/fa rats. It was of special interest that the PCA showed that plasma from Zucker fa/fa rats fed the baked salmon diet showed more similarity to Long-Evans rats than did Zucker fa/fa rats fed the control diet. For both strains, urine and plasma amino acid compositions were not reflective of dietary amino acid compositions, which is in accordance with our recent study on the effects of cod protein intake on amino acids in Zucker fa/fa rats(Reference Drotningsvik, Midttun and McCann17).
Both rat strains showed higher urine concentrations of 1-MeHis and 3-MeHis when fed the baked salmon diet compared with the control diet. This is in line with our recent report showing higher urine concentrations of these compounds in rats fed cod protein compared with a casein-whey protein mixture(Reference Drotningsvik, Midttun and McCann17). Similar, but not identical, patterns were seen in both Zucker fa/fa rats (higher plasma concentrations of 1-MeHis and 3-MeHis) and Long-Evans rats (higher plasma concentration of 1-MeHis) fed the baked salmon diet compared with their respective control group. Anserine (a dipeptide of β-alanine and 1-MeHis) degrades to 1-MeHis and is found in rat muscle and many types of fish, including Atlantic salmon, but not in cows’ milk and human skeletal muscle(Reference Christman30, Reference van Waarde54–Reference Crush56), whereas 3-MeHis is released upon breakdown of actin and myosin(Reference Marliss, Wei and Dietrich57). Since these methylhistidines are not re-utilised for protein synthesis or metabolised, they are excreted in the urine, and their urinary levels have been proposed as a useful biomarker of meat intake(Reference Altorf-van der Kuil, Brink and Boetje58). We propose that the higher plasma and urine concentrations of 1-MeHis and 3-MeHis in Zucker fa/fa rats fed the baked salmon diet are probably a result of salmon intake, and not degradation of muscle proteins. This suggestion is supported by similar growth patterns in the two dietary groups and the reported lower serum aspartate aminotransferase concentration in the baked salmon group in the Zucker fa/fa rat experiment(Reference Vikoren, Drotningsvik and Mjos31). Similarly, we propose that the higher urine concentrations of 1-MeHis and 3-MeHis in Long-Evans rats fed the baked salmon diet compared with the control diet originated from the fish intake. Urinary 1-MeHis and 3-MeHis concentrations have been observed to increase in response to muscle tissue intake from both meat(Reference Altorf-van der Kuil, Brink and Boetje58) and cod(Reference Drotningsvik, Midttun and McCann17), and in the present study, we found similar effects after salmon fillet intake. We found a higher 1-MeHis content compared with 3-MeHis in baked salmon, and this was reflected in a more pronounced effect on urine and plasma concentrations of 1-MeHis relative to 3-MeHis in both rat strains fed the baked salmon diet compared with the control diet. Further studies should investigate the effects of other diets containing proteins from fish muscles and other protein sources on urinary concentrations of these biomarkers.
Other candidate biomarkers of fish intake include the small molecules, TMAO and creatine, which are also cleared from plasma by the kidneys and excreted in urine. Like methylhistidines, these compounds can also be obtained from the diet or produced by the body. Farmed Atlantic salmon contains both creatine(Reference Clark59) and TMAO(Reference Dyer60), whereas casein does not contain these compounds(Reference Drotningsvik, Midttun and McCann17); however, TMAO and its precursors are also found in egg and red meat, but in much lower amounts compared with fish(Reference Cho, Taesuwan and Malysheva61, Reference Miller, Corbin and da Costa62). We have recently shown that Zucker fa/fa rats fed baked salmon had better kidney function compared with those fed a control diet(Reference Vikoren, Drotningsvik and Mwakimonga18), but still, plasma TMAO concentration was similar between both diet groups. The higher urine TMAO concentration in Zucker fa/fa rats indicated that glomerular filtration was sufficient to avoid the accumulation of TMAO in circulation in the baked salmon group, thus indicating that the high urine TMAO concentration reflected a high dietary intake of TMAO from salmon. Findings from Long-Evans rats support this, as no differences were seen in plasma TMAO concentration between rats fed the baked salmon diet or control diet, with a markedly higher urine TMAO concentration in the former group. These findings are in line with other reports showing higher TMAO concentrations in urine and/or circulation after fish protein or fish intake(Reference Drotningsvik, Midttun and McCann17, Reference Cho, Taesuwan and Malysheva61, Reference Yazdekhasti, Brandsch and Schmidt63–Reference Cheung, Keski-Rahkonen and Assi65), thus supporting the use of TMAO as a biomarker of fish intake. Creatine could also be a valuable biomarker of fish intake in certain experimental settings such as the present study, where controls received only dairy protein, and higher creatinine concentrations were found in urine and plasma in baked salmon diet-fed rats of both strains. However, since creatine is also found in animal muscles, this compound may not be a useful biomarker specific to fish intake.
The present study has some strengths and limitations. Strengths include the measurement of many markers of renal function, amino acids, metabolites of amino acids and both water- and fat-soluble vitamins. A limitation of the present study is that since the Long-Evans experiment was conducted after the Zucker fa/fa study, the experimental conditions may have been different. We made great efforts to avoid such differences by controlling the temperature and humidity, using the same cages, equipment and frozen feeds, and the same staff members handled and euthanised the rats.
To conclude, urine concentrations of most amino acids and their metabolites as well as plasma concentrations of the majority of vitamins were higher, whereas plasma concentrations of several amino acids (especially arginine), total glutathione and most tryptophan metabolites were lower in Zucker fa/fa rats compared with Long-Evans rats fed the control diet, probably as a consequence of impaired renal function and slower chylomicron clearance in Zucker fa/fa rats. Dietary consumption of salmon led to lower urine concentrations of several established renal function markers in Zucker fa/fa rats, indicating a better kidney function, but did not affect renal function markers in Long-Evans rats. A trend towards lower urine concentrations of amino acids was seen in both rat strains fed the salmon diet when compared with control groups, but this was more pronounced in Long-Evans rats, and did not reflect dietary amino acid content, demonstrating that different rat strains may respond differently to the same diet. The present study also showed that, despite differences in tubular function between rat strains, plasma concentrations of 1-MeHis and creatine, and urine concentrations of 1-MeHis, 3-MeHis, creatine and TMAO are promising biomarkers of salmon intake. Future studies should investigate whether these biomarkers of fish intake are valid when assessing compliance in clinical trials, which is subjected to reporting bias.
Acknowledgements
This work was supported by the Norwegian Seafood Research Fund (FHF, grant no. 900842). Salmon fillets were provided by Lerøy Seafood Group (Bergen, Norway). These sponsors were not involved in the design of the study, data collection, analysis and interpretation of data, writing of the article, or in the decision to submit the article for publication.
A. D., L. A. V., G. M. and O. A. G. formulated the research question and designed the study. A. D., L. A. V. and O. A. G. conducted the animal study. A. D., Ø. M., A. M., P. M. U. and O. A. G. analysed the data and performed statistical analyses. O. A. G. drafted the manuscript and take primary responsibility for the final content. All authors contributed to the writing and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001284









