Rabies virus (RABV), a member of the Lyssavirus genus (family Rhabdoviridae), consists of a single-stranded, negative-sense RNA genome. The virus is neuroinvasive and infection generally leads to an acute encephalitis. Epidemiologically, RABV is distributed throughout the world, with the exception of nation islands. Furthermore, RABV has a wide host range including terrestrial mammals and several bat species. In Asia and Africa, the disease is mainly diagnosed in dogs, hence most human rabies infections result from exposure to infected dogs.
Limpopo province (Fig. 1 a), was the first entry point of rabies in the 1950s, apparently from Angola [Reference Swanepoel, Coetzer and Tustin1], and this region has experienced periodical rabies outbreaks involving domestic (dogs) and wildlife (black-backed jackals) species since then [Reference Brückner and Hurter2–Reference Zulu, Sabeta and Nel4]. More recently (2005/2006), a dog rabies outbreak that occurred in this province resulted in at least 20 human deaths [Reference Cohen5] and was subsequently traced back to southern Zimbabwe. No rabies cases were reported from Limpopo province for many years prior to the 2005/2006 outbreak. Following this outbreak, massive dog vaccination campaigns were mounted resulting in a significant reduction of human rabies cases, from 22 in 2006 to three in 2010 [Reference Cohen5].

Fig. 1. (a) Map of South Africa showing the nine provinces and the geographical location of Limpopo province. (b) Local and district municipalities of Limpopo province.
Dog rabies outbreaks in Limpopo province commonly occur in the north and northeastern districts due to the presence of susceptible dog populations in rural areas (see Fig. 1 a, b). The recent dog rabies outbreak in Limpopo was followed by comprehensive dog vaccinations by the Department of Veterinary Services.
Table 1. Virus isolates included in the study
In this investigation, dog and wildlife rabies trends in northern Limpopo between 2004 and 2009 (Fig. 2 a, b), and genetic relationships of selected dog RABVs were established. Eighteen dog RABVs, all previously shown to contain RABV antigen [Reference Dean, Abelseth, Atanasiu, Meslin, Kaplan and Koprowski6], were selected from the archive at the Onderstepoort Veterinary Institute (OVI), Pretoria, and included in this study. Total viral RNA was extracted and the cytoplasmic domain of the glycoprotein and the G–L intergenic region of each virus isolate was amplified and sequenced as described previously [Reference Zulu, Sabeta and Nel4, Reference Cohen5] (BigDye® Terminator v. 3.1, Applied Biosystems, USA) and the G(+) and L(−) primers.
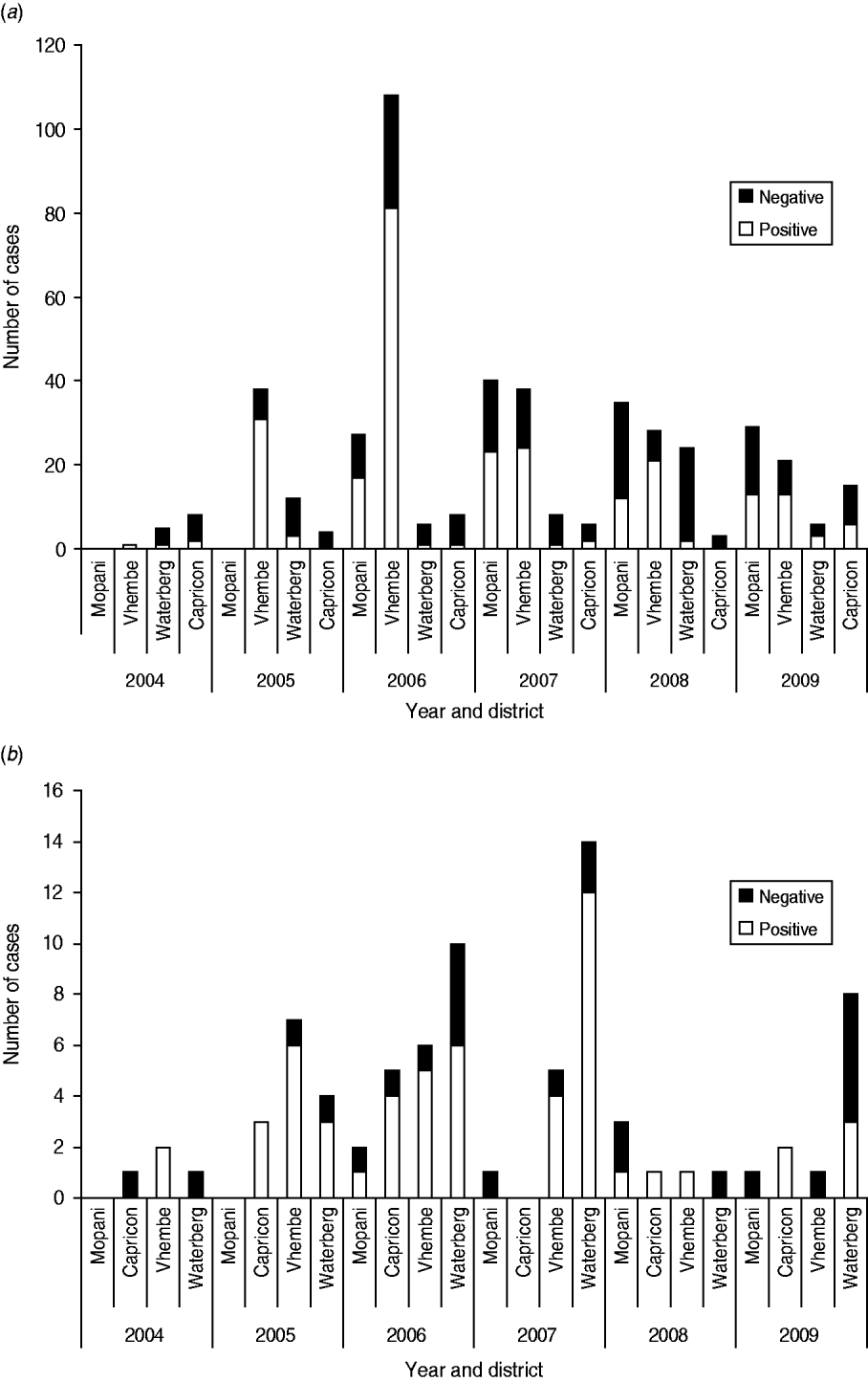
Fig. 2. Number of (a) positive dog rabies cases and (b) positive jackal rabies cases in Limpopo, 2004–2009, according to municipal district (data obtained from the Records of Onderstepoort Veterinary Institute, Pretoria, South Africa).
Nucleotide sequences were analysed using standard software packages for multiple alignments [Reference Higgins, Salemi and Vandamme7, Reference Saitou and Nei8] and phylogenetic analysis was based on an alignment of a 592-nt sequence region for the construction of the Neighbour-joining tree [Reference Higgins, Salemi and Vandamme7]. The phylogenetic analyses demonstrated that the RABVs recently recovered from domestic dogs in eastern Limpopo clustered together with RABVs responsible for the 2005/2006 outbreak [(1i); Fig. 3] [Reference Cohen5], and are of the canid-associated Africa 1b variant [Reference Bourhy, Kissi and Tordo9].
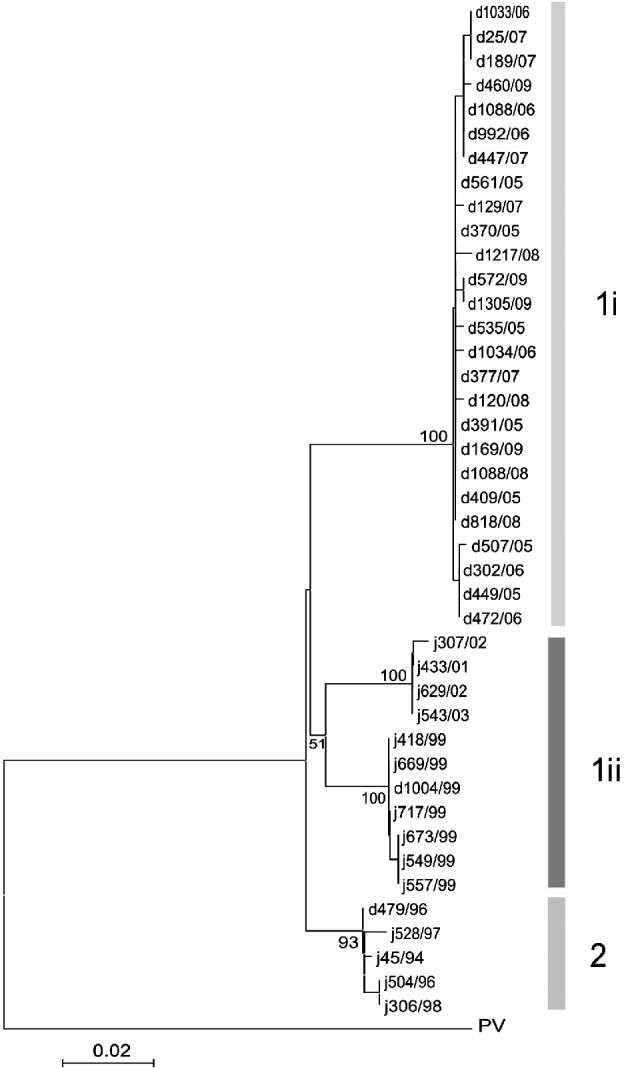
Fig. 3. Phylogenetic tree based on an alignment of a 592-bp region of the G–L intergenic region of 42 RABV isolates obtained from domestic dog (d) and jackal (j) species from Limpopo province. The Pasteur Virus (PV) strain was used to root the tree.
A case surveillance data analysis (for 2004–2009) from the study region (Fig. 2a) demonstrated an initial increase in the number of dog rabies cases in the Vhembe district reaching a peak in 2006 which coincided with the human rabies outbreak [Reference Cohen5, 10]. No samples were submitted from Mopani district in 2004 and 2005, but in 2006 (n=17) and 2007 (n=23) rabies cases were recorded, and a marked reduction was noted in 2008 and 2009 (in Mopani district) parallel to observations in neighbouring Vhembe district. Whether these rabies cases in Mopani district were due to spillover from Vhembe is not clear. Although the Waterberg and Capricorn districts generally had fewer reported cases of dog rabies (n=5) during the same period under review, an increase in submissions was noted in 2008 (Waterberg, n=24) and 2009 (Capricorn, n=15). These two districts are generally associated with game ranches and less human settlements compared to Vhembe and Mopani districts hence the reduced number of dog rabies cases.
Case surveillance data confirmed RABV infection in the black-backed jackal (BBJ) species C. mesomelas in Vhembe, Mopani, Capricorn and Waterberg districts. Capricorn district also recorded positive cases in this species in 2005, 2006 and 2009. Rabies in BBJ is commonly reported in the Waterberg district and is independent of dog rabies. This observation is consistent with ecological conditions that exist here and are believed to be conducive to the proliferation of the BBJ species. No parallel decrease in rabies cases was observed in BBJ as seen in dogs (2005–2007), a possible indication that spatial factors influence rabies dynamics in this wild carnivore species [Reference Zulu, Sabeta and Nel4, Reference Bingham11].
The reduction in the number of rabies-positive cases is most likely a result of vaccination campaigns that were mounted in response to the 2005/2006 rabies outbreak. This situation is likely to persist for some years in future, provided that vaccination campaigns are appropriately targeted and performed annually. Based on the surveillance data presented here (Fig. 2 a) it is possible that an increase in vaccination coverage levels and sustainability of vaccination campaigns could eliminate or control rabies.
In many developing nations of Africa and Asia, vaccination coverage levels are often quite low, and range from 4% to 60% [Reference Cohen5] depending on accessibility to dogs for these programmes. However, in order to achieve herd immunity against rabies, the World Health Organization (WHO) recommends that at least 70% of a population should be vaccinated annually. Nonetheless, the efficacy of vaccination campaigns are rarely evaluated by means of monitoring herd immunity in South Africa and the rest of Africa. Similarly, in the Eastern Cape province (South Africa), although vaccination of dogs and cats has been implemented since 1996, the practice has neither curbed the spread nor prevented the number of domestic rabies cases [Reference Van Stittert12] and this underscores the challenge that rabies control is facing not only in Limpopo, but in many other parts of the country. Although rabies prevalence appears to be in gradual decline, it could be that residual foci may still persist in some isolated areas and could potentially serve as sources of subsequent outbreaks in the presence of susceptible dog populations [Reference Johnson13].
It is true that parenteral vaccination still remains the most effective approach in reducing the spread of canine rabies in Africa [Reference Kaare14]. It should be further emphasized that only well coordinated and comprehensive vaccination campaigns are capable of eliminating dog rabies in the sub-region and these approaches will greatly assist in preventing future rabies outbreaks in dogs. Naturally, ongoing surveillance is required to detect residual foci or re-infection, particularly in those areas where rabies has been or is believed to have been eliminated [Reference Johnson13].
Phylogenetic data presented here demonstrate an expansion of a cluster of viruses previously shown to have originated from Zimbabwe and further underline the maintenance of the same variant in dog populations in Limpopo province. There is a definite potential of rabies to spillover to wildlife species (cluster 1ii) and the opportunistic nature of the RABV strain enhances the ability of RABV to cross species barries fairly easily, making it very difficult to eliminate rabies in this region.
Domestic and wildlife species in communal areas clearly remain the major sources for rabies outbreaks. The inconsistency and infrequency of vaccination campaigns and lack of evaluation of their effectiveness thereof makes it more difficult to eliminate or control rabies foci in these regions. Furthermore, the lack of awareness in communities about rabies compounds the problem, resulting in small numbers of animals being presented for vaccination. Biannual vaccinations with campaigns repeated every 6–8 months, particularly in areas with high dog populations, should be considered but the practicality of such approaches present potential problems given the cost limitations associated with these approaches.
ACKNOWLEDGEMENTS
This work was funded by the Department of Agriculture Fisheries and Forestry (DAFF) [grant no. OVI 04/16 (C171)]. The authors express their gratitude to Rahana Dwarka for critically reviewing the manuscript.
DECLARATION OF INTEREST
None.






