Introduction
Resveratrol is a natural polyphenol compound found in several plants, such as grapes, peanuts and berries. It was first isolated in 1939, but only gained considerable attention a few decades later, when the French paradox was described(Reference Cooper, Chopra and Thurnham1). Recently, the use of different in vitro and in vivo experimental approaches as well as experiments in cells isolated from animals and humans has revealed that resveratrol exerts antioxidant, anti-inflammatory and anti-proliferative effects(Reference Nunes, Danesi and Del Rio2,Reference Gambini, Ingles and Olaso3) . Resveratrol also prevents metabolic diseases by ameliorating glucose and lipid homoeostasis and reducing fat accumulation and inflammatory biomarkers(Reference van der Spuy and Pretorius4,Reference Timmers, Hesselink and Schrauwen5) . However, much less attention has been directed to the effects of resveratrol on urogenital tract (UGT) diseases, which manifest mainly with lower urinary tract symptoms (LUTS), prostatic disorders (benign prostatic hyperplasia (BPH) and prostatitis) and erectile dysfunction (ED). Epidemiological studies have shown a positive correlation between LUTS, prostatic dysfunction and ED(Reference Matsuda, Kobayashi and Fukura6,Reference Nakamura, Fujimura and Nagata7) . These UGT diseases typically share common pathophysiological mechanisms, including an unbalanced oxidative state in which increased production of reactive oxygen species (ROS) is favoured and antioxidant capacity is reduced, as demonstrated in the bladder, urethra, prostate and corpus cavernosum(Reference Calogero, Burgio and Condorelli8–Reference Calmasini, de Oliveira and Alexandre14). By targeting these mechanisms, a number of studies have confirmed that therapy with resveratrol ameliorates UGT diseases. Therefore, in preparation for this review, we searched the literature for pre-clinical studies evaluating the efficacy of resveratrol on LUTS, BPH, ED and non-cancerous prostatic diseases in animal models and in human and animal isolated cells and, herein, provide up-to-date knowledge on the efficacy of resveratrol in UGT diseases.
Putative mechanisms of action of resveratrol
The antioxidant effect of resveratrol is clearly the most studied property of this polyphenol. Resveratrol acts as an antioxidant through at least two different pathways; that is, resveratrol increases antioxidant system efficacy and/or reduces ROS production in the targeted tissue. At the molecular level, resveratrol reduces nicotinamide adenine dinucleotide phosphate (NADPH) activity by inhibiting gp91 phox translocation from the cytosol to the membrane, thus impairing NADPH assembly(Reference Chow, Hshu and Wang15). Resveratrol also reduces NADPH oxidase mRNA expression and increases protein expression of superoxide dismutase(Reference Spanier, Xu and Xia16).
The mechanism by which resveratrol modulates gene expression is still poorly understood; however, it has been proposed that sirtuin-1 (sirt-1) activation by resveratrol plays a key role in this process. Sirt-1 is an important protein linked with longevity and numerous intracellular genetic regulations. Transcription factors such as nuclear factor κB (NF-κB), tumour suppressor p53 and Forkhead box class O (FOXO) are all targets of sirt-1. Furthermore, sirt-1 is implicated in heterochromatin formation and histone hypoacetylation, thereby producing gene repression(Reference Vaquero, Scher and Erdjument-Bromage17). Sirt-1 seems to work in synergy with AMP-activated protein kinase (AMPK) to promote cell metabolism adaptations through activation of transcription factors and/or co-activators such as peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α) and FOXO, which are also implicated in sirt-1-induced gene expression(Reference Nikolai, Pallauf and Huebbe18). However, whether the modulation of gene expression by resveratrol in UGT also involves sirt-1 activation remains unclear. Furthermore, in UGT, pathways in addition to the sirt-1 pathway have been shown to be activated by resveratrol, suggesting a complex and non-specific mechanism of action for this polyphenol, which open fields for future studies.
Protective effects of resveratrol on male urogenital tract dysfunction
LUTS, ED, BPH and prostatitis are the most common UGT diseases. The main risk factors for these diseases include arterial hypertension, obesity, diabetes and ageing, which itself increases inflammation and oxidative status, thereby worsening the incidence and/or progression of UGT diseases(Reference Calmasini, Silva and Alexandre19–Reference Xiong, Zhang and Tan21). Within this context, resveratrol has emerged as a potential candidate for the treatment of such diseases. Herein, we detailed the current data on resveratrol actions in male UGT disorders, emphasising LUTS, prostatic diseases and ED. Table 1 and Fig. 1 summarise the main effects and the proposed mechanisms of resveratrol in the abovementioned diseases.
Table 1. The in vivo effects of resveratrol in animal models of UGT dysfunctions
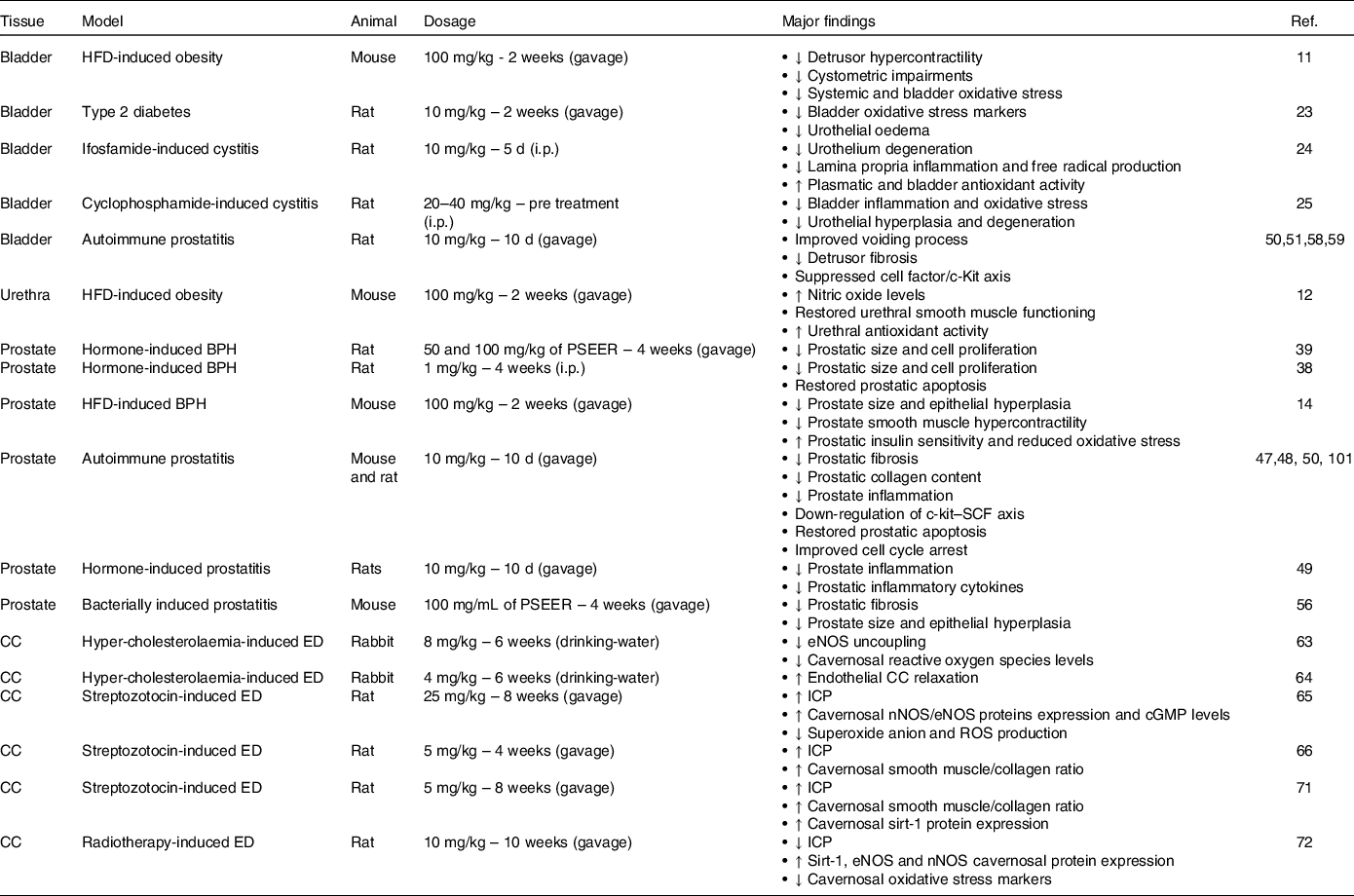
BPH, benign prostatic hyperplasia; cGMP, cyclic guanosine monophosphate; CC, corpus cavernosum; ED, erectile dysfunction; eNOS, endothelial nitric oxide synthase; HFD, high-fat diet; ICP, intra-cavernosal pressure; i.p., intraperitoneal; nNOS, neuronal nitric oxide synthase; PSEER, peanut sprout extract enriched with resveratrol; ROS, reactive oxygen species.
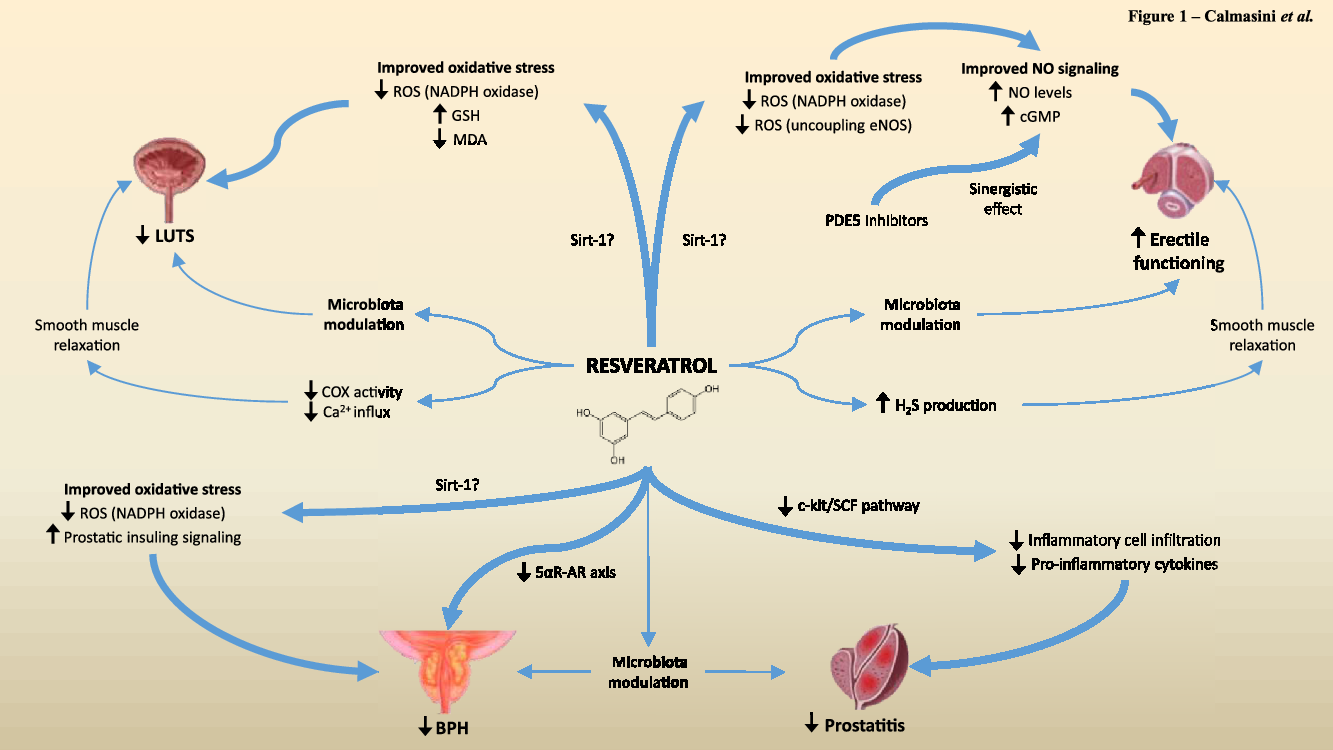
Fig. 1. Schematic representation of the suggested pathways by which resveratrol acts in the UGT and its beneficial effects. Strong evidence (represented by the thick blue arrows) indicates that resveratrol may act in the UGT by reducing oxidative stress, inflammation and cell proliferation-related pathways. A few studies (thin blue arrows) indicate that resveratrol exhibits a direct smooth muscle inhibitory effect and/or is capable of modulating the gut microbiota, resulting in improvement of UGT diseases.
Lower urinary tract symptoms
Normal urinary continence requires a complex interaction between the brain, nervous system and pelvic organs, namely the bladder, urethra and prostate. When any component of this system loses normal function, the micturition process can be affected and the patient may experience LUTS, characterised by bladder storage and/or filling symptoms and consisting of urinary frequency, nocturia, urgency and stream problems, thus negatively affecting the patient’s quality of life(Reference Steers22). Metabolic diseases such as type 2 diabetes and obesity are among the most important risk factors for LUTS, and experimental models of both of these pathological conditions have been used to test the efficacy of resveratrol as a pre-clinical approach, as illustrated in Table 1. In high-fat diet (HFD)-fed obese mice, 2-week administration of resveratrol at 100 mg/kg increased the antioxidant activity in isolated bladder, resulting in attenuation of overactive bladder, as evidenced by the reductions of non-voiding contractions, urinary frequency and detrusor smooth muscle hypercontractility(Reference Alexandre, Calmasini and de Oliveria11). Similarly, in the urethral tissue of obese mice, resveratrol increased antioxidant activity and nitric oxide (NO) production and normalised urethral smooth muscle hypercontractility, thus restoring urethral relaxation(Reference Alexandre, Calmasini and Sponton12). In a rat model of type 2 diabetes induced by a low dose of streptozotocin (40 mg/kg) followed by a HFD for 2 weeks, resveratrol at 10 mg/kg/d for 14 d reduced oxidative stress markers, such as malondialdehyde, 4-hydroxy-2-nonenal and 8-hydroxy-2-deoxyguanosine, and decreased serum glucose levels(Reference Tsounapi, Honda and Hikita23). Considering that resveratrol treatment also reduces body weight and that it improves metabolic parameters, it is unclear whether the amelioration of obesity-induced bladder dysfunction in HFD-fed animals was the result of a direct antioxidant effect in UGT tissues or was a consequence of reduced body weight. Furthermore, the exact molecular mechanisms by which resveratrol improves bladder and urethral oxidative balance in metabolic disease models have yet to be elucidated.
In a similar fashion, resveratrol abrogated cystitis-induced bladder dysfunction by increasing antioxidant defences. In ifosfamide-induced rat cystitis, an inflammatory condition closely related to LUTS, resveratrol, given at 10 mg/kg, intraperitoneally (i.p.) for 5 d, reversed vesical epithelium degeneration and lamina propria inflammation. These effects were attributed to increased glutathione levels and reduced myeloperoxidase activity in the bladder induced by resveratrol treatment(Reference Sehirli, Sakarcan and Velioglu-Ogunç24). Additionally, in cyclophosphamide-induced rat cystitis, in a preventive approach, resveratrol at 20 and 40 mg/kg also reduced the oxidative status of the bladder, resulting in less epithelial degeneration and desquamation(Reference Keles, Bozkurt and Cemek25).
The effects of resveratrol in the bladder have also been addressed using in vitro assays (Table 2). For instance, resveratrol at 4 mM reduced the oxidative stress induced by hydrogen peroxidase (H2O2) in rabbit bladder smooth muscle and mucosa(Reference Francis, Leggett and Schuler26). Notably, in all the in vivo and in vitro studies mentioned above, the high efficacy of resveratrol was associated with reduced systemic and/or tissue oxidative stress biomarker expression. In fact, oxidative stress has been implicated in a number of lower urinary tract disorders; however, it is important to remember that a causal relationship between increased oxidative stress and LUTS has not been shown, and hence, more specific analyses, identifying the particular reactive species involved in LUTS genesis, are needed to prove this causal relationship. Therefore, the antioxidant effects of a particular compound, such as resveratrol, can be tested against specific reactive species(Reference Andersson27).
Table 2. The in vitro effects of resveratrol in human and animal UGT tissues/cells
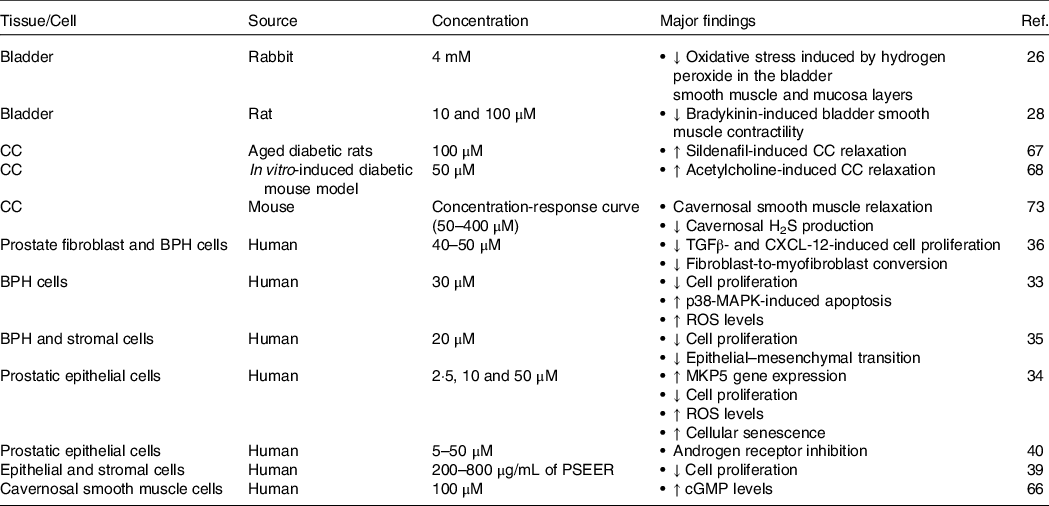
BPH, benign prostatic hyperplasia; CC, corpus cavernosum; cGMP, cyclic guanosine monophosphate; CXCL-12, CXC motif chemokine 12; H2S, hydrogen sulphide; PSEER, peanut sprout extract enriched with resveratrol; ROS, reactive oxygen species; TGFβ, transforming growth factor β.
Finally, we cannot neglect the fact that resveratrol efficacy in treating or preventing LUTS may be related to its inhibitory effect on smooth muscle reactivity. Resveratrol at 10 and 100 μM reduced bradykinin-induced rat detrusor contraction in a concentration-dependent manner(Reference Tsuda, Nakahara and Mori28). Specifically, at 100 μM, it reduced detrusor contractility by 74 %, which was associated with a reduction in cyclooxygenase activity and prostaglandin E2 generation and with the inhibition of Ca2+ entry through L-type Ca2+ channels(Reference Tsuda, Nakahara and Mori28). In human and rodent bladder smooth muscle, these Ca2+ channels play crucial roles in agonist-induced detrusor contractility(Reference Luptak, Kocmalova and Franova29,Reference Leiria, Sollon and Calixto30) ; therefore, they may be important targets for resveratrol, but further studies are required to verify this supposition. Table 2 summarises the main effects of resveratrol in the bladder and urethra.
Prostatic diseases
The prostate is a gland in the male genital tract that plays an important role during the fertilisation process. However, during a male’s lifetime, this gland can be affected by several pathological conditions, mostly cancer, BPH and/or prostatitis. Considering that the effects of resveratrol on prostatic cancer have been reviewed in detail in the literature(Reference Mokbel and Wazir31), we focus here on BPH and prostatitis.
Benign prostatic hyperplasia
BPH is a non-cancerous prostatic dysfunction characterised by abnormal epithelial and stromal cell proliferation. Prostate overgrowth and smooth muscle hypercontractility associated with BPH may lead to urethral narrowing, bladder outlet obstruction and LUTS development(Reference Devlin, Simms and Maitland32). Considering the high epidemiological prevalence of BPH in ageing men (reaching 90 % in 90-year-old men) and the small number of commercially available drugs, the search for new molecules and pathways is crucial to improve the BPH treatment.
The ability of resveratrol to suppress cell growth has been explored extensively, especially in UGT-related proliferative diseases such as BPH. In the BPH-1 cell line, 30 μM resveratrol reduced cell proliferation by 90 %, which was attributed to an increased apoptosis rate induced by p38 MAPK activation and FOXO3a repression(Reference Li, Hu and Lu33). In human prostatic epithelial cells, lower resveratrol concentrations (2·5–10 μM) also reduced cell proliferation by inducing cellular senescence(Reference Tsounapi, Honda and Hikita23). Interestingly, some of the proposed mechanisms accounting for the resveratrol-induced anti-proliferative effects are related to increased intracellular ROS production that culminates with FOXO3a repression and cellular senescence(Reference Zhang, Wu and Schoene34). This suggests that resveratrol may act through different mechanisms depending on cell type and drug concentration and incubation time.
A recent study carried out with epithelial (BPH-1) and stromal (WPHY-1) prostatic cell lines demonstrated that resveratrol treatment at 20 μM for 72 h suppressed prostatic cell proliferation by interacting with long non-coding RNAs (lncRNAs)(Reference Chen, Xu and Liu35). More specifically, in both these cell lines, resveratrol reduced the gene expression of lncRNAs DIO3 opposite strand (DIO3OS), a gene implicated in BPH pathogenesis. In addition, the authors demonstrated that DIO3OS expression is up-regulated by transforming growth factor (TGF) β1 in human prostatic cells. In vitro incubation with TGFβ1 (5 ng/mL for 72 h) resulted in an approximately two-fold increase in DIO3OS gene expression in BPH-1 and WPHY-1 cells, which was suppressed by resveratrol(Reference Chen, Xu and Liu35). Similarly, in prostate fibroblast cells obtained from BPH patients, resveratrol (40–50 μM) reduced cell proliferation through a mechanism involving TGFβ inhibition(Reference Gharaee-Kermani, Moore and Macoska36).
There are several BPH animal models described in the literature, but androgen-induced BPH, in which the animals are supplemented with testosterone, associated or not with oestradiol, for 2–4 weeks, is one of the most employed models(Reference Zhang, Zhang and Tang37). After the BPH hormonal induction period, the prostate exhibits macroscopically increased size concomitant with histological alterations evidenced by significant epithelial layer hyperplasia. Using the hormonally induced BPH model in Sprague–Dawley rats, a previous study showed that resveratrol treatment (1 mg/kg. i.p. for 4 weeks) reduced both prostate size and protein expression of proliferating cell nuclear antigen, and promoted tissue apoptosis by reducing Bcl-2 family protein expression(Reference Chung, Cheon and An38). Notably, the efficacy of resveratrol in reducing BPH features was comparable to that of the 5α-reductase inhibitor finasteride, a Food and Drug Administration-approved drug used clinically to treat BPH; however, the molecular mechanisms by which resveratrol regulates protein expression in the prostate were not explored and deserve further study.
Prostatic cell proliferation in testosterone-induced rat BPH was also significantly reduced by a peanut sprout extract enriched with resveratrol (PSEER) administered at 50 and 100 mg/kg for 4 weeks by gavage(Reference Chung, Cheon and An38). PSEER-treated rats also exhibited a reduced prostate weight ratio and dihydrotestosterone levels, along with attenuation of the increase in epithelial layer thickness. Moreover, the expression of Ki-67, a marker for cell proliferation, was reduced after PSEER treatment, especially in the epithelial layer of the prostate tissue. Interestingly, PSEER treatment was as effective as finasteride in reducing prostate weight and epithelial hyperplasia, suggesting that these two compounds have similar mechanisms of action(Reference Chung, Cheon and An38).
In fact, resveratrol has anti-androgenic effects. The 5α-reductase-androgen receptor axis was suppressed by PSEER treatment, explaining in part the prevention of prostate enlargement in testosterone-induced BPH in rats(Reference Chung, Cheon and An38). The anti-androgenic mechanism of resveratrol seems to involve at least two pathways: (i) the inhibition of 3-hydroxysteroid dehydrogenase, an enzyme that catalyses essential steps in the formation of all classes of active steroid hormones, and (ii) allosteric androgen receptor agonist, inhibiting in vitro androgen receptor activation by dihydrotestosterone in a concentration-dependent manner(Reference Lundqvist, Tringali and Oskarsson39). Intriguingly, this inhibitory effect was more pronounced in rats (IC50 3·87 ± 0·06 μM) than in humans (IC50 8·48 ± 0·04 μM), which may help explain the low efficacy of resveratrol in some translational studies(Reference Li, Chen and Zhu40). This negative modulation of the androgen pathway by resveratrol may be of particular relevance to BPH conditions because the reduction in dihydrotestosterone levels is a standard approach to BPH treatment.
With respect to the male reproductive system, clinical studies indicate that obese patients are more likely to develop BPH(Reference Gacci, Corona and Vignozzi41). Although the mechanism of obesity-induced prostatic dysfunction is not completely understood, high levels of oxidative stress are implicated in this process. Accordingly, obese animals have been used to better understand BPH physiopathology and to test new drugs, especially antioxidant drugs. Mice fed a HFD for 12 weeks exhibited prostate overgrowth and prostatic epithelial hyperplasia associated with increased ROS levels. A 2-week treatment with resveratrol (100 mg/kg, once daily by oral gavage) reversed the HFD-induced oxidative stress in the prostate, which was accompanied by reduced prostatic overgrowth and epithelial hyperplasia. Resveratrol also reduced prostatic smooth muscle hypercontractility in obese mice(Reference Calmasini, de Oliveira and Alexandre14). Similarly, resveratrol exerts its protective effect in UGT cells in part by reducing ROS production, thereby improving the oxidative balance, as demonstrated in prostate epithelial cells(Reference Conte, Kisslinger and Procaccini42).
Obesity-associated ROS production has also been implicated in insulin resistance and hyper-insulinaemia. In HFD obese rats, hyper-insulinaemia was associated with BPH(Reference Vikram, Jena and Ramarao43). Similarly, the prostate of HFD-fed obese mice presented defective insulin action and increased levels of ROS, both of which were improved by resveratrol(Reference Calmasini, de Oliveira and Alexandre14). The exact link between preserved insulin signalling in the prostate and BPH pathophysiology in resveratrol-treated animals is still unclear. In vascular and bladder tissues, insulin stimulates NO synthesis through IRS-1/PI3K-Akt/eNOS pathway activation(Reference Leiria, Sollon and Calixto30,Reference Liu, Jiang and Zhang44) . Therefore, it is possible that impaired obesity-induced insulin signalling in the prostate results in low levels of NO, favouring the contractile machinery and cell proliferation of the prostate.
In addition to ameliorating UGT diseases, resveratrol exerts some effects under healthy conditions. In normal epithelial and stromal cells, 24-h in vitro incubation with PSEER reduced the cell count and viability in a concentration-dependent manner. An initial effect of PSEER was observed at 200 μg/mL in both cell types, reaching 50 % inhibition at 800 μg/mL(Reference Song, Hwang and Chung45). PSEER suppressed cell proliferation by arresting the cell cycle in the G1 phase. The molecular mechanisms proposed for this inhibitory effect involve decreased expression of the proteins cyclin D1 and CDK4 (which are related to G1 cell cycle progression) and increased p21WAF1 protein expression (a negative cell cycle regulator). In addition, the expression of fibroblast growth factor (FGF), 5α-reductase and AR was reduced after incubation with PSEER, suggesting a mechanism independent of oxidative stress(Reference Song, Hwang and Chung45).
Prostatitis
Prostatic inflammation, also referred to as prostatitis, has recently been implicated in BPH pathogenesis and may contribute to LUTS(Reference De Nunzio, Presicce and Tubaro46). Importantly, prostatitis may affect men of all ages, and it is estimated that one-half of all men will face this condition during life.
The approved pharmacological treatments for prostatitis involve anti-microbial therapy and anti-inflammatory drugs. Therefore, drugs such as resveratrol, which exhibits an anti-inflammatory profile, have been tested experimentally to treat this condition. In rats, a 10-d treatment with resveratrol (10 mg/kg per gavage) reduced autoimmune prostatitis-induced mast cell infiltration in the prostate(Reference Zeng, He and Yu47). Using the same animal model of prostatitis, a previous study demonstrated reduced prostatic leucocyte infiltration after resveratrol treatment (10 mg/kg daily per gavage)(Reference He, Zeng and Yu48). Similarly, in a rat model of 17-β-oestradiol-induced prostatitis, a 10-d treatment with resveratrol (10 mg/kg daily per gavage) reduced the infiltration of inflammatory cells in the prostate(Reference Qian, Gu and Guan49). The abovementioned studies indicate that resveratrol promotes beneficial effects in experimental models of prostatitis by suppressing inflammatory cell infiltration in the prostate, even in a short-term treatment of 10 d. The attenuation of prostatitis by resveratrol may account for histological improvements, as evidenced by reduced epithelial layer height and fibrosis, as well as by epithelial and stromal hyperplasia(Reference He, Zeng and Yu48,Reference Qian, Gu and Guan49) .
One of the proposed mechanisms to explain the reduction of inflammatory cell infiltration in the prostatic tissue by resveratrol treatment is the suppression of the prostatic c-kit-stem cell factor (SCF) axis, an important pathway related to inflammation and carcinogenesis(Reference He, Zeng and Yu50). The suppression of the c-kit/SCF axis by resveratrol is accompanied by reductions in prostatic inflammation and leucocyte infiltration(Reference He, Zeng and Yu51). In addition, resveratrol decreases pro-inflammatory cytokine levels in the prostate, such as interleukin (IL)-6, IL-8, tumour necrosis factor (TNF)α and TGFβ, which may be associated with the reduced presence of inflammatory cells(Reference Qian, Gu and Guan49). Among these cytokines, TGFβ plays a pivotal role in prostatitis-induced fibrosis. This cytokine has been associated with the conversion of fibroblasts to myofibroblasts, which contribute to prostate remodelling and collagen deposition(Reference Paulis52). The exact mechanism by which resveratrol inhibits TGFβ activity has not been completely elucidated; however, evidence has implicated at least two different pathways: upstream inhibition of microRNA (especially miR-17) and downstream inhibition of Smad complex proteins(Reference Ashrafizadeh, Najafi and Orouei53,Reference Zhang, Lu and Ong’achwa54) . Furthermore, in hepatocytes, the positive relationship between the SCF axis and TGFβ has been demonstrated to act as a positive feedback loop involving STAT3 and Smad2 activation(Reference Rojas, Zhang and Wang55). Therefore, it is possible that this same positive loop plays a role in prostatic inflammation and that resveratrol may exert its therapeutic effect by inhibiting this loop(Reference He, Zeng and Yu48).
Resveratrol-enriched extracts have also been tested in prostatitis assays. PSEER (containing 14·85 μg/mL resveratrol) suppressed prostate enlargement and stromal fibrosis induced by intraurethral injection of Escherichia coli in mice. After mice were treated with 200 μL of PSEER for 4 weeks, the number of colony-forming units in the prostate was reduced from 2·4 × 105 to 0·6 × 105, which may explain the structural amelioration seen in PSEER-treated mice(Reference Pyo, Lee and Lee56).
Due to the anatomical proximity among the prostate, bladder and urethra, prostatitis has been shown to negatively impact lower urinary tract functioning(Reference Aydogdu, Gocun and Aronsson57). In rats, the induction of autoimmune prostatitis led to voiding dysfunction, as evidenced by increases in bladder capacity, voiding pressure and residual volume. Oral treatment with resveratrol at 10 mg/kg for 10 d attenuated the impaired voiding process by mechanisms involving the suppression of the c-kit/SCF axis in the bladder(Reference Yu, Jiang and He58). Resveratrol also reduced TGFβ-induced bladder fibrosis secondary to prostatitis by down-regulating TGFβ, Wnt and β-catenin protein expression. Histological analysis revealed that prostatitis led to bladder smooth muscle disarrangement and increased α-SMA protein expression, a marker for myofibroblasts. Resveratrol restored the control levels of all the protein expressed in the bladder, lowering the fibrotic area(Reference He, Zeng and Yu59). However, considering that resveratrol also promoted changes in the prostate by reducing prostatic inflammation, it is difficult to ascertain whether the improvements by resveratrol on prostatitis-induced bladder impairments reflect a local vesical action or just a consequence of the ameliorated prostatitis condition.
The efficacy of resveratrol in prostatitis-induced LUTS in rats was also assessed in the absence and presence of solifenacin, a competitive and selective muscarinic receptor (M3) antagonist, clinically approved by the Food and Drug Administration and other regulatory organisations worldwide to treat LUTS. The combination of resveratrol plus solifenacin produced a synergistic effect, further reducing all the abnormal cystometric parameters in comparison with resveratrol alone. Corroborating the literature, prostatitis led to overexpression of bladder c-kit and SCF proteins, which were reduced to the same extent by treatment with resveratrol alone or in combination with solifenacin, indicating different targets for both drugs(Reference Yu, Jiang and He58).
Erectile dysfunction
Erectile dysfunction is characterised by the incapacity of achieving or maintaining an erection sufficient for intercourse. The activation of the NO-soluble guanylyl cyclase-cyclic GMP (NO–sGC–cGMP) axis is crucial to induce corpus cavernosum smooth muscle relaxation and penile erection(Reference Calmasini, Klee and Webb60). Reduced NO bioavailability in the corpus cavernosum due to abnormal NO–cGMP signalling has been implicated in ED pathophysiology(Reference Silva, Alexandre and Calmasini61). Epidemiological data show that ED is closely related to obesity, diabetes and dyslipidaemia, supporting a causal link between these conditions and ED(Reference Leisegang, Henkel and Agarwal62). As reported in the literature, the high efficacy of resveratrol in ameliorating ED has been largely confirmed by using different experimental approaches. Similar to lower urinary tract smooth muscle, resveratrol treatment increases cavernosal relaxation and improves ED mainly by reducing oxidative stress, thus ameliorating the antioxidant activity of the cavernosal tissue (as detailed below). This improved oxidative status by resveratrol treatment in the corpus cavernosum also involves enhanced activities of endothelial (eNOS) and neuronal nitric oxide synthase (nNOS) enzymes.
Hyper-cholesterolaemic model of erectile dysfunction and resveratrol
Using a hyper-cholesterolaemia-induced ED model in rabbits, a previous study showed that both preventive and therapeutic oral treatments with resveratrol (8 mg/kg/d for 6 weeks) ameliorated impaired cavernosal endothelium-dependent relaxation. This beneficial effect was associated with a resveratrol-induced reduction in NADPH oxidase activity in the corpus cavernosum of hyper-cholesterolaemic animals. The improved oxidative status induced by resveratrol also ameliorated the eNOS/NO pathway, reducing the levels of uncoupling eNOS(Reference Murat, Korhan and Kizer63). Similarly, a lower resveratrol dose (4 mg/kg/d for 6 weeks, oral) improved endothelium-dependent corpus cavernosum relaxation in hyper-cholesterolaemic rabbits(Reference Soner, Murat and Demir64). Interestingly, the hyper-cholesterolaemic diet did not alter the endothelium-independent cavernosal relaxation induced by sodium nitroprusside, corroborating the idea that resveratrol acts as an endothelial protector in cavernosal tissue(Reference Soner, Murat and Demir64).
Diabetic models of erectile dysfunction
In streptozotocin-induced diabetes, the oral administration of resveratrol at 25 mg/kg/d for 8 weeks ameliorated ED by increasing both the cavernosal cGMP levels and the nNOS/eNOS expression in diabetic rats(Reference Bai and An65). Moreover, resveratrol reduced ROS production in the corpus cavernosum from the diabetic group, reinforcing an important antioxidant mechanism triggered by this polyphenol. Interestingly, the combination of resveratrol (25 mg/kg/d) plus the phosphodiesterase-5 (PDE5) inhibitor sildenafil (5 mg/kg for 8 weeks) resulted in a synergistic effect by further improving ED in diabetic rats, which was also attributed to the antioxidant properties and up-regulation of the NO–cGMP pathway(Reference Bai and An65). The same synergism between resveratrol and another PDE5 inhibitor (vardenafil) was observed in humans(Reference Fukuhara, Tsujimura and Okuda66). These findings indicate that this synergistic effect may be associated with the activation of the NO–cGMP pathway itself, not a non-specific molecule-related effect. This synergism was also reported using corpus cavernosum in vitro. In aged diabetic rats, in vitro incubation of corpus cavernosum with resveratrol at 100 μM for 45 min enhanced sildenafil-induced relaxation(Reference Dalaklioglu, Bayram and Tasatargil67). A lower concentration (50 μM) of resveratrol also improved acetylcholine-induced corpus cavernosum relaxation in diabetic mice(Reference Boydens, Pauwels and Decaluwé68).
One of the proposed mechanisms by which resveratrol up-regulates the NO–cGMP pathway activity in the corpus cavernosum is the activation of sirt-1, an important NOS modulator in several vascular and nonvascular tissues(Reference Donato, Magerko and Lawson69). Sirt-1 is expressed in the human and rodent corpus cavernosum(Reference Freitas, Rodrigues and Tomada70,Reference Yu, Wan and Qiu71) , and its expression is reduced in the cavernosal tissue of diabetic rats, which may be implicated in ED(Reference Yu, Wan and Qiu71). In addition, oral intake of resveratrol at doses of 5 and 10 mg/kg/d for 8 weeks is capable of up-regulating sirt-1 protein expression in ED models of diabetes and radiotherapy(Reference Yu, Wan and Qiu71,Reference Sener, Tavukcu and Atasoy72) .
Finally, it is important to highlight that resveratrol may also induce cavernosal relaxation by mechanisms in addition to increasing NO production. For instance, resveratrol directly relaxed the mouse corpus cavernosum with potency at an application of approximately 1 mM through mechanisms involving increased hydrogen sulphide (H2S) production(Reference Yetik-Anacak, Dereli and Sevin73), suggesting that H2S mediated cavernosal relaxation at high concentrations of resveratrol. Collectively, these literature data indicate that resveratrol has a protective effect on erectile function in several pathological conditions and that this polyphenol may be a valuable alternative treatment applied in association with PDE5 inhibitors or in conditions where PDE5 inhibitors do not work effectively as monotherapy.
Pharmacokinetics of resveratrol
Resveratrol comprises three hydroxyl groups and two phenolic rings that confer high lipophilic properties. This molecular characteristic of resveratrol confers a high oral absorption rate (approximately 70 % in humans) but rather low bioavailability(Reference Sergides, Chirila and Silvestro74). This apparent paradoxical effect may be explained by at least two complementary pathways: (i) resveratrol is extensively metabolised in the liver before reaching circulation and (ii) this polyphenol may enter the enterohepatic cycle, which may retard and/or decrease its oral absorption(Reference Crozier, Jaganath and Clifford75). After a single oral dose of resveratrol of 25 mg, low plasmatic levels were detected in humans (less than 10 ng/mL or approximately 40 nM); however, when considering resveratrol in addition to its metabolites, the overall concentration was found to be 500 ng/mL or 2 μM(Reference Boocock, Patel and Faust76). The major conjugated metabolites produced during resveratrol metabolism are sulphate and glucuronide, which are suggested to contribute to the beneficial effects of resveratrol treatment in experimental and clinical studies.
In all the aforementioned studies involving animal models, the resveratrol dosage used in the in vivo protocols ranged between 1 and 100 mg/kg/d (Table 1). It was reported that oral administration of 2 mg/kg resveratrol to rats achieves peak plasma concentrations of 2·6 μM at 10 min after intake(Reference Emília, Buenafuente and Casals77). Using 50 mg/kg orally in rats, the plasma concentration of resveratrol was as high as 10 μM(Reference Marier, Vachon and Gritsas78). However, when we consider the high concentrations of resveratrol used in the in vitro assays (between 10 and 100 μM) and its reduced oral bioavailability, only a few studies suggested that the in vivo actions could be explained by a direct effect of resveratrol on the targeted tissue (Table 2).
Despite its low bioavailability and tissue distribution, resveratrol exhibits an array of beneficial effects in humans and animals. It has been suggested that resveratrol can act directly in the liver during its first pass, which would not be dependent on its bioavailability(Reference Huang, Lang and Chen79). Moreover, resveratrol may produce some of its beneficial effects by acting directly on the gut cells. For example, intra-duodenal infusion of resveratrol in HFD-fed mice enhanced insulin sensitivity and lowered hepatic glucose production through a mechanism involving the sirt-1 receptor and AMPK protein activation in the gut(Reference Cote, Rasmussen and Duca80).
Resveratrol as a microbiota modulator
The microbiota of the gut is the largest in the human body in terms of diversity and number of microbes and is composed mainly of five different phyla, namely Bacteroidetes, Firmicutes, Verrucomicrobia, Actinobacteria and Proteobacteria(Reference Man, Huige and Ning81). The ratio between the phyla generally varies between individuals according to the lifestyle and other environmental factors that impact the host organism. Gut microbiota dysbiosis is associated with a number of diseases, exerting a pivotal role in the health–disease balance. Metabolic diseases, such as type 2 diabetes and obesity, have been closely related to low microbiota diversity and altered microbiota composition. For instance, a reduced number of Bacteroidetes is found in the gut of animal models of metabolic syndrome, while the levels of Firmicutes species are increased. The altered ratio, in addition to the low microbiota diversity, increases calorie harvesting, which may contribute to adiposity. Some species such as Firmicutes, are capable of metabolising polysaccharides that are excreted in a balanced gut microbiota(Reference Hernández-Ceballos, Cordova-Gallardo and Mendez-Sanchez82).
Recently, growing evidence has supported a role for resveratrol as a microbiota modulator(Reference Chaplin, Carpéné and Mercader83). Resveratrol is capable of promoting gut microbiota changes by inhibiting Enterococcus faecalis growth, as well as promoting Lactobacillus and Bifidobacterium populations, both of which are involved in gut permeability and integrity(Reference Qiao, Sun and Xia84). Therefore, it is possible that resveratrol may exert its beneficial effects in part by improving microbiota diversity, especially under impaired metabolic conditions. In fact, mice fed a HFD to induce obesity and treated with resveratrol at 200 mg/kg/d for 12 weeks exhibited an increased number of Lactobacillus and Bifidobacterium (both belonging to the Actinobacteria phylum) and a decreased number of Enterococcus faecalis in the gut. Each of these bacterial genera is negatively and positively associated with body weight gain, respectively. Furthermore, the ratio between Bacteroidetes and Firmicutes bacteria, which negatively correlates with body weight, was increased after resveratrol treatment(Reference Qiao, Sun and Xia84).
In addition to the changes in body weight, resveratrol also improves glucose homoeostasis by a mechanism involving gut microbiota modulation. Obese mice fed a diet containing resveratrol exhibited reduced fasting glucose and glucose tolerance, an effect partially lost when the obese mice were treated with antibiotics to deplete the microbiota(Reference Hui, Liu and Huang85). Experiments involving faecal microbiota transplantation have also been conducted to further explore the role of resveratrol-induced microbiota modulation and its relationship with glucose homoeostasis. The faecal microbiota obtained from control mice fed a resveratrol-supplemented diet was transplanted to HFD-fed obese mice(Reference Kim, Parajuli and Sung86). These authors found that faecal microbiota transplantation improved glucose homoeostasis in the obese group, as evidenced by the reduced area under the curve (AUC) in the glucose tolerance test. Interestingly, the sterile faecal content from control mice fed resveratrol also improved blood glucose levels in the obese group, suggesting that post-biotics also contribute to resveratrol-induced metabolic improvements(Reference Roje, Elek and Palada87).
Concerning UGT specifically, the resveratrol efficacy in ameliorating UGT diseases through gut microbiota modulation has been poorly addressed; however, a few studies have shown an important link between UGT and gut microbiota. Under physiological conditions, the gut microbiota is important for maintaining bladder structure and functioning. The mRNA expression of ninety-seven genes was found to be up- or down-regulated in the bladder of germ-free mice, which may be involved in the reduced bladder size and weight in these animals(Reference Roje, Elek and Palada87). The abundance of Faecalibacterium along with the reduced number of Bifidobacterium was recently reported in patients with overactive bladder syndrome(Reference Okamoto, Hatakeyama and Imai88). Similarly, a negative correlation between Lachnospiraceae, Blautia and LUTS in adult men was found(Reference Holland, Karr and Delfino89). The cross-talk between gut microbiota and UGT dysfunctions was also reported in inflammation-induced rat dysbiosis. The gut inflammation induced by colonic instillation of 2,4,6-trinitrobenzenesulphonic acid was associated with impaired detrusor contractility with no accompanying inflammatory signs(Reference Noronha, Akbarali and Malykhina90). Additionally, patients diagnosed with irritable bowel syndrome are 2·12- and 2·38-fold more likely to develop organic and psychogenic ED, respectively(Reference Hsu, Lin and Kao91).
The mechanisms by which gut microbiota modulates UGT under physiological and pathological conditions may involve neuronal modulation (afferent and efferent nerves), neurotransmitter release, and control of food intake(Reference Cani and Knauf92). All these effects induced by the gut microbiota may contribute to better metabolic, hormonal and neurogenic functioning of the host. Therefore, it is likely that at least some of the beneficial effects of resveratrol in UGT dysfunction may be due to gut microbiota modulation, but surprisingly, no study has explored this possibility until now.
Future directions
To the best of our knowledge, there is just one clinical trial evaluating the efficacy of resveratrol in BPH patients. Long-term oral treatment with resveratrol (1000 mg, 4 months) in middle-aged men with metabolic syndrome indeed reduced the plasma levels of the androgen precursors androstenedione, dehydroepiandrosterone and dehydroepiandrosterone sulphate but had no significant effect on prostate size or the levels of prostate-specific antigen, testosterone and dihydrotestosterone(Reference Kjær, Ornstrup and Poulsen93). On the other hand, resveratrol given orally for 2 months to patients with prostatic fibrosis (but with normal prostate volume) secondary to chronic prostatic inflammation resulted in reduced leucocyte infiltration as well as lowered Chronic Prostatic Symptom Index (by 3·5 points) and International Prostate Symptom Score (by 7 points), indicating better UGT functioning(Reference Vicari, Arancio and Catania94).
One critical issue about resveratrol that may explain the reduced number of clinical trials involving this polyphenol in the UGT is its low bioavailability, which reflects its low distribution to tissues. In an attempt to overcome this problem, several strategies have been proposed, such as the synthesis of resveratrol analogues, drug association and the development of pharmaceutical techniques. Acetylation or methylation of the three hydroxyl groups in the resveratrol molecule is a commonly used strategy reported to increase the plasma concentration by 74·5 % after intragastric administration to rats. Increases in half-life (234 %) and volume of distribution (176 %) along with reduced total body clearance (17·8 %) were also observed with these modified compounds(Reference Liang, Liu and Wang95).
With regard to the association of resveratrol with other drugs, a double-blind placebo-controlled and randomised multicentre study was performed to evaluate the co-administration of resveratrol and the cyclooxygenase inhibitor meloxicam in patients with knee osteoarthritis(Reference Hussain, Marouf and Ali96). The authors reported that resveratrol 500 mg plus meloxicam 15 mg, both orally administered once per day, improved osteoarthritis-associated symptoms, such as knee physical function and pain, compared with meloxicam offered as a monotherapy. This combination was also considered safe based on clinical and biochemical analysis(Reference Hussain, Marouf and Ali96).
Different pharmaceutical formulations of resveratrol have also been developed to overcome its low bioavailability. In human volunteers, an oral formulation containing resveratrol solubilised in a lipid solution exhibited a twelve-fold increase in maximum plasma concentration and an eight-fold increase in AUC compared with dry powder of resveratrol capsules. Moreover, in HFD-fed mice, this same formulation reduced liver, colon and hypothalamus inflammation compared with dry powder of resveratrol(Reference Amiot, Romier and Anh97). Curiously, high-fat meals reduced AUC and the maximal plasma concentration of resveratrol in humans. Therefore, despite the high resveratrol lipophilicity, large amounts of fat reduced its bioavailability; hence, resveratrol is recommended to be taken with normal or low-fat meals(Reference la Porte, Voduc and Zhang98).
Nanoparticles loaded with resveratrol have also been tested. Oral administration to rats of a nanoparticle composed of resveratrol/hydroxylpropylmethylcellulose/poloxamer 407 (1:4:1) led to a ten-fold increase in maximum plasma concentration and a three-fold increase in AUC of resveratrol compared with resveratrol(Reference Ha, Sim and Lee99). A lipid-based nanocarrier system composed of a dioctadecyldimethylammonium bromide: monoolein liposomal system (1:2) was also shown to improve the stability and cellular penetration of resveratrol(Reference Barbosa, Santos-Pereira and Soares100). However, despite these innovative formulations of resveratrol, no study has tested these compounds in the healthy or diseased UGT.
Considering the low number of clinical studies addressing the effects of resveratrol on UGT dysfunctions, the results obtained from basic science should be interpreted from a human perspective, which may help to better understand the underlying pathophysiology of UGT diseases. For instance, resveratrol-induced c-kit/SCF pathway inhibition has been shown to play a role in prostate and bladder impairments in animal models(Reference He, Zeng and Yu101). This pathway has already been demonstrated in the human prostate and is activated in the prostate of BPH patients(Reference Cardoso, Figueira and Correia102,Reference Imura, Kojima and Kubota103) . Therefore, drugs targeting the c-kit/SCF pathway, such as resveratrol, may be an interesting approach for future clinical trials involving UGT diseases. Similarly, the activation of sirt-1 in animal models is another proposed mechanism by which resveratrol exerts its beneficial effects in pre-clinical studies involving UGT diseases, such as ED(Reference Yu, Wan and Qiu71,Reference Sener, Tavukcu and Atasoy72) . Selective sirt-1 agonists have been developed and successfully tested in animal models of metabolic diseases; however, clinical trials have failed to demonstrate the efficacy of these molecules in ameliorating the glucose homoeostasis in type 2 diabetic patients(Reference Baksi, Kraydashenko and Zalevkaya104). However, the testing of selective sirt-1 agonists in human UGT has yet to be demonstrated. Nonetheless, this highlights the complex task in translating results from animals to humans but encourages further clinical trials of resveratrol or its modified molecules in UGT diseases. In this context, the results of analyses on the pre-clinical data from animals or isolated cells, treated resveratrol or sirt-1 agonists not only help to understand the molecular basis underlying resveratrol effects, but also guide additional clinical trials of UGT disease treatments.
Conclusion
Resveratrol is a polyphenol compound largely explored in recent decades as a therapeutic drug in a number of human and animal diseases. Its mechanisms of action are not specific (not exactly determined) but may involve receptor activation, antioxidant activity and microbiota modulation. Despite all the beneficial effects shown with in vitro assays and animal models, the efficacy of resveratrol in humans is still a matter of debate. In UGT diseases specifically, resveratrol ameliorates LUTS, BPH, prostatitis and ED in animal models, but there are still few studies in human tissues and insufficient clinical trials to establish resveratrol efficacy. Therefore, future studies designed to investigate the resveratrol actions on human UGT dysfunctions are required.
Acknowledgements
We thank FAPESP for the financial support.
Financial support
This work was supported by the São Paulo Research Foundation (FAPESP; grant numbers 2019/09912-9 and 2020/06254-8). FAPESP had no role in the design, analysis or writing of this article.
Conflict of interest
There are no conflicts of interest.
Authorship
Participated in manuscript design: C.F.B., A.E.
Drafted the manuscript: C.F.B., S.F.H., A.E.C.
Edited and revised the manuscript: C.F.B., A.E.







