Introduction
Bipolar disorder (BD) is a chronic psychiatric disorder with recurrences [1], leading to a lower quality of life and an elevated risk of suicide [Reference Hofer, Mizuno, Wartelsteiner, Wolfgang Fleischhacker, Frajo-Apor and Kemmler2,Reference Fajutrao, Locklear, Priaulx and Heyes3]. The complexity of the BD phenotype causes high rates of misdiagnosis [Reference Benazzi4,Reference Ghaemi, Boiman and Goodwin5] and, consequently, delay in correct diagnosis and adequate treatment [Reference Ghaemi, Boiman and Goodwin5]. This has led to an immense recent research interest in uncovering endophenotypes (i.e., state-independent intermediate phenotypes) to improve the diagnostic accuracy of BD.
Aberrant emotional reactivity and regulation have been posited as promising endophenotypes for BD [Reference Miskowiak, Kjærstad, Meluken, Petersen, Maciel and Köhler6] that are related to impaired interpersonal and occupational functioning [7–9]. However, extant behavioral studies have provided conflicting findings with respect to which of these emotional cognitive domains are affected and whether such changes are state- or trait-related (for a systematic review on emotional cognition in BD, see [Reference Miskowiak, Seeberg, Kjaerstad, Burdick, Martinez-Aran and Bonnin10]). Behavioral paradigms of self-reported emotional states in response to pleasant and aversive stimuli have generally failed to detect significant differences between remitted patients with BD and controls [11–16]. It is plausible that these behavioral measures of emotion processing fail to detect differences between patients with BD and healthy controls (HCs) because of insufficient sensitivity of subjective ratings to detect abnormal brain function. In contrast, functional magnetic resonance imaging (fMRI) studies have consistently found aberrant neural activation in patients with BD during explicit and implicit processing of emotional faces and words during acute mood episodes and in remission [Reference Townsend and Altshuler17,Reference Chen, Suckling, Lennox, Ooi and Bullmore18]. However, the high cost of fMRI renders this an unfeasible tool to support diagnostic evaluations in clinical practice. Identification of alternative less costly methods with high sensitivity to abnormal brain responses may therefore represent more feasible tools that can more readably be implemented in the clinical assessments.
Eye-tracking and facial emotion analysis are highly sensitive to emotion processing abnormalities and can more readily be implemented to aid diagnostic accuracy due to their lower costs and easier applications in the clinic. Indeed, eye-tracking studies of facial emotion processing using pro/antisaccade paradigms identified trait-related oculomotor abnormalities, including more errors and slower saccadic responses, in remitted and symptomatic patients [19–21] (although, see also [Reference Peckham, Johnson and Tharp22], which showed no abnormal viewing patterns in a free-viewing paradigm). Similarly, eye-tracking studies found trait-related negative bias in BD, as reflected by more fixations and time spent viewing threatening images in remitted and symptomatic patients [Reference Garcia-Blanco, Salmeron and Perea23,Reference Garcia-Blanco, Salmeron, Perea and Livianos24]. In a recent study, we found that remitted patients with BD exhibited subtle abnormalities in visual gaze patterns and facial displays of emotion when viewing aversive images [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16]. Specifically, patients looked more away from these images, which may be an implicit emotion regulation strategy to compensate for their heightened emotional reactivity [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16]. In contrast, a study using a free-viewing eye-tracking paradigm showed no aberrant eye-movements in BD [Reference Purcell, Lohani, Musket, Hay, Isaacowitz and Gruber14]. Two studies of facial emotions also found more incongruent facial expressions in remitted patients with BD during presentations of emotional pictures and film clips (i.e., expressions did not match the valence of the emotion-eliciting stimuli) [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16,Reference Bersani, Polli, Valeriani, Zullo, Melcore and Capra25]. However, one study found no abnormal positive or negative facial displays of emotions during neutral, happy, or sad film-clips compared with controls [Reference Gruber, Hay and Gross26]. The employed film clips in the latter study may have been suboptimal for detection of abnormal emotion processing and regulation in BD. Specifically, film clips tapping into risk-taking and thrill-seeking behavior may be more sensitive to such abnormalities given the clinical presentation of increased impulsivity and risk-taking in BD [Reference Holmes, Bearden, Barguil, Fonseca, Serap Monkul and Nery27,Reference Swann, Pazzaglia, Nicholls, Dougherty and Moeller28]. Also, while static images have limited ecological validity [Reference Parsons29], emotional film clips have close resemblance to real-world scenarios and are thus likely to elicit stronger emotional reactivity.
In this exploratory study, we therefore aimed to investigate subtle behavioral differences in emotional reactivity between remitted BD patients and HCs during highly emotional film clips including risk-taking and thrill-seeking scenes using eye-tracking, facial emotion analysis, and self-rated emotional reactivity. Based on the above findings, we hypothesized that patients with BD would: (I) gaze more away from emotional—particularly negative—film clips as an implicit attempt to compensate for their heightened emotional reactivity, (II) display aberrant (e.g., incongruent) facial expressions during all the emotional film clips in general and more facial displays of positive emotions (happiness and surprise) during film clips tapping into risk-taking/thrill-seeking/winning, and (III) experience stronger positive and negative emotional reactions to the pleasant/thrill-related/risk-taking and the aversive/sad/social anxiety-relevant film clips, respectively.
Materials and Methods
Participants
Thirty-eight patients with BD and 40 HCs were recruited as part of the larger Bipolar Illness Onset cohort study that aims to identify illness biomarkers in newly diagnosed patients with BD [Reference Kessing, Munkholm, Faurholt-Jepsen, Miskowiak, Nielsen and Frikke-Schmidt30]. All participants were screened with the Schedules for Clinical Assessment in Neuropsychiatry to confirm BD diagnosis in patients and ascertain the absence of a psychiatric disorder in HCs, and depressive and manic symptoms were rated with the 17-item Hamilton Depression Scale (HDRS-17) [Reference Hamilton31] and Young Mania Rating Scale (YMRS) [Reference Young, Biggs, Ziegler and Meyer32]. All patients with BD, 15–70 years of age, from Clinic for Affective Disorders at Psychiatric Centre, Copenhagen were consecutively referred to the study after confirmed ICD-10 diagnosis of BD by their treating psychiatrist. They were included in the study if they were in full or partial remission defined as total scores of ≤14 on the HDRS-17 and YMRS, respectively. Age- and gender-matched HCs were recruited through the Copenhagen University Hospital blood bank or through online advertisement. They were included if they had no personal or first-degree family history of psychiatric illness or substance abuse. Informed consent was obtained from all participants prior to inclusion in the study. Exclusion criteria for all participants were a history of brain injury, neurological disorders including dementia, severe somatic illness, current substance abuse, and having received electroconvulsive therapy in the previous 3 months. Furthermore, participants were excluded if the eye-tracking calibration was not successfully achieved.
Procedure
Study participation involved a single test session of approximately 2 h at The Psychiatric Centre Copenhagen, Copenhagen University Hospital. During this time, participants were given two experimental tasks: an emotional picture task, for which the results have been reported previously [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16] and a paradigm involving emotional film clips. In the latter paradigm, participants were presented with a set of positive and negative emotional film clips on a 14-in. laptop computer screen. Their eye movements and facial expressions were recorded using the Tobii X2-30 eye-tracker (Tobii Inc., Stockholm, Sweden) and laptop camera, and the data were processed using the iMotions software (iMotions Inc., MA, USA). The eye-tracker was initially calibrated using a seven-point calibration, and the participants were instructed to sit comfortably approximately 50 cm from the screen, not to talk, breathe normally, and to keep their eyes on the screen during the entirety of the task.
Experimental paradigm
Participants were presented with seven emotional film clips; including a neutral, a happy, and a sad film clip. The latter four film clips were specifically chosen to tap into the BD phenotype and involved winning an Olympic medal (winning), a thrill-seeking car race (racing), walking on top of a skyscraper without safety equipment (risk-taking), and a socially uncomfortable situation (socially anxious) (see Table 1 for a description of the emotional film clips). The film clips lasted between 90 and 110 s and were presented in a random order. The total duration of the task was 13 min. The task was administered using iMotions software platform version 6.4 on a Lenovo T470s (Beijing, China) laptop computer.
Table 1. Narratives of the seven emotional film clips

Measures of emotional reactivity to film stimuli
Self-reported positive and negative affect
After viewing each film clip, participants were instructed to rate their level of positive and negative affect, respectively, on a 100-point visual analog scale presented on the screen ranging from none (0) to a lot (100).
Eye-tracking
Total gaze time, fixation time, and fixation count within the area of interest were recoded using the Tobii X2-30 eye-tracker while participants viewed the film clips. This eye-tracking device uses near-infrared light directed at the pupils to generate reflections in the corneas tracked by an infrared camera [Reference Farnsworth33] at a sampling rate of 30 Hz (±2 Hz). The total gaze and fixation times were calculated as the percentage of time participants gazed/fixated within the AOI, while fixation count reflected the number of fixations within the AOI. The AOI was defined as the whole computer screen. Hence, the data reflect how much time is spent actually viewing the film clips rather than looking away. The fixation algorithm was duration dispersion-based, in which gaze points with a 1° radius for a minimum duration of 100 ms with 50% of the samples available is continuously searched for. The fixation centroid (x, y) was recalculated for each gaze point that was added to the fixation. All data were extracted using the iMotions software.
Facial emotion recordings
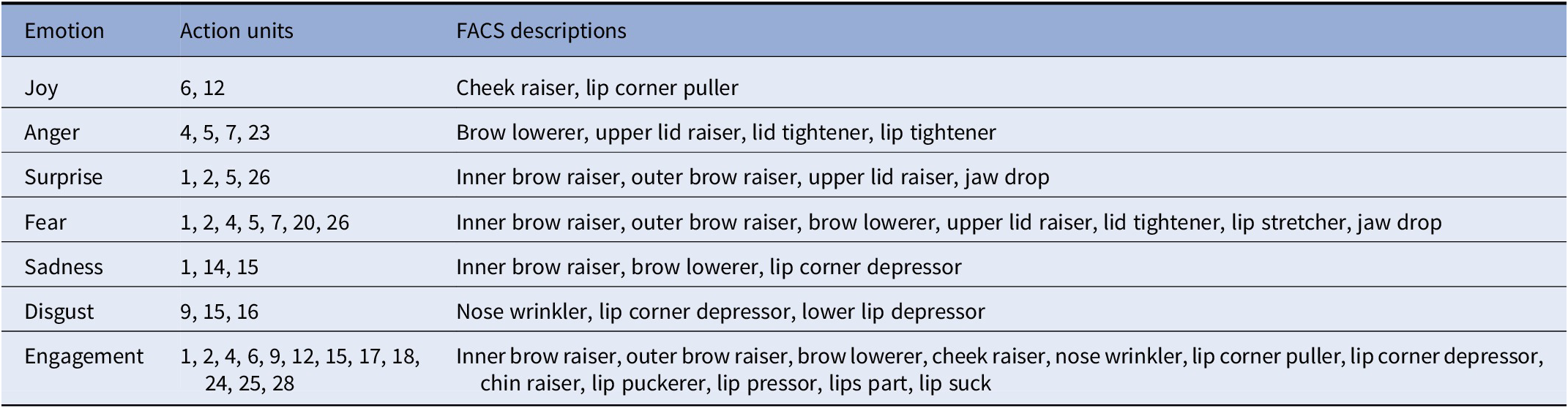
Participants’ facial expressions were measured using the Affectiva (MA, USA) AFFDEX algorithm. This algorithm detects the face and its action units to outline the facial expression metrics by comparing facial features with a database of normative distributions of feature characteristics [34]. The Affectiva AFFDEX algorithm is based on the Facial Action Coding System (FACS) [Reference Ekman and Friesen35] and uses 14 facial expression metrics to classify the six basic emotions (joy, surprise, anger, fear, sadness, and disgust). We also included a measure of engagement (i.e., facial expressiveness) and combined positive and negative facial expressions (see Table 2 for action units associated with each facial emotion classification). Within the iMotions software platform, the raw data for the six emotions and engagement/expressiveness were calculated to obtain a binary result with a threshold of 10, denoting that facial expressions with at least a 10% likelihood that a human assessor would rate the emotion corresponding to the AFFDEX algorithm were accepted. This threshold was set to detect subtle changes in facial emotions triggered by the film clips and was in accordance with the default settings in the iMotions software. Facial expressions that did each the threshold were considered neutral or a lack of facial expression. All facial expression data were calculated as the percentage of time the facial expression was displayed for each film clip.
Table 2. Emotions with corresponding action units and Facial Action Coding System (FACS) descriptions [Reference Farnsworth33,34]
Statistical analysis
We conducted a series of repeated-measures analyses of variance with group (BD, HC) as between-subjects factor and emotional reactivity for the seven film clips as within-subjects factor. Statistically significant interactions were followed up with independent samples t-tests to examine the origin of the interaction and post hoc analyses of covariance adjusting for depressive and manic symptoms (HDRS-17 and YMRS scores) and years of education. Self-report variables were arcsine-transformed prior to analyses. Finally, we conducted exploratory post hoc correlational analyses between the variables where we found statistically significant group differences and (a) patients’ medication status (yes/no antidepressants, antipsychotics, anticonvulsants, and lithium) and (b) subsyndromal symptoms (HDRS-17 and YMRS scores). Analyses were performed with Statistical Package for Social Sciences (version 22.0, IBM, NY, USA). All effects are reported as significant at ps ≤ 0.05 (two-tailed), and effect sizes for significant results are reported as partial eta squared (ƞp 2) and Cohen’s d.
Results
Participant characteristics
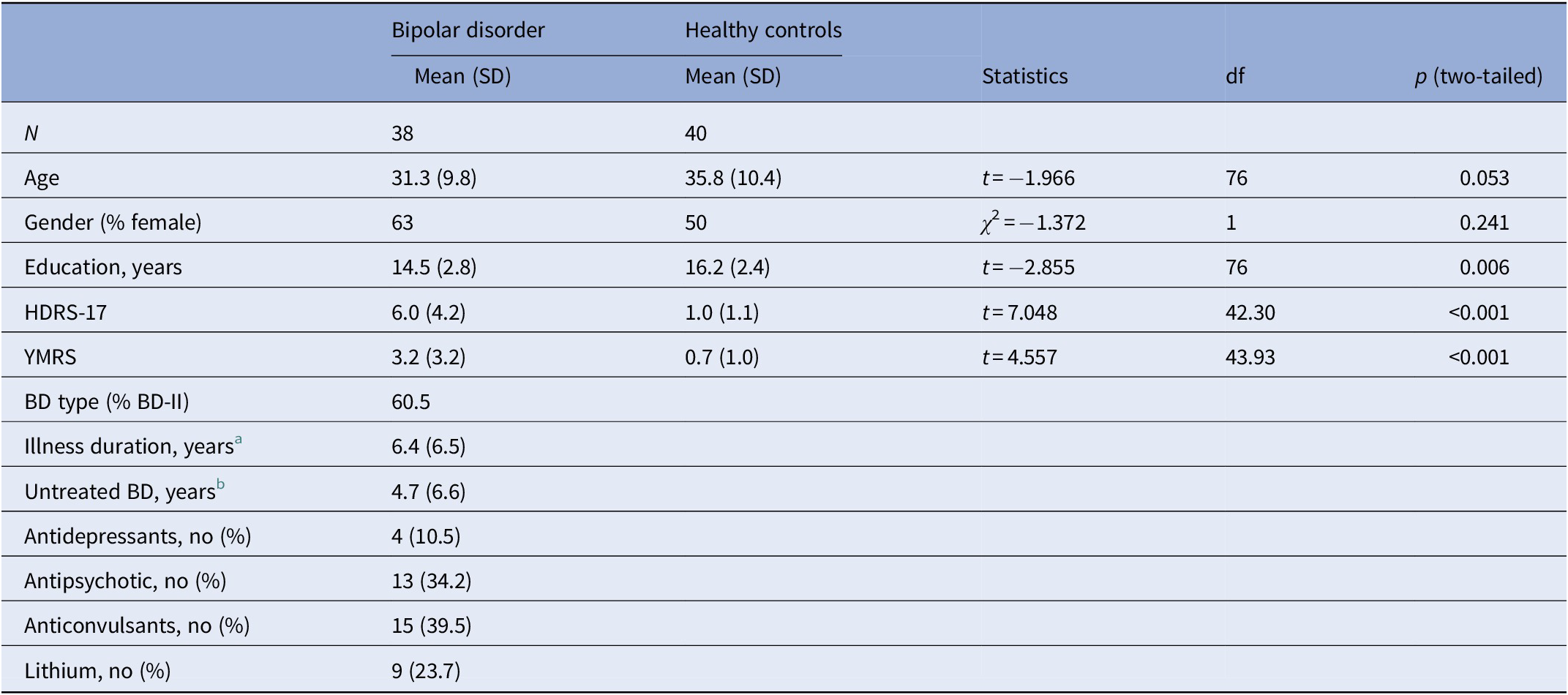
Six participants (three BD patients and three HCs) were excluded due to incomplete data, resulting in a final sample of 38 patients with BD in full or partial remission and 40 HCs. The two groups were matched for age and sex (ps > 0.05). However, patients had undergone fewer years of education (p = 0.01) and displayed more subsyndromal depression and mania symptoms (ps ≤ 0.001; Table 3).
Table 3. Participant demographic characteristics
a Defined as time from first manic, hypomanic, or mixed episode to date of test.
b Defined as time from first manic, hypomanic, or mixed episode to date of diagnosis.
Eye-tracking
Patients with BD generally spent less time gazing at all film clips compared to HCs (main effect of group: F(1, 76) = 8.17, p = 0.005, ƞp 2 = 0.097). There was also a significant group by film clip interaction (F(6, 456) = 2.32, p = 0.03, ƞp 2 = 0.03); patients with BD spent less time gazing at the neutral (t = 2.81, df = 60.94, p = 0.007, d = .64), happy (t = 2.50, df = 47.13, p = 0.02, d = .57), sad (t = 2.31, df = 56.68, p = 0.02, d = .53), winning (t = 2.56, df = 65.05, p = 0.01, d = .58), racing (t = 2.34, df = 46.13, p = 0.02, d = .55), socially anxious (t = 2.62, df = 52.36, p = 0.01, d = .60), but not risk-taking (p = 0.83) film clips compared to HCs. These group effects rendered nonsignificant after adjustment for subsyndromal symptoms and years of education (ps ≥ 0.12; covarying only for subsyndromal symptoms: ps ≥ 0.12). There were no significant differences between the groups in fixation time or numbers of fixations at the film clips (ps ≥ 0.50).
Facial expressions
Facial expression analyses revealed a trend toward BD patients generally exhibiting more fearful facial expressions when viewing film clips than HCs (F(1, 75) = 8.13, p = 0.08, ƞp 2 = 0.041), which rendered significant after adjustment for subsyndromal symptoms and years of education (F(1, 72) = 15.35, p = 0.01, ƞp 2 = 0.081; Figure 1). This increase in facial displays of fear occurred in the absence of differences between groups in their expressions of joy, surprise, anger, sadness, disgust, or engagement (ps ≥ 0.22). Due to technical issues, facial expression data were lost from one BD patient.

Figure 1. Patients with bipolar disorder (BD) generally exhibited more subtle fearful facial expressions when viewing emotional film clips compared to healthy controls (HCs). Values represent the mean percent fearful facial expressions in patients (BD) and HCs, respectively. Error bars represent standard error of the mean.
Self-reported positive and negative emotional reactivity
The results revealed a significant group by film clip interaction (F(6, 456) = 2.17, p = 0.045, ƞp 2 = 0.03) for self-reported positive emotional reactions to the film clips, which prevailed after adjustment for subsyndromal symptoms and years of education (p = 0.01). This was driven by less positive emotion ratings by patients with BD for the film clips depicting winning (t = 2.28, df = 76, p = 0.03, d = .52) and racing (t = 2.24, df = 70.15, p = 0.03, d = .51) and a trend toward less positive emotion ratings in response to the happy film clip (t = 1.85, df = 76, p = 0.07, d = .42). There were no significant differences between patients and HCs on self-reported positive reactions to sad, risk-taking, or socially anxious film clips (ps ≤ 0.15). However, there was a significant interaction for self-reported negative emotional reactivity (F(6, 456) = 2.88, p = 0.009, ƞp 2 = 0.04), which was driven by BD patients rating the film clip depicting risk-taking as eliciting more negative emotions (t = 2.03, df = 76, p = 0.046, d = .46) compared with HCs. This interaction was reduced to trend-level in a post hoc analysis adjusted for symptom severity and education (p = 0.10). No other group differences reached statistical significance (ps ≥ 0.12).
Correlations with medicine and subsyndromal symptoms
No significant correlations were found between medication status (whether patients were taking lithium, anticonvulsants, antidepressants, or antipsychotics) and self-reported emotional reactivity or gaze time (ps ≥ 0.08). Patients’ more fearful facial expressions when viewing the film clips depicting winning and racing was associated with use of anticonvulsants (winning: r = 0.34, p = 0.04; racing: r = 0.33, p = 0.045), while more fearful facial expressions when viewing the socially anxious film clip was associated with use of antidepressants (r = 0.44, p = 0.007).
Across all participants, more subsyndromal depressive symptoms were associated with (a) more fearful facial expressions during the socially anxious film clip (r = 0.24, p = 0.036); (b) less gaze time during the neutral (r = −0.23, p = 0.04), sad (r = −0.23, p = 0.04), racing (r = −0.29, p = 0.01), happy (r = −0.23, p = 0.04), and socially anxious (r = −0.41, p < 0.001) film clips; (c) less self-reported positive reactions to winning (r = −0.23, p = 0.04) and happy (r = −0.28, p = 0.01) film clips, and (d) increased self-reported negative reactions to risk-taking (r = 0.24, p = 0.03). Subsyndromal mania symptoms did not correlate with fearful facial expressions, gaze times, or self-reported emotional reactions (ps ≥ 0.25).
Discussion
The present study investigated for the first time eye movements, facial displays of emotion, and self-reported emotional reactivity in response to highly emotional film clips in remitted newly diagnosed BD patients compared with HCs. Consistent with our hypothesis, patients with BD gazed more away during all emotional film clips, although this finding rendered nonsignificant after adjustment for subsyndromal mood symptoms. In contrast, we found no support for our hypothesis that patients would display more extreme positive and negative emotional reactivity for the pleasant/thrill-related/risk-taking film clips and the aversive/sad/social anxiety film clips, respectively. Instead, patients demonstrated a general negative bias on both facial emotion recordings and self-reported emotional response, as reflected by stronger facial displays of fear during all emotional film clips combined with less positive emotion ratings in response to the winning and happy film clips and more negative emotion ratings for the risk-taking/thrill-related film clip.
The finding that patients gaze more away from emotional film clips is consistent with our previous finding from an overlapping cohort that patients gazed away from aversive pictures [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16]. As participants had been specifically instructed to keep their eyes on the screen even if the scenes played out were unpleasant, this could represent an implicit (nonconscious) emotion regulation strategy in these remitted patients that compensates for their heightened emotional reactivity. Our finding that this difference between BD patients and HCs disappeared after adjustment for subsyndromal depression and mania symptoms, coupled with the significant correlation between eye gaze and subsyndromal depressive symptoms, is also consistent with our previous finding that patients inclination to gaze away from aversive pictures was associated with subsyndromal symptoms [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16]. Also, research has suggested that depressed BD patients are less attentive to positive cues, as evidenced by studies showing that depressed BD patients were slower and less accurate at identifying happy words [Reference Holmes, Erickson, Luckenbaugh, Drevets, Bain and Cannon36], exhibited attentional inference by positive words [Reference Jongen, Smulders, Ranson, Arts and Krabbendam37], and spend less time gazing at happy images [Reference Garcia-Blanco, Salmeron, Perea and Livianos24]. Hence, possible implicit attempts to regulate emotional states by controlling gaze direction are likely a state-dependent feature related to subsyndromal symptoms rather than being a trait-related endophenotype of BD.
Few studies to date have investigated subtle behavioral responses to emotional stimuli in remitted patients with BD using facial emotion analysis techniques. Our results showing that patients with BD exhibited subtle aberrant facial expressions during emotional film clips are in accordance with previous studies that have found aberrant facial expressions, specifically increased surprised facial expressions during presentation of neutral and unpleasant images [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16] and less appropriate facial expressions when viewing emotional film clips in remitted patients with BD compared to HCs [Reference Bersani, Polli, Valeriani, Zullo, Melcore and Capra25]. This is consistent with the results of the present study, in which patients with BD expressed more fear, even during film clips considered positive (i.e., happy and winning). Interestingly, there was an association between greater use of anticonvulsants and more fearful facial expressions in patients with BD during the winning and racing film clips involving competition, risk-taking, and thrill-seeking behavior. It is well-established that there is a close association between facial displays of emotion and the emotion that is subsequently experienced at a conscious level [Reference James38]. It could therefore be speculated that this may be a neuropsychological mechanism by which anticonvulsants counteract escalation of positive emotion into manic episodes, although this post hoc finding should be interpreted with caution.
Results from previous studies investigating reactivity to emotional stimuli using self-report measures of valence in patients with BD have been inconsistent. In accordance with the results of our study showing no differences between patients and HCs on self-reported emotional reactivity to happy, sad, and neutral film clips, a majority of previous studies report no aberrant self-reported emotion reactivity to unpleasant, pleasant, and neutral images [Reference Aminoff, Jensen, Lagerberg, Andreassen and Melle11,Reference Broch-Due, Kjærstad, Kessing and Miskowiak16,39–41]. Other studies, however, have found that remitted patients with BD report greater positive emotional responses in response to happy, sad, and neutral film clips and neutral pictures, respectively [Reference Gruber, Harvey and Purcell42,Reference M'Bailara, Demotes-Mainard, Swendsen, Mathieu, Leboyer and Henry43]—possibly reflecting a state-dependent feature of mania only observable in samples of BD-I patients. Our study is the first to suggest that film clips associated with mania-like behavior, such as competition and thrill-seeking behavior (i.e., film clips depicting winning and racing) elicited less positive or more negative emotions in newly diagnosed remitted patients with BD compared to HCs.
Our results suggest that patients with BD demonstrated a trait-related negative bias—both at the overt self-report level and within more subtle measures of facial displays of emotion. This negative bias was evident in our sample of partially/fully remitted patients who were also newly diagnosed with BD, hence further supporting negative bias being trait-related in BD. Indeed, adaptive emotion processing depends on amygdala responses to negative information in healthy populations [Reference Dannlowski, Ohrmann, Bauer, Kugel, Arolt and Heindel44]. Yet hyper-activation of the amygdalae has been associated with trait-related negative bias during processing of neutral and sad faces and emotion regulation in BD (e.g., [Reference Townsend and Altshuler17,Reference Rich, Vinton, Roberson-Nay, Hommer, Berghorst and McClure45,Reference Almeida, Versace, Hassel, Kupfer and Phillips46]). Attentional bias toward negative emotions has also been extensively reported in patients with unipolar depression (UD) and therefore appears not to be specific to BD [Reference Miskowiak and Carvalho47]. In our patient sample, the majority (60.5%) suffered from BD-II, which—given its significant resemblance with UD—may explain the observed negative emotional bias. There is therefore a need for studies to delineate whether the negative bias in emotion reactivity pertains specifically to UD and BD-II patients or to mood disorders in general. Differences in aberrant emotional reactivity in patients with UD and BD-II compared with BD-I may not increase the diagnostic accuracy, which is mostly inappropriate for BD-II, but could help improve treatment selection for BD patients once they have been diagnosed similarly to the recent use of the polarity index [Reference Popovic, Reinares, Goikolea, Bonnin, Gonzalez-Pinto and Vieta48].
A strength of the present study was that it included a relatively large sample of patients and controls (N = 78) compared with previous studies (N = 30–46) [Reference Broch-Due, Kjærstad, Kessing and Miskowiak16,Reference Bersani, Polli, Valeriani, Zullo, Melcore and Capra25,Reference Gruber, Harvey and Purcell42,Reference Gruber, Hay and Gross49], and that patients were in either full or partial remission. Nevertheless, several limitations should be noted. First, a majority (66%) of the patient sample were taking psychotropic medication at time of testing. The use of antidepressants, antipsychotics, and anticonvulsants have been linked with unfavorable cognitive side-effects, in part because of their anticholinergic and antidopaminergic actions [Reference Cullen, Ward, Graham, Deary, Pell and Smith50,Reference Goldberg and Burdick51]. Yet, excluding medicated patients would yield a limited sample size (N = 13) as well as possibly reduce the generalizability of the results given the frequent use of psychotropic medication in BD. Second, not all patients were in full remission. However, post hoc sensitivity analyses in patients who were in full remission (defined as HDRS-17 and YMRS ≤7) revealed no changes in the results in comparison to the results of the analyses with adjustment for subsyndromal symptoms and education level. That is, for eye-tracking analyses, results rendered nonsignificant when limiting the sample to patients in full remission (p = 0.21). However, for facial expressions, there was a significant effect of group (F(1,60) = 4.29, p = 0.043), driven by remitted patients with BD exhibiting more fear when watching the film clips. For self-reported emotional reactivity, there was a significant interaction for positive emotions (F(4.5, 277.6) = 2.38, p = 0.04), driven by remitted patients with BD rating racing (t = 2.78, df = 60.9, p = 0.007) and winning (t = 2.09, df = 61, p = 0.041) as eliciting more negative emotions than HCs. All other effects of group remained nonsignificant (ps ≥ 0.10). Third, we did not adjust for multiple comparisons given the exploratory approach of the study. Results are therefore hypothesis-generating in nature. Fourth, the cross-sectional design of the study limits causal inferences. Fifth, a higher sampling rate of the eye-tracker as well as self-ratings of emotions before each emotional film clip would have given more detailed and precise results. Sixth, baseline self-reported emotions ratings conducted prior to the administration of the emotional film clips would have elucidated whether the results were attributable to the emotional film clips or reflected a stable emotion. Finally, given that the negativity bias found in patients with BD in the current study is typically considered a feature of UD, and that the most common misdiagnosis of BD is UD [Reference Goodwin and Jamison52], an additional limitation of this study is the lack of a UD comparison group. Future studies should investigate subtle behavioral responses during emotion reactivity between remitted patients with BD and UD versus HCs in order to elucidate whether our findings are BD-specific or a general feature of mood disorders.
Conclusions
In conclusion, the present study found subtle behavioral differences in facial expressions and self-report measures of emotions in response to emotional film clips between newly diagnosed remitted patients with BD and HCs. Specifically, patients with BD displayed a trait-related negative bias in facial expressions and self-rated emotional reactivity to happy/thrill-related/risk-taking film clips. The present preliminary findings suggest that inclusion of highly sensitive behavioral measures like eye-tracking and facial emotion analysis could have the potential to improve clinical decision making on diagnostic accuracy in the clinical settings. Future studies should therefore compare patients with BD to patients with UD to investigate whether these subtle differences are specific to BD or to mood disorders in general.
Conflict of Interest
K.M. has received consultancy fees from Lundbeck, Allergan, and Janssen in the past 3 years. L.V.K. has, within the preceding 3 years, been a consultant for Sunovion and Lundbeck. H.L.K., C.K.J., and I.B.-D. report no conflicts of interest. The study was funded by grants from the Mental Health Services, Capital Region of Denmark, The Danish Council for Independent Research, Medical Sciences (DFF-4183-00570), Weimans Fund, Markedsmodningsfonden (the Market Development Fund 2015-310), Gangstedfonden (A29594), Helsefonden (16-B-0063), Innovation Fund Denmark (the Innovation Fund, Denmark, 5164-00001B), Copenhagen Center for Health Technology (CACHET), EU H2020 ITN (EU project 722561), Augustinusfonden (16-0083), Lundbeck Foundation (R215-2015-4121). The sponsors had no role in the study design, collection, analysis, or interpretation of the data, in writing the report or in the decision to submit the work for publication.







Comments
No Comments have been published for this article.