Fusobacterium are obligate, anaerobic, Gram-negative bacilli which inhabit the oral, gastrointestinal, upper respiratory tract, and vaginal mucosa as part of normal flora. Fusobacterium infections occur through disruption of mucosal surfaces from trauma, tumour, or prior infection, and can then progress to oropharyngeal disease, pleuropulmonary infection, and bacteraemia [Reference Su1]. Fusobacterium bacteraemia (FB) accounts for 0·19–0·90% of all human bacteraemia cases, predominantly from a gastrointestinal (GI) source, and is associated with a variable mortality of 0–47·4% [Reference Su1–Reference Yang5]. Immunocompromised patients, the elderly, diabetics, patients with renal insufficiency, or patients with congestive heart failure are at increased risk for developing FB, and carry a worse prognosis [Reference Su1, Reference Epaulard4].
FB can be associated with Lemierre's syndrome, an acute and deadly septic thrombophlebitis of the internal jugular vein in previously healthy young adults. While Lemierre's syndrome is predominantly associated with F. necrophorum, polymicrobial infection is common, and cases have been documented with other Fusobacterium spp., Bacteroides spp., Staphylococcus haemolyticus, S. hominis, and S. epidemidis, Streptococcus viridans and group C streptococci, Proteus spp., and Klebsiella pneumoniae [Reference Karkos6]. Lemierre's syndrome occurs in 2–20% of cases of FB, carries a mortality of 5–9%, and can occur without concomitant FB in up to 40% of cases [Reference Su1, Reference Huggan and Murdoch3, Reference Karkos6, Reference Hagelskjaer Kristensen and Prag7].
We performed a retrospective descriptive study of patients with FB in a teaching hospital in Ohio. This is the second study focusing on FB in adults to be conducted in the USA in 30 years [Reference Henry, DeMaria and McCabe8]. We intended to analyse which patient variables are more frequently associated with FB, and which patient variables predict mortality.
A 10-year retrospective study of patients with FB was conducted at Summa Health System – a 658-bed community teaching hospital in northeastern Ohio. Cases of FB were identified by reviewing the microbiology database for blood cultures collected from January 2001 to December 2010. Demographic information, medical comorbidities, clinical presentation and clinical findings, laboratory data [white blood cell (WBC) count, haemoglobin and haematocrit, and creatinine), radiological findings, treatment measures, and clinical outcomes were collected from all patient charts. We also identified the most likely source of bacteraemia for each patient based on the history, clinical findings, and radiological reports. Bivariate Fisher's exact test and bivariate t test were used to statistically analyse relationships between patient characteristics and outcomes.
Nineteen patients with FB were identified during the 10-year study period. A total of 18 035 blood culture bottles demonstrated infection, and 21 bottles were positive for Fusobacterium spp., representing 0·11% of positive cultures. The mean age of presentation was 58·6 years (range 24–97 years) with an equal sex distribution. All patients with FB had at least one comorbid condition (Table 1).
Table 1. Characteristics of Fusboacterium bacteraemia patients
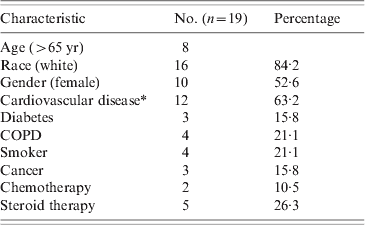
COPD, Chronic obstructive pulmonary disease.
* Cardiovascular disease: coronary artery disease, history of thromboembolic events, or hypertension which is not secondary to a non-cardiovascular aetiology.
Most (94·7%) patients presented with clinical evidence of infection. However, in a single patient the positive blood culture was found incidentally. None of these cases presented as Lemierre's syndrome. The most common presenting variables in FB included GI complaints (63·2%) – defined as persistent nausea or abdominal pain, vomiting or haematemesis, diarrhoea or haematochezia; hyperthermia (47·4%) – defined as temperature ⩾37·8 °C; leukocytosis (63·2%) – defined as WBC count ⩾11 000 cells/μl; and elevated creatinine level (42·1%) – defined as creatinine ⩾1·2 mg/dl.
The most likely infectious sources for the 19 cases based on history, symptomatology, physical examination, and radiological findings included GI (63·2%), genitourinary (GU) (10·5%), respiratory (5·3%), and indeterminate (21·0%). The presenting conditions of the patients with a presumed GI infectious source included acute liver failure, colitis with peritonitis, cholecystitis with alcoholic cirrhosis, colonic perforation, cholangitis, hepatic metastases with peritonitis, two patients with gastroenteritis, two patients with diverticulitis and two patients with hepatic abscesses. For the two patients with a likely GU infectious source, the precipitating events were pelvic inflammatory disease and pyelonephritis. The patient with a likely pulmonary source of infection presented with post-obstructive pneumonia. The four patients with an indeterminate infectious source presented with myocardial infarction, uncontrolled schizophrenia, pregnancy with Escherichia coli urinary tract infection, and a stroke.
Polymicrobial bacteraemia occurred in five (26·3%) cases. Eighteen (94·7%) of 19 patients were treated with antibiotics. Penicillins (57·9%), quinolones (47·4%), and metronidazole (31·6%) were those most frequently administered. Three (15·8%) patients were treated with antibiotic regimens lacking in vitro activity against Fusobacterium infection. One patient died before antibiotics could be administered.
Four (21·1%) patients died within 30 days of hospitalization, 13 (68·4%) patients required admission to the intensive care unit (ICU) at the time of FB diagnosis, and seven (36·8%) patients received mechanical ventilation. The mean length of hospital stay (LHS) was 13·8 days (range 1–50 days). The factors demonstrating a significantly increased mortality included creatinine ⩾1·2 mg/dl and altered mental status, with P values of 0·018 and 0·037, respectively (Table 2). No factors were significantly associated with ICU admission or mechanical ventilation. Variables associated with increased LHS included cardiovascular disease (CVD), diabetes, and ICU admission, with P values of 0·002, 0·001 and 0·038 respectively. CVD is defined here as coronary artery disease, history of thromboembolic events, or hypertension which is not secondary to a non-cardiovascular aetiology.
Table 2. Variables associated with increased mortality in Fusobacterium bacteraemia patients

COPD, Chronic obstructive pulmonary disease; ICU, intensive care unit.
* Immunosuppression: current chemotherapy, systemic steroid use, or HIV infection (CD4 <250).
† Cardiovascular disease: coronary artery disease, history of thromboembolic events, or hypertension which is not secondary to a non-cardiovascular aetiology.
‡ P < 0·05.
The first US case series of FB was described in 1983 [Reference Henry, DeMaria and McCabe8]. At least seven other similar case series focusing specifically on FB have been published elsewhere since then [Reference Su1–Reference Yang5, Reference Bourgault9, Reference Nohrstrom10]. FB is a rare clinical event, ranging from 0·19% to 0·90% of blood cultures, with a predilection towards male patients aged >50 years [Reference Su1, Reference Su2, Reference Su4, Reference Su8–Reference Brook11]. By comparison, the incidence of FB in this study was considerably lower at 0·11%. The mean age in this study was 58·6 years with a roughly equal gender distribution. These data conform to the trend of an increasing mean age of FB patients over the last 20 years [Reference Su1, Reference Huggan and Murdoch3–Reference Yang5, Reference Henry, DeMaria and McCabe8, Reference Bourgault9].
Every patient in this study had at least one comorbidity, with CVD being present in over half of the patients. Other studies commonly document an association with neutropenia or malignancies in FB patients, ranging from 30·8% to 45·6% of the cases [Reference Su1, Reference Epaulard4, Reference Yang5]. The frequency of immunosuppression in our FB population was comparable at 36·8%, with two patients on chemotherapy, five patients on systemic steroid therapy, and one patient positive for HIV (CD4 <240 cells/ml).
A wide spectrum of disease presentation is frequently noted in FB studies, ranging from febrile neutropenia to Lemierre's syndrome [Reference Fanourgiakis2, Reference Huggan and Murdoch3, Reference Nohrstrom10]. In our study, the most common clinical manifestations of FB were GI complaints (63·2%), hyperthermia (47·4%), sepsis (31·6%), and mental status changes (26·3%). While GI infection is a common source of FB, the infectious source remains unknown in up to 38·6% of cases [Reference Su1, Reference Yang5, Reference Bourgault9]. GI infection was the most common presumed infectious source of FB in our study (63·2%) with an equal distribution between hepatobiliary and colonic sources, but the presumed infectious source was unknown in 21% of our patients. Several studies associate FB with GU or obstetric infection in 5–35% of cases [Reference Su1, Reference Henry, DeMaria and McCabe8–Reference Chaim and Mazor12]. We found a similar association in 10·5% of our cases. It should be noted that the presumed infectious source for the pregnant patient presenting with E. coli urinary tract infection could not be categorically determined, as a GI and obstetric source were equally likely.
Polymicrobial bacteraemia was present in 26·3% of our FB cases. This is comparable to the rates reported in other studies, ranging from 29·8 to 43·1% of cases [Reference Su1, Reference Epaulard4, Reference Yang5, Reference Bourgault9]. The high frequency and heterogeneity of polymicrobial bacteraemia associated with FB correlates with the diverse normal microbial flora of each disrupted mucosal surface [Reference Guarner and Malagelada13, Reference Marshall14].
Speciation data from the Fusobacteria cultures could not be attained due to the retrospective nature of this study, so no relationship between Fusobacterium spp. and outcome measures could be determined. However, multiple studies demonstrate that F. nucleatum has the greatest prevalence, and F. necrophorum possesses the greatest disease severity [Reference Citron, Poxton, Baron and Murray15]. Antibiotic susceptibility and β-lactamase production assay were likewise not performed for the blood samples growing Fusobacterium spp., although one microbiological study demonstrated sensitivity to metronidazole in 100%, penicillin with β-lactamase inhibitors in 84%, penicillin alone in 75·6%, clindamycin in 88%, moxifloxacin in 81% and carbapenems in 95% of the cases [Reference Liu16]. Given the unavailability of sensitivities, appropriateness of antibiotics was defined as an adequate time-course with any of the antibiotics listed above.
The overall 30-day mortality after FB diagnosis was 21·1%, which is comparable to other studies ranging from 0% to 47·4% [Reference Su1–Reference Huggan and Murdoch3, Reference Yang5, Reference Nohrstrom10, Reference Brook11]. In our study, we found that elevated creatinine levels and mental status changes correlated significantly with a higher mortality rate in FB patients. It should be considered that many of the factors analysed, such as leukocytosis and elevated creatinine levels, are common signs of severe infection and may not be specific to FB itself. ICU admission was required in 68·4% of cases and the LHS averaged nearly 2 weeks (13·8 days), indicating that patients are generally in very poor health at the time of FB diagnosis. While it cannot be determined from this study whether this finding is strictly the result of poor pre-existing health status or the direct effects of FB, 92·3% of patients requiring ICU had ⩾3 comorbidities, compared to only 33·3% of non-ICU patients. No significant correlation existed between the analysed factors and ICU admission, but patients presenting to the ICU generally had sepsis and a presumed GI source of infection. However, ICU admission, along with CVD and diabetes were significantly associated with increased LHS.
While all cases of polymicrobial sepsis resulted in ICU admission or death, no statistically significant association was found (P = 0·338, 0·272, respectively), which is consistent with previous studies [Reference Su1, Reference Yang5, Reference Bourgault9]. Despite the lack of significant association, microbiological studies suggest that other bacteria enhance the virulence of Fusobacterium [Reference Nohrstrom10]. It is also notable that a presumed GI infectious source was present in 100% of patients who died and in 84·6% of patients requiring ICU admission, despite the lack of statistically significant associations with these outcome measures (P = 0·245, 0·377 respectively).
This study, as well as 6/8 of the reviewed studies specific to FB, determined possible risk factors for FB and poor outcomes through univariate analysis or subjective description [Reference Fanourgiakis2–Reference Epaulard4, Reference Henry, DeMaria and McCabe8–Reference Nohrstrom10]. However, the multiple logistic regression analysis necessary to determine independent risk factors is largely unattainable for most studies of FB, given its low prevalence and the large sample size needed to prove significance. Only two of the reviewed FB studies had a prevalence and sample size large enough to perform multivariate analysis [Reference Su1, Reference Yang5].
In summary, we present the second study of FB cases in the USA in 30 years. This infection occurred in patients with comorbid conditions, most commonly of a cardiovascular nature. GI complaint was the most common presentation and GI mucosa was the most common presumed source of infection. Factors associated with poorer outcomes include elevated creatinine level, changed mental status, CVD, diabetes, and ICU admission.
ACKNOWLEDGEMENTS
The authors acknowledge the Microbiology Department at Summa Health System for their technical support.
DECLARATION OF INTEREST
None.




