Craniopharyngiomas are epithelial neoplasms of the sellar and suprasellar regions, which are benign histologically but difficult to manage clinically, with frequent local invasion and high rate of recurrence, particularly with subtotal resection.Reference Buslei, Rushing, Giangaspero, Paulus, Burger, Santagata, Louis, Ohgaki and Wiestler1,Reference Crotty, Scheithauer and Young2 They are thought to arise from remnants of the Rathke pouch and consist of two types, the adamantinomatous craniopharyngioma (ACP) and papillary craniopharyngioma (PCP) variants.Reference Le, Towfighi, Kapadia and Lopes3 While ACPs are more common and have a bimodal age distribution with most cases seen in children and older adults,Reference Alomari, Kelley and Damisah4 PCPs are seen almost exclusively in adults.Reference Crotty, Scheithauer and Young2,Reference Alomari, Kelley and Damisah4,Reference Borrill, Cheesman, Stivaros, Kamaly-Asl, Gnanalingham and Kilday5 ACPs are characterized by ameloblastoma-like epithelium with peripheral palisading, stellate reticulum admixed with wet keratin, and occasional calcifications and xanthomatous change.Reference Crotty, Scheithauer and Young2,Reference Schlaffer, Buchfelder, Stoehr, Buslei and Hölsken6 The majority of ACPs show mutations in the CTNNB1 gene leading to the translocation of β-catenin protein from the membrane to the nucleus that can be demonstrated on immunohistochemistry.Reference Schweizer, Capper and Hölsken7 PCPs show nonkeratinizing stratified squamous epithelium with fibrovascular cores and typically no wet keratin or calcifications. They may occasionally show goblet cells and rarely ciliated columnar or cuboidal cells.Reference Crotty, Scheithauer and Young2 Over 95% of PCPs harbor the BRAF V600E mutation that can be detected on immunohistochemistry using antibodies against the mutant epitope (clone VE1).Reference Schweizer, Capper and Hölsken7 The differential diagnosis of lesions with squamous epithelium in the sellar region includes Rathke cleft cyst (RCC) and epidermoid and dermoid cysts. Epidermoid and dermoid cysts demonstrate keratinizing stratified squamous epithelium with dry keratin in contrast to the nonkeratinizing squamous epithelium in PCPs and wet keratin in ACPs, with dermoid cysts additionally showing dermal adnexal structures.Reference Buslei, Rushing, Giangaspero, Paulus, Burger, Santagata, Louis, Ohgaki and Wiestler1,Reference Harrison, Morgello and Post8 RCCs occur predominantly in adults and are rare in children.Reference Schlaffer, Buchfelder, Stoehr, Buslei and Hölsken6 They are also thought to arise from Rathke pouch remnants and are lined by cuboidal or columnar cells, often with cilia and occasionally goblet cells, and may show xanthomatous change.Reference Le, Towfighi, Kapadia and Lopes3 Approximately 5–39% of cases undergo squamous metaplasia resulting in significant histologic overlap with PCP that makes differentiating between the two difficult, especially on small biopsies.Reference Le, Towfighi, Kapadia and Lopes3,Reference Schlaffer, Buchfelder, Stoehr, Buslei and Hölsken6 There have been cases initially diagnosed as RCCs that were reclassified as PCPs based on recurrence or BRAF mutational analysis.Reference Crotty, Scheithauer and Young2,Reference Schlaffer, Buchfelder, Stoehr, Buslei and Hölsken6,Reference Schweizer, Capper and Hölsken7 The distinction is important in management as RCCs demonstrate an excellent prognosis with a 90% remission rate after partial resection or fenestration.Reference Schweizer, Capper and Hölsken7 We report a rare case of PCP in a young child in which molecular testing was critical for diagnosis.
A previously healthy 6-year-old female presented to an outside hospital for a 1-month history of mild cough, fevers, intermittent nausea and vomiting, problems with balance, and 1 week of left-sided headaches. She was also found to have acute visual loss in the left eye on examination. Computed tomography of the head demonstrated a sellar/suprasellar mass with hyperdensity concerning for hemorrhage. She was subsequently transferred to our institution where magnetic resonance imaging showed a 4.9-cm T2 heterogeneous, multilobulated, and partially calcified sellar/suprasellar mass with peripheral enhancement causing regional mass effect and surrounding edema (Figure 1). She underwent endonasal resection which demonstrated thick yellow fluid under pressure with no evidence of hemorrhage. Histologic examination showed nonkeratinizing squamous epithelium with many infiltrating neutrophils adjacent to anterior pituitary tissue (Figure 2A) and focal ciliated columnar epithelium with rare goblet cells (Figure 2B).

Figure 1: (A) Coronal T1 post-contrast magnetic resonance image demonstrates a large multilobulated sellar/suprasellar mass (arrow) that shows peripheral enhancement and (B) heterogenous T2 signal (arrow) on the sagittal T2 weighted image with surrounding edema.
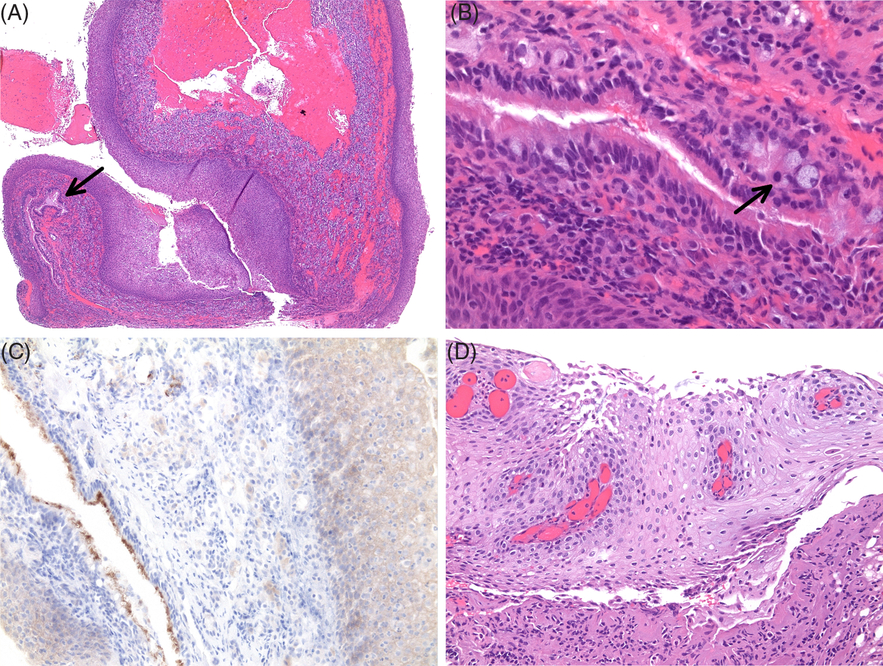
Figure 2: (A) Initial resection showing nonkeratinizing squamous epithelium and columnar epithelium (arrow) adjacent to anterior pituitary tissue (hematoxylin and eosin, 40×). (B) Ciliated columnar epithelium with rare goblet cells (arrow) (400×). (C) VE1 immunostain is positive in the squamous epithelium and negative in the columnar epithelium with nonspecific staining of cilia and anterior pituitary tissue (200×). (D) Resection of residual tumor showing nonkeratinizing squamous epithelium with fibrovascular cores (200×).
Immunohistochemistry for CAM5.2 and CK7 was positive in the columnar epithelium with focal to patchy staining of the superficial layers of the squamous epithelium. CK20 was negative in both the squamous and columnar epithelium. The adjacent normal anterior pituitary tissue showed focal staining with CK7, CAM5.2, and CK20. VE1 immunostain was positive in the squamous epithelium and negative in the columnar epithelium (Figure 2C). There was nonspecific staining of the cilia and anterior pituitary cells, as described previously.Reference Schweizer, Capper and Hölsken7 Sanger sequencing demonstrated a c.1799T>A (BRAF V600E) mutation. Immunostaining for β-catenin showed only membranous staining, confirmed by next-generation sequencing that demonstrated no mutations within exon 3 of the CTNNB1 gene. The patient subsequently underwent a pterional craniotomy for resection of residual tumor 1.5 months later that showed nonkeratinizing squamous epithelium with fibrovascular cores (Figure 2D). There was also prominent xanthomatous inflammation with calcifications, features more often seen in ACP and RCC,Reference Le, Towfighi, Kapadia and Lopes3 although these changes may partly be attributable to the initial surgery.
There is significant histologic and immunophenotypic overlap between PCPs and RCCs, despite attempts at differentiation with cytokeratin immunohistochemistry. While all craniopharyngiomas have been negative for CK20, two-thirds of RCCs are also negative and both express CK8.Reference Le, Towfighi, Kapadia and Lopes3 Our case initially did not show fibrovascular cores characteristic of PCPs, raising the consideration of RCC with squamous metaplasia as RCCs can undergo marked squamous metaplasia such that the squamous epithelium becomes dominant.Reference Schweizer, Capper and Hölsken7 PCPs can rarely show ciliated or goblet cells, but they are usually admixed with or in the superficial layer overlying the squamous epithelium,Reference Schlaffer, Buchfelder, Stoehr, Buslei and Hölsken6 whereas the current case demonstrated a separate component of ciliated columnar epithelium adjacent to anterior pituitary tissue reminiscent of RCC. It has been hypothesized that PCP may arise from RCC by acquiring a BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutation as the presence of squamous metaplasia in RCC has been associated with increased recurrence, which may reflect a continuum between RCCs and PCPs.Reference Crotty, Scheithauer and Young2,Reference Borrill, Cheesman, Stivaros, Kamaly-Asl, Gnanalingham and Kilday5–Reference Schweizer, Capper and Hölsken7 Our case of PCP may possibly have had components of RCC or remnants.
The prognosis of PCPs is dependent on the extent of resection with significantly higher rates of recurrence after incomplete resection compared to gross total resection.Reference Buslei, Rushing, Giangaspero, Paulus, Burger, Santagata, Louis, Ohgaki and Wiestler1,Reference Crotty, Scheithauer and Young2 Although radiation therapy is commonly utilized to treat residual disease or progression in adults, the efficacy of conventional treatment in pediatric cases is unclear because of their rarity.Reference Borrill, Cheesman, Stivaros, Kamaly-Asl, Gnanalingham and Kilday5 Furthermore, there is significant morbidity associated with radiation in children.Reference Borrill, Cheesman, Stivaros, Kamaly-Asl, Gnanalingham and Kilday5 Recently, BRAF inhibitors such as vemurafenib and dabrafenib have been tried with success in treating PCPs in adults and may be a potential treatment option in children as well.Reference Borrill, Cheesman, Stivaros, Kamaly-Asl, Gnanalingham and Kilday5
In conclusion, this case highlights that PCPs very rarely occur in children and illustrates the importance of testing for BRAF mutations as craniopharyngiomas and RCCs show overlapping histology but have significant differences in management and prognosis, especially with the emergence of targeted therapy using BRAF inhibitors with promising results reported in adults.Reference Borrill, Cheesman, Stivaros, Kamaly-Asl, Gnanalingham and Kilday5
Acknowledgements
We thank Mayo Clinic Laboratories for CTNNB1 mutation analysis and NeoGenomics Laboratories, Inc. for BRAF mutation analysis.
Statement of Authorship
Study concept and design: SM and JKD; data acquisition and review/editing of the manuscript: SM, RR, TM, KDLR, TK, SF-G, and JKD.
Disclosures
The authors have nothing to disclose.




