People with schizophrenia display pronounced difficulties in recognising negative facial affect, such as fearful expression. Reference Mandall, Padney and Prasad1,Reference Kohler, Turner, Bilker, Brensinger, Siegel, Kanes, Gur and Gur2 The neural basis of this deficit and its relationship to symptoms of psychosis are still unclear, although correlations with both positive and negative psychotic symptoms have been reported. Reference Schneider, Gur, Gur and Shtasel3,Reference Gur, Kohler, Ragland, Siegel, Lesko, Bilker and Gur4 Visual perception of facial fear in healthy individuals involves face-responsive visual regions, i.e. the fusiform gyrus and the cortex surrounding the superior temporal sulcus as well as the amygdala. Reference Morris, Friston, Buchel, Frith, Young, Calder and Dolan5,Reference Surguladze, Brammer, Young, Andrew, Travis, Williams and Phillips6 We used functional magnetic resonance imaging (fMRI) to examine the hypothesis that activation within these regions would be restricted in people with schizophrenia during processing of facial fear. More specifically, as amygdala activation has been associated with emotional salience and the formation of positive psychotic symptoms, Reference Fudge, Powers and Haber7 we predicted that the participants with schizophrenia relative response in the amygdala would demonstrate a positive relationship with positive psychotic symptoms. As visual cortical perception deficits in patients with schizophrenia have been associated with negative symptoms, Reference Potkin, Alva, Fleming, Anand, Keator, Carreon, Doo, Jin, Wu and Fallon8,Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park9 we also predicted that activation within the extrastriate visual regions would correlate negatively with the severity of negative symptoms in the participants with schizophrenia.
Method
Eleven people (all dextral) Reference Annet10 with a DSM–IV diagnosis of schizophrenia 11 were recruited from the Maudsley Hospital, London. All of these individuals, of whom nine were men and two were women, were receiving out-patient treatment with stable dosages of antipsychotic medication at the time of the study, with a chlorpromazine equivalent daily dosage of 523 mg (s.d.=455, range 166–1666); eight patients were treated with conventional and three with atypical antipsychotics. The severity of negative and positive symptoms of the patients was assessed with the Positive and Negative Syndrome Scale (PANSS). Reference Kay, Fiszbein and Opler12 All the PANSS ratings were completed by L.A.M. after appropriate training.
Nine healthy dextral individuals (five men, four women) comparable for age and years of education were recruited as a comparison group from the local population. Exclusion criteria for all participants were illicit substance or alcohol misuse within the past 2 years, a history of neurological illness, head injury or other significant medical illness. All participants had normal pre-morbid IQ scores, estimated with the National Adult Reading Test; Reference Nelson13 control group participants had significantly higher scores (t=3.49, d.f.=18, two-tailed P=0.003). Written informed consent was obtained for all participants and the study was approved by the Institute of Psychiatry research ethics committee.
Imaging study
Procedure
The participants took part in an event-related fMRI experiment while viewing grey-scale images depicting prototypical facial expressions of fear and sadness, a neutral facial expression and a fixation cross. The facial stimuli were from the standard set of prototypical facial expressions of the six basic emotions by Ekman & Friesen. Reference Ekman and Friesen14 The ‘sad face’ processing data are not discussed in this paper.
The sequence of the four stimuli (fearful face/sad face/neutral face/fixation cross) was randomised and common to all participants. Twenty stimuli were presented per condition, depicting either a face or the fixation cross, each presented for 3 s with an inter-stimulus interval of 6 s, and the duration of the experiment was 8 min. This was an implicit emotional processing task, with participants indicating the gender of the face by moving a joystick. No response was required to the fixation cross.
Data acquisition and image analysis
Data were acquired using a 1.5 T scanner at the Maudsley Hospital, London, and analysed with software developed at the Institute of Psychiatry, London, using a standard non-parametric approach. Whole-brain analysis of variance was used to estimate significant within-group and between-group effects. A detailed description of the data acquisition and image analysis is available as a data supplement to the online version of this paper. A correlational analysis was used to examine associations between PANSS positive and negative sub-scale scores and activation within the brain regions specified in our a priori hypotheses: the fusiform gyrus, the superior temporal gyrus and the amygdala.
Results
The demographic and clinical characteristics of the participants are shown in Table 1.
Table 1 Demographic and clinical characteristics of the sample

| Schizophrenia group (n=11) | Control group (n=9) | |||
|---|---|---|---|---|
| Mean (s.d.) | Range | Mean (s.d.) | Range | |
| Age, years | 35 (9) | 20-53 | 32 (6) | 25-45 |
| NART IQ | 106.18 (10.41) | 87-123 | 119.44 (5.03) | 111-125 |
| Education, years | 13 (2) | 10-16 | 15 (3) | 10-19 |
| Duration of illness, years | 12 (9) | 2-33 | ||
| PANSS score | ||||
| Positive scale | 16 (6.72) | 7-28 | ||
| Negative scale | 13.91 (5.54) | 7-22 | ||
| Total score | 58.91 (17.72) | 31-85 | ||
Behavioural results
There was no significant difference in performance between the patient and control groups in the gender discrimination task (Mann–Whitney U=38.0, z=–0.90, two-tailed P=0.37 for fearful expression; Mann–Whitney U=37.5, z=–0.94, P=0.35 for neutral facial expression).
Imaging results
Within-group comparisons
The whole-brain analysis of variance (ANOVA) revealed the following within-group significant differences:
-
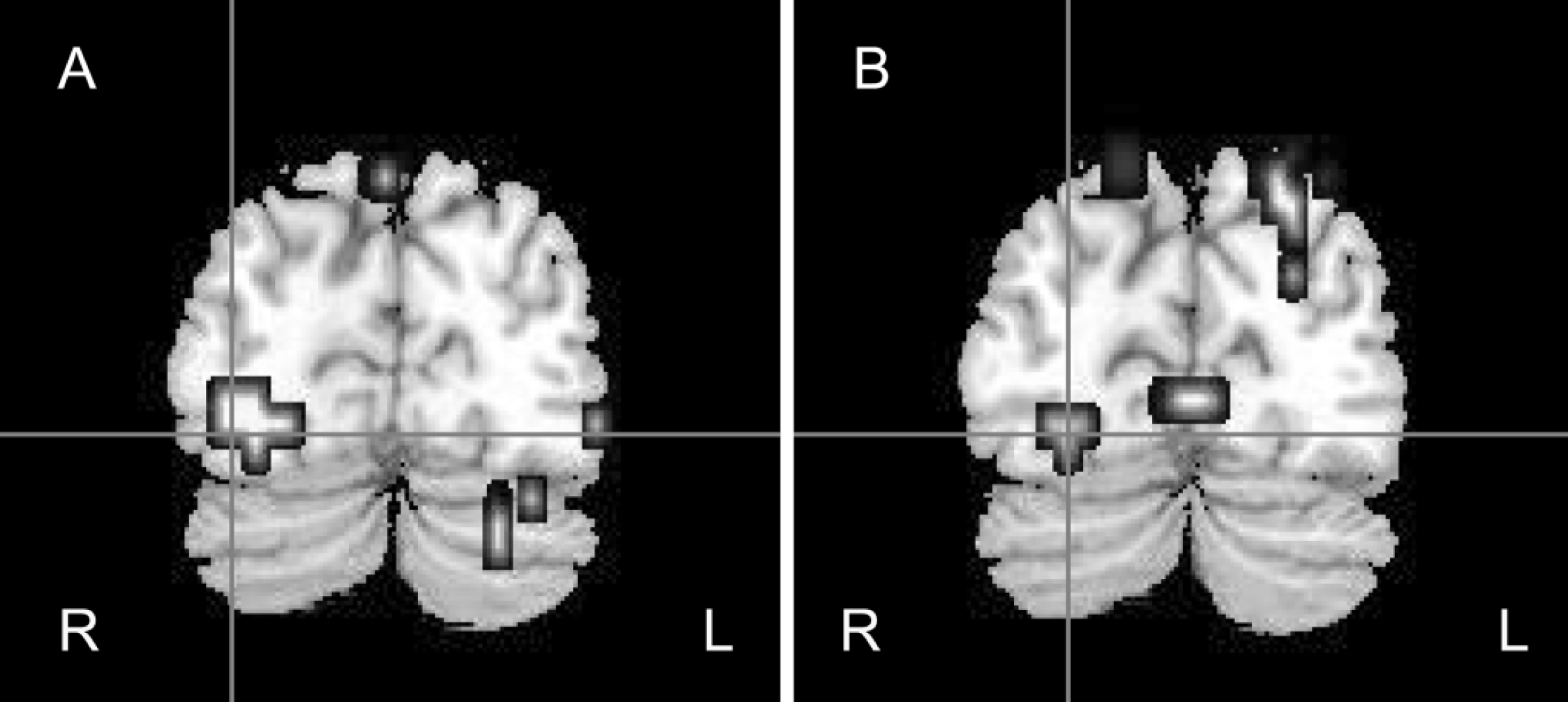
• Control group: fearful v. neutral faces. In the control group the processing of fearful faces elicited significantly greater activation within the bilateral amygdala, right-sided parahippocampal gyrus, fusiform gyrus, superior temporal gyrus, middle occipital and temporal gyri and right insula than did processing of neutral faces (Table 2; Figs 1(a), 2(a)).

Fig. 1 Coronal view of the brain showing right fusiform gyrus responses to fearful v. neutral faces (a) in the control group (x=32, y=–74, z=–13) and (b) the between-group differences, where control participants demonstrated greater activation than the participants with schizophrenia (x=25, y=–77, z=–13). R, right hemisphere; L, left hemisphere.

Fig. 2 Sagittal view of the brain showing right amygdala responses to fearful v. neutral faces (a) in the control group (x=25, y=–7, z=–7) and (b) the between-group differences, where control participants demonstrated greater activation than participants with schizophrenia (x=22, y=–7, z=–13).
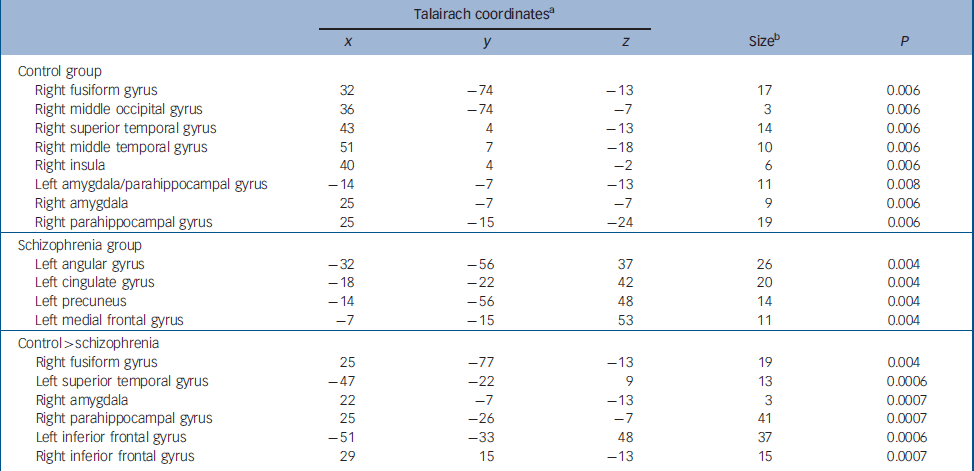
Table 2 Clusters showing significant activation differences to fearful v. neutral faces in healthy participants and patients

Talairach coordinatesa x y z Sizeb P Control group Right fusiform gyrus 32 -74 -13 17 0.006 Right middle occipital gyrus 36 -74 -7 3 0.006 Right superior temporal gyrus 43 4 -13 14 0.006 Right middle temporal gyrus 51 7 -18 10 0.006 Right insula 40 4 -2 6 0.006 Left amygdala/parahippocampal gyrus -14 -7 -13 11 0.008 Right amygdala 25 -7 -7 9 0.006 Right parahippocampal gyrus 25 -15 -24 19 0.006 Schizophrenia group Left angular gyrus -32 -56 37 26 0.004 Left cingulate gyrus -18 -22 42 20 0.004 Left precuneus -14 -56 48 14 0.004 Left medial frontal gyrus -7 -15 53 11 0.004 Control > schizophrenia Right fusiform gyrus 25 -77 -13 19 0.004 Left superior temporal gyrus -47 -22 9 13 0.0006 Right amygdala 22 -7 -13 3 0.0007 Right parahippocampal gyrus 25 -26 -7 41 0.0007 Left inferior frontal gyrus -51 -33 48 37 0.0006 Right inferior frontal gyrus 29 15 -13 15 0.0007 -
• Patient group: fearful v. neutral faces. The processing of fearful faces elicited significantly greater activation within the left medial frontal gyrus, the left angular gyrus, the cingulate gyrus, and left precuneus than did processing of neutral faces (Table 2).
Between-group comparisons
The whole-brain ANOVA revealed the following between-group significant differences:
-
• Control v. patient groups: activation to fearful faces. Control participants demonstrated significantly greater activation than did the participants with schizophrenia during the processing of fearful faces within the right fusiform gyrus, the left superior temporal gyrus, the bilateral inferior frontal gyri, the right amygdala and right parahippocampal gyrus (Table 2; Figs 1(b), 2(b)).
Correlational analyses
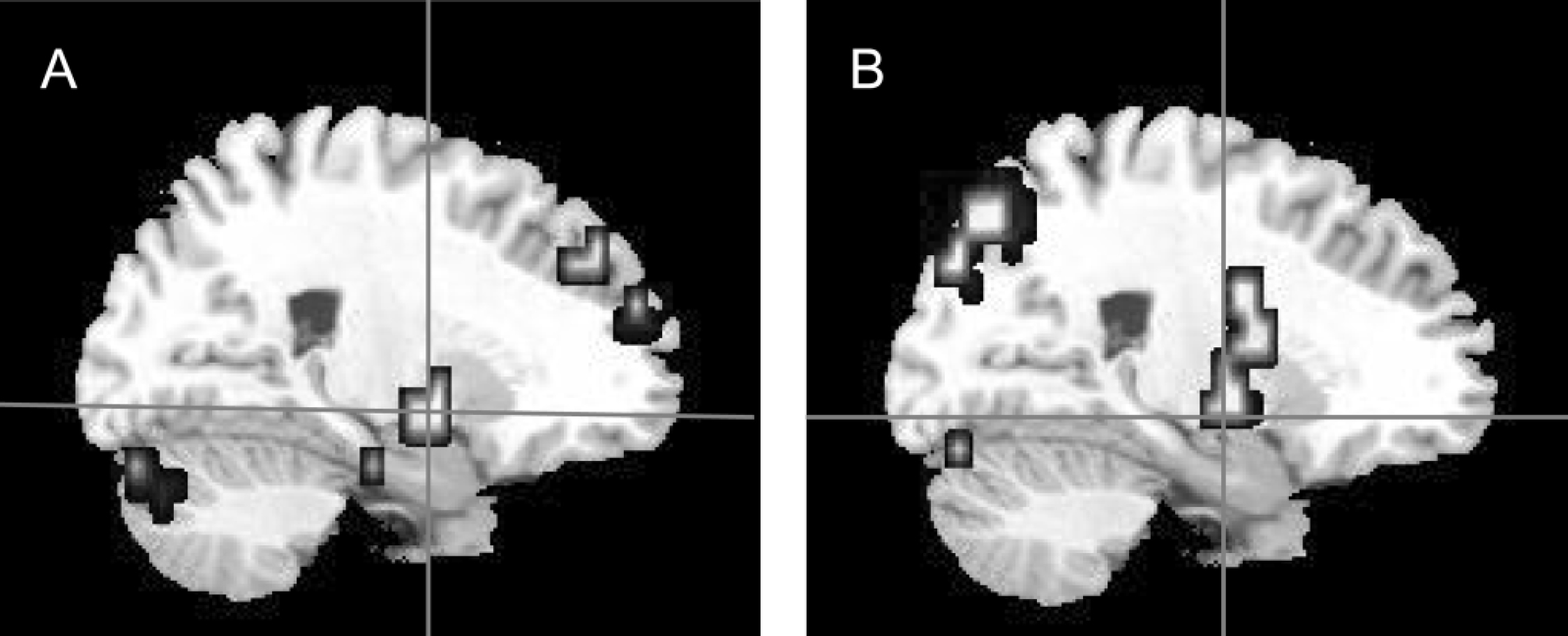
Correlational analyses in the patient group revealed a significant negative correlation between the PANSS negative sub-scale score and activation within the left superior temporal gyrus during the processing of fearful faces (Pearson r=–0.84, P=0.001; Spearman's rho=–0.74, P=0.01) (Fig. 3). This correlation remained significant after controlling for the patients' IQ (r=–0.805, d.f.=9, P=0.005) and gender (r=–0.847, d.f.=9, P=0.002). No other correlation between measured brain activity and PANSS scores was statistically significant.

Fig. 3 Sagittal view of the brain showing between-group differences in neural response of left superior temporal gyrus to fearful faces, where control participants demonstrated greater responses than participants with schizophrenia (x=–47, y=–22, z=9). The graph shows the correlation between the Positive and Negative Syndrome Scale (PANSS) negative sub-scale scores and the mean blood oxygen level dependent (BOLD) signal change in the left superior temporal gyrus of participants with schizophrenia (Pearson r=–0.84, P=0.001; Spearman's rho=–0.74, P=0.01).
Discussion
Visual processing of facial fear elicited bilateral amygdala activation in healthy participants, with significantly greater activation within the right amygdala compared with the participants with schizophrenia, consistent with earlier reports. Reference Phillips, Williams, Senior, Bullmore, Brammer, Andrew, Williams and David15,Reference Gur, McGrath, Chan, Schroeder, Turner, Turetsky, Kohler, Alsop, Maldjian, Ragland and Gur16 This dysfunctional amygdala activation might relate to the fear recognition deficits manifested by patients with schizophrenia. Reference Gur, McGrath, Chan, Schroeder, Turner, Turetsky, Kohler, Alsop, Maldjian, Ragland and Gur16 The amygdala has been postulated to have a key role in modulating the dopaminergic system. Reference Fudge and Emiliano17 Disruption of dopamine regulation is considered a key factor in the generation of psychotic symptoms by attributing salience and reinforcing aberrant brain circuitry. A previous positron emission tomography (PET) study Reference Taylor, Liberzon, Decker and Koeppe18 demonstrated a positive correlation between the left amygdala response of patients with schizophrenia and the severity of positive symptoms. However, contrary to our prediction, severity of positive symptoms was not associated with a commensurate increase in activation of the amygdala. It is possible that amygdala activation might be linked non-specifically with the illness or medication, rather than the symptom profile. An alternative explanation might be that there is insufficient variation in the positive symptoms of participants with schizophrenia to show a clear correlation, and longitudinal examination of a more symptomatic group of patients could clarify this point.
Healthy participants demonstrated greater activation within the right fusiform gyrus and superior temporal gyrus in response to fearful compared with neutral faces. This finding supports enhancement of extrastriate visual cortices activation by emotionally salient stimuli. Reference Morris, Friston, Buchel, Frith, Young, Calder and Dolan5,Reference Surguladze, Brammer, Young, Andrew, Travis, Williams and Phillips6 In contrast, participants with schizophrenia showed significantly reduced activation within these regions during facial fear processing compared with healthy controls. This is consistent with earlier findings, Reference Paradiso, Andreasen, Crespo-Facoro, O'Leary, Watkins, Boles Ponto and Hichwa19–Reference Taylor, Phan, Britton and Liberzon21 and may reflect a deficit in the visual processing of facial fear in people with schizophrenia.
Participants with schizophrenia demonstrated a left lateralised reduction in superior temporal gyrus activation, similar to the findings of Johnston et al. Reference Johnston, Stojanov, Devir and Schall22 The left lateralised reduction in superior temporal gyrus activation in our study may represent some reversal of the normal right lateralised temporal lobe response to facial fear in people with schizophrenia and may be indicative of impaired function of the left superior temporal gyrus during fear processing in schizophrenia. This might reflect a more general emotional deficit, as an abnormal left lateralised temporal lobe activation has been previously demonstrated in response to emotional prosody. Reference Mitchell, Elliott, Barry, Cruttenden and Woodruff23
The cortex surrounding the superior temporal sulcus, along with the fusiform gyrus, is associated with social cognition. Specifically, these regions are involved in the visual perception of socially relevant stimuli. Reference Adolphs24 Findings from neuroimaging, electrophysiological and single-cell recording studies have associated the visual perception of biologically salient motion with the activation of the posterior aspects of the superior temporal sulcus and the adjacent superior temporal gyrus. Reference Allison, Puce and McCarthy25,Reference Gallagher and Frith26 The perception of the changeable aspects of the faces such as facial emotion, gaze direction and lip movements also elicits activation within the superior temporal sulcus and the adjacent superior temporal gyrus, even in response to implied motion evidenced by static visual stimuli, such as facial expressions. Reference Allison, Puce and McCarthy25 A deficit in biological motion perception may result in deficient social perception, and cognition and social functioning. People with schizophrenia and severe negative symptoms exhibit a pronounced difficulty in the recognition of facial emotions, Reference Gur, Kohler, Ragland, Siegel, Lesko, Bilker and Gur4 particularly facial fear. Reference Kohler, Turner, Bilker, Brensinger, Siegel, Kanes, Gur and Gur2,Reference Van't Wout, Aleman, Kessels, Cahn, de Haan and Kahn27 They also exhibit impairments in social cognition tasks such as theory of mind tasks, also associated with negative symptoms. Reference Corcoran, Mercer and Frith28–Reference Pickup and Frith30 The social cognition deficits may contribute to the social functioning deficits of the patients with negative symptoms. Reference Addington and Addington31
The cortical processing of motion-related visual stimuli is impaired in people with schizophrenia and this impairment is more intense in those with severe negative symptoms. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park9 This deficit has been associated with the middle temporal cortical visual area, which responds to both scrambled motion and biological motion sequences. The association of activation of the superior temporal cortex, which responds selectively to all aspects of biological motion, with negative symptoms has not been investigated. Consistent with our hypothesis, the severity of negative symptoms of the participants with schizophrenia correlated negatively with the degree of activation attenuation within the left posterior superior temporal gyrus. Our finding indicates an association between impaired extrastriate cortical visual processing of facial fear and negative symptoms in people with schizophrenia. This association may underlie the previously reported pronounced difficulties of patients with high levels of negative symptoms in the recognition of fearful faces.
The neural response within the right fusiform gyrus in the participants with schizophrenia in our study did not correlate with the severity of negative symptoms. This is at odds with an earlier PET study, Reference Potkin, Alva, Fleming, Anand, Keator, Carreon, Doo, Jin, Wu and Fallon8 which showed decreased glucose metabolic rate in the right fusiform area of patients with predominantly negative symptoms and a negative correlation with the negative symptoms of the patients. The finding of Potkin et al Reference Potkin, Alva, Fleming, Anand, Keator, Carreon, Doo, Jin, Wu and Fallon8 was regarded as consistent with the difficulties of the patients with negative symptoms in identifying the emotional content of faces and scenes. The differences in these results may be secondary to the different time frames between event-related fMRI and PET, as the occipital cortices are associated with early visual processing.
Deficits in visual tasks in the participants with schizophrenia could be attributed to their attentional deficits, which could lead to lower engagement with the task during the scanning session. However, the findings of our study are not likely to derive from the patients' attentional impairments, since their performance in the gender discrimination task did not differ significantly from the control group's performance.
Methodological considerations
There are several potential limitations to this study. First is the issue of generalisability of these results from the analysis of a relatively small number of participants. A non-parametric statistical approach to the analysis of the imaging data is preferred, and we have used stringent thresholds for all of our analyses to minimise any type I errors; the significance threshold was set to give less than one false-positive cluster over the whole brain. This would suggest that our findings are relatively robust. Second, participant characteristics–including symptoms and medication in the patient group–may also influence the between-group variability. Ideally, we would have recruited unmedicated patients for this study; however, pragmatically these were difficult to ascertain. Examining for medication effects within the patient group, we found no significant correlation between the medication dose and the blood oxygen dependent level response values within the right amygdala, right fusiform gyrus and left superior temporal gyrus. Similar findings have also been demonstrated in unmedicated patients. Reference Taylor, Phan, Britton and Liberzon21 One possibility is that activation differences between patients and controls are secondary to differences in performance. The gender discrimination task we used placed minimal performance demands on the participants, Reference Phan, Wager, Taylor and Liberzon32 thereby reducing the possibility that performance differences might confound our results.
In summary, we have confirmed that patients with schizophrenia demonstrated reduced neural responses within extrastriate visual cortices and amygdala during processing of facial fear. A left lateralised reduction of superior temporal gyrus activity correlated with increasing severity of negative symptoms. This association may underlie the previously reported pronounced difficulties of people with schizophrenia and high levels of negative symptoms in the recognition of fearful faces.
Acknowledgements
S.S.S. was supported by an Advanced Clinical Training Fellowship from the Wellcome Trust and L.A.M. was funded by the Psychiatry Research Trust.








eLetters
No eLetters have been published for this article.