Introduction
The ongoing severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) epidemic led to the implementation of mitigation strategies worldwide. To understand SARS-CoV-2 dynamics under various mitigation strategies, it is important to study the role of age because variations in transmission by age suggest differential impact of various measures such as physical distancing and workforce-related policies, which in turn has implications for epidemic control.
The rates of SARS-CoV-2 infection seem to vary by age. Serological studies found the highest seroprevalence in younger adults and older adolescents in England [Reference Ward1], Switzerland [Reference Stringhini2], Germany [Reference Streeck3] and New York State outside of the greater New York City area [Reference Rosenberg4]. A serological study in Spain found higher seroprevalence in older individuals than in younger ones [Reference Pollán5].
Control measures may have differential effectiveness in different age groups [Reference Zhang6], and the groups for which the measures are more effective may vary across populations. Social distancing seems to have been less effective among younger adults and older adolescents in Germany [Reference Goldstein and Lipsitch7] and among persons aged 50–59 years in the Netherlands [Reference Backer8].
In Spain, a national lockdown was instituted on 15 March and was further strengthened to include work restrictions to non-essential workers between 30 March and 14 April [9]. This intervention had a pronounced effect on transmission that led to decreasing case counts in all regions of the country. However, much less information is available on the relative incidence of different age groups during the different stages of the lockdown. For example, age groups that were overrepresented among those who continued non-remote work between 15 and 29 March could have had an increased relative incidence during that period.
Here, we estimate changes in detected incidence of SARS-CoV-2 infection by age group after the implementation of the different lockdown measures. We applied previously developed methodology [Reference Goldstein and Lipsitch7, Reference Goldstein10, Reference Goldstein11] to assess the changes in the incidence of age groups between 15 and 69 years after control measures were implemented in Spain.
Methods
Data sources
On 20 May we obtained information on coronavirus disease 2019 (COVID-19) cases reported from 1 March through 30 April to the National Epidemiological Surveillance Network (RENAVE) via the platform SiViEs (see Fig. 1, Results). We retrieved data on reported PCR-confirmed cases with available information on the date of symptoms onset. We excluded healthcare workers due to significant non-community transmission among them.
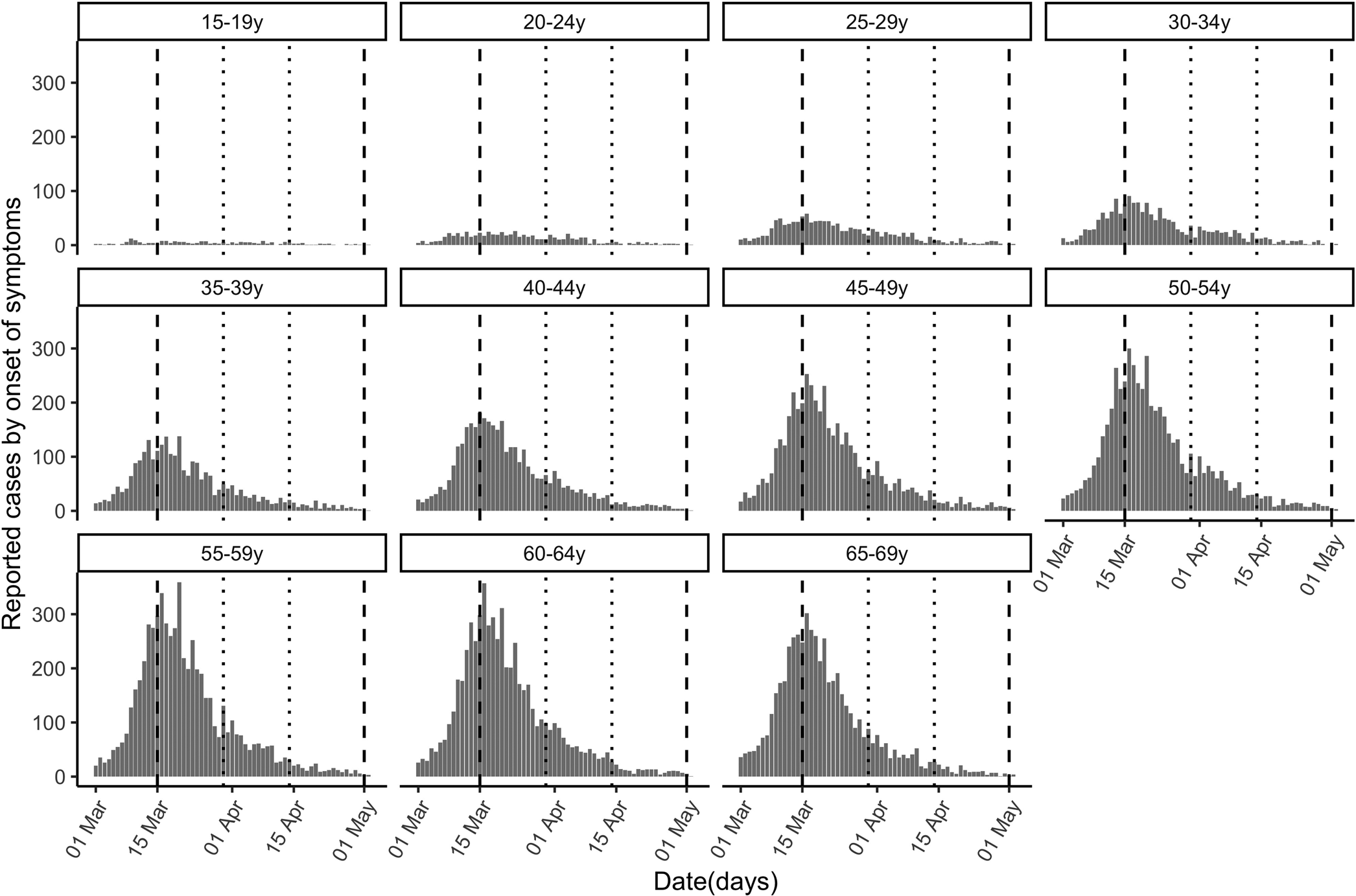
Fig. 1. Cases of COVID-19 (reported by the day of symptom onset) by age group between 1 March and 30 April, 2020 in Spain. Vertical dashed lines demarcate the complete lockdown period (15 March−30 April) and dotted lines the strengthened lockdown period (30 March−14 April).
Relative change in SARS-CoV-2 infection by age group
Daily counts (by date of symptom onset) of reported COVID-19 cases in individuals aged 15 through 69 years are plotted in Figure 1. We excluded children under 15 years because of potential temporal changes in diagnosis/ascertainment of cases, and individuals 70 years and older because significant non-community transmission of infection in long-term care facilities (LTCFs) likely affected their relative share of cases [12–14]. In sensitivity analyses, we restricted the calculations to (a) two regional clusters to explore heterogeneity based on seroprevalence levels and (b) hospitalised cases to evaluate the impact of potential changes in ascertainment, under the assumption that the detection of severe cases requiring hospitalisation is relatively insensitive to changes in diagnostic criteria.
We selected three periods: 1–10 March (five days before the national lockdown, as some social distancing started on 10 March), 25 March−3 April (starting ten days after implementation of the initial lockdown to detect changes in symptoms onset) and 8–17 April (starting ten days after implementation of the strengthened lockdown). During the initial lockdown, but not during the strengthened lockdown, non-essential workers were allowed to work when remote work was not possible [9].
Using the methodology in [Reference Goldstein and Lipsitch7], we computed the age-specific proportion ratios for each of the lockdown periods (25 March−3 April or 8–17 April) relative to the pre-lockdown period (1–10 March) as follows: for each age group g, let E(g) be the number of COVID-19 cases in age group g and $\sum\nolimits_h {E\lpar h\rpar }$![]() the total number of cases in age groups h = 1 (15–19 years) to h = 11 (65–69 years) during the pre-lock down period. Similarly, let L(g) and $\sum\nolimits_h {L\lpar h\rpar }$
the total number of cases in age groups h = 1 (15–19 years) to h = 11 (65–69 years) during the pre-lock down period. Similarly, let L(g) and $\sum\nolimits_h {L\lpar h\rpar }$![]() be the corresponding numbers during the initial lockdown. The proportion ratio (PR) statistic in age group g is
be the corresponding numbers during the initial lockdown. The proportion ratio (PR) statistic in age group g is
that is, the ratio between the proportion of cases in age group g in the initial lockdown period and the proportion of cases in age group g in the pre-lockdown period. We repeated these calculations after replacing the cases in the initial lockdown period by the cases in the strengthened lockdown period.
In sensitivity analyses, we computed the age-group PR statistic (a) disaggregating the cases in two groups depending on regional seroprevalence estimates [Reference Murtagh and Legendre15] and (b) including only hospitalised cases, which would be less affected by potential changes in ascertainment given higher severity. The Supplementary Material describes the methods to obtain confidence bounds for the PR statistic [Reference Altman16].
Results
Table 1 classifies the confirmed cases by age group and period. The higher number of cases in older individuals compared with younger ones does not necessarily reflect differences in incidence because infections are more severe in older individuals, and the likelihood of reporting of infection is higher for severe infections. Figure 1 plots the epidemic curves of daily COVID-19 cases by age group (15–69 years) between 1 March and 30 April, 2020 (n = 73 650).
Table 1. Number of reported COVID-19 cases with available information on the date of symptom onset in different age groups for different time periods
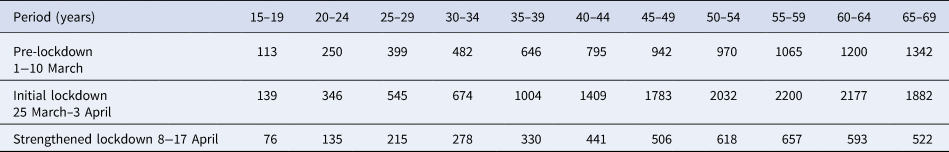
Case counts increased a great deal with age, likely reflecting higher case ascertainment for older individuals.
After the initial lockdown period
Figure 2 plots the PR estimates for the initial lockdown period compared with the pre-lockdown period. The highest estimates correspond to individuals aged 50–54 years (PR = 1.21; 95% CI 1.12,1.30) and 55–59 years (PR = 1.19; 1.11,1.27). PR estimates for individuals aged 15–44 years and 60–69 years were significantly lower than the estimates for those aged 50–59 years (Supplementary Material, online Supplementary Table S2).
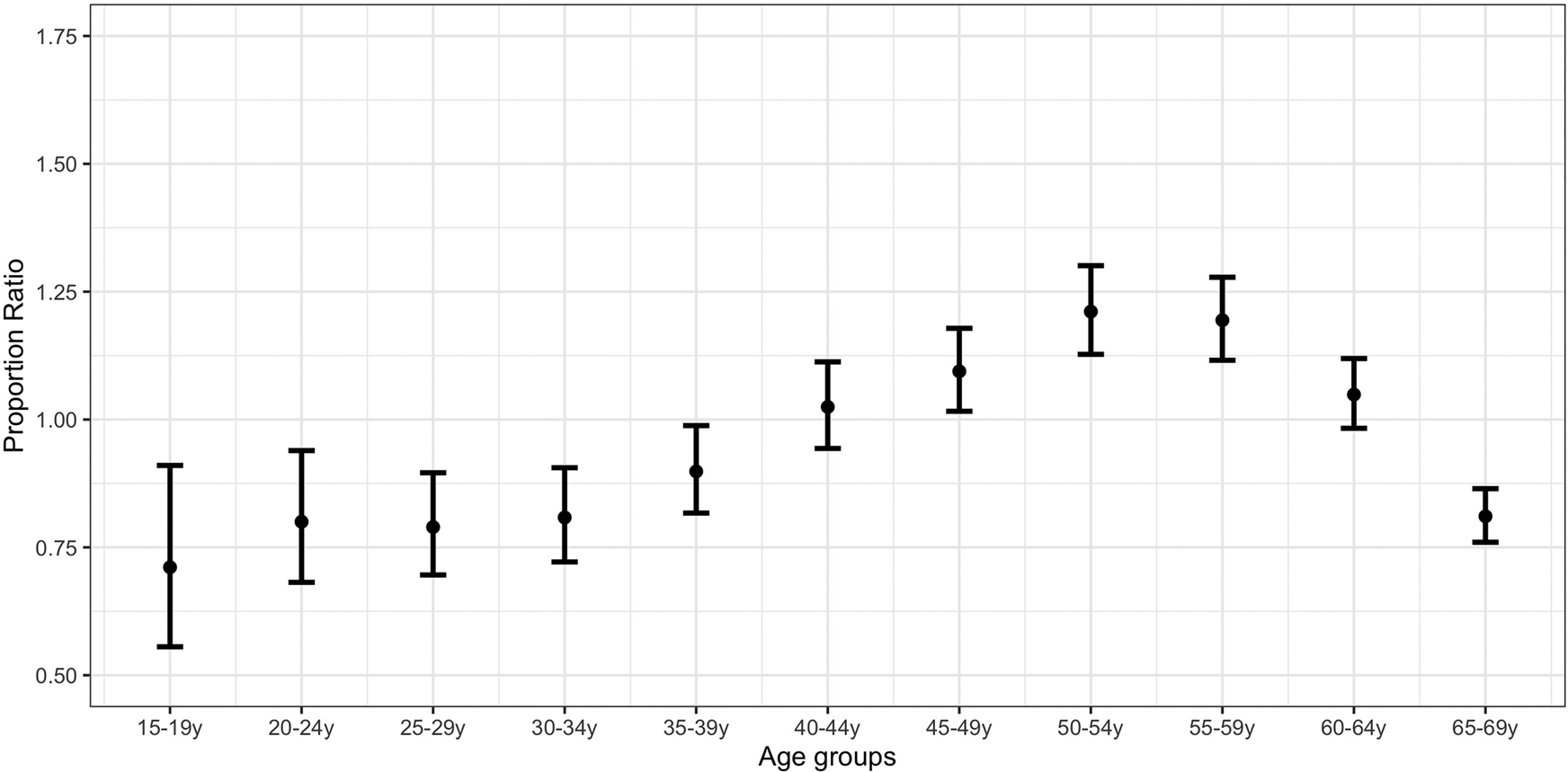
Fig. 2. Proportion ratio estimates of confirmed COVID-19 cases by age group in Spain for the period 25 March–3 April vs. 1−10 March.
After the strengthened lockdown period
Figure 3 plots the PR estimates for the strengthened lockdown period vs. the pre-lockdown period. The highest estimates correspond to individuals aged 15–19 years (PR = 1.26; 0.95,1.68), 50–54 years (PR = 1.20; 1.09,1.31) and 55–59 years (PR = 1.16; 1.06,1.27). PR estimates for individuals aged 50–54 years were significantly higher than the estimates for those aged 35–49 years and 60–69 years (Supplementary Material, online Supplementary Table S3).
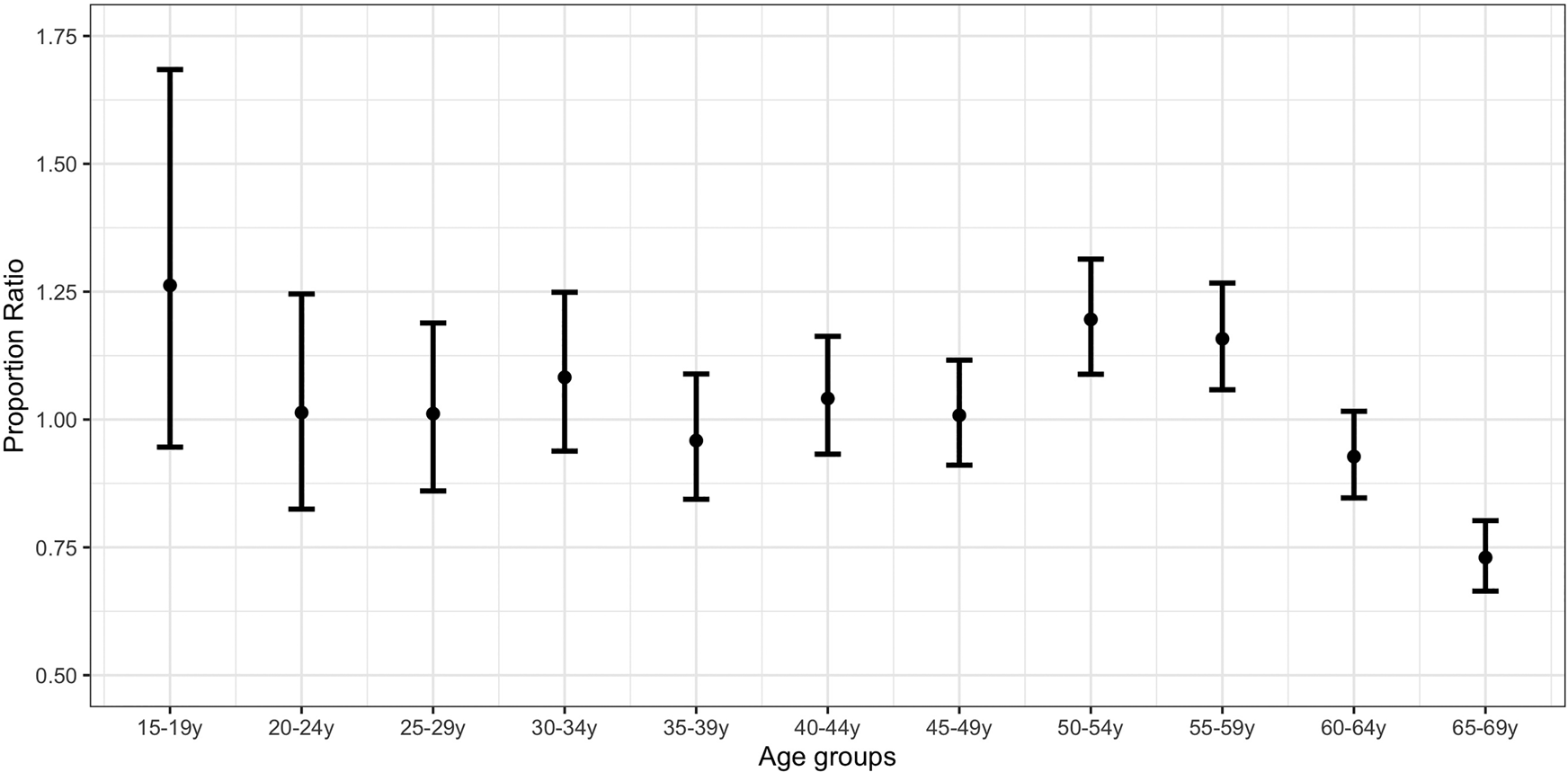
Fig. 3. Proportion ratio estimates of confirmed COVID-19 cases by age group in Spain for the period 8−17 April vs. 1−10 March.
A comparison of Figures 2 and 3 suggests an increase in the proportion of COVID-19 cases for the strengthened lockdown period compared with the initial lockdown period in younger age groups (up to 34 years) relative to the middle ones (35–64 years).
In sensitivity analyses, PR estimates did not materially change across regions with different seroprevalence or when we restricted the analysis to hospitalised cases (Supplementary Material, Sections S1–S2).
Discussion
We applied the methodology described in [Reference Goldstein and Lipsitch7, Reference Goldstein10, Reference Goldstein11] to show that the relative incidence of detected SARS-CoV-2 infections in different age groups changed during the lockdown periods in Spain. Compared with the pre-lockdown period, individuals aged 40–64 years, and particularly those aged 50–59 years, had an elevated relative incidence during the first lockdown period. The corresponding relative incidence of younger adults/older adolescents, as well as persons aged 50–59 years, increased during the strengthened lockdown period compared with the pre-lockdown period.
These differences by age group might be explained by a number of factors. First, the elevated relative incidence in middle-aged adults during the first lockdown period, when non-essential workers were allowed to work, is consistent with the higher employment rates in Spain in middle-aged adults compared with younger adults [17]. Second, adherence to social distancing may vary with age: relative increases for younger adults/older adolescents during the second lockdown period might reflect lower adherence to lockdown measures, whereas perception for risk of severe disease could have led to stronger individual adherence for persons aged 60–69 years. Finally, household transmission in the context of the high proportion of multigenerational households in Spain may also have contributed to the observed patterns. Further work is needed to understand those issues to better inform future mitigation efforts.
Our results are aligned with findings in other European countries. Persons aged 50–59 years were least impacted in terms of mixing/number of contacts with people following the introduction of social distancing measures in the Netherlands [Reference Backer8], which is in agreement with the elevated relative incidence in persons (excluding healthcare workers) aged 50–59 years throughout the lockdown period in Spain. Our findings for the strengthened lockdown period in Spain echo those from other countries: a higher relative incidence of infection in younger persons was found in England [Reference Ward1], Switzerland [Reference Stringhini2] and Germany (Figure 6A in [Reference Streeck3]), and the highest proportion ratio estimates for the post lockdown period in Germany are in younger persons [Reference Goldstein and Lipsitch7].
Our findings could be affected by age-differential changes in case ascertainment, over time or across regions. However, there is no evidence of fundamental diagnostic changes for the adult population during the lockdown period in Spain, where the focus was on testing the more severe cases. Moreover, our sensitivity analysis restricted to hospitalised cases, which are less likely affected by changes in ascertainment, as well as region-specific analyses (with splitting into regions reflecting places with higher and lower-medium seroprevalence) yielded estimates that were consistent with those of the main analysis.
In summary, our paper provides evidence for an elevated relative incidence of individuals aged 40–64 years during the initial lockdown period, when non-essential work was allowed, and for an elevated relative incidence of younger adults/older adolescents when only essential workers continued to work. These results suggest that age structure is an important factor in the effect of lockdown interventions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820002551
Data availability
The data that support the findings of the study are available as aggregates in Table 1 of the main text (and at the end of the supplementary material, online Supplementary Table S1 for sensitivity analysis). Line list data are available upon request from the National Center of Epidemiology, Carlos III Institute of Health, Madrid, Spain.
Acknowledgements
We thank all the contributors to the surveillance and investigation of COVID-19 epidemic in Spain, including colleagues at local and regional Public Health Services, as well as the Centre of Health Alerts and Emergencies at the Ministry of Health, the National Centre of Epidemiology and the National Centre of Microbiology at the Carlos III Institute of Health.
Financial support
P.M.D. was supported by the Fellowship Foundation Ramon Areces. Marc Lipsitch and Edward Goldstein were supported by Award Number U54GM088558 from the National Institute of General Medical Sciences and Lipsitch also by the Morris-Singer Fund. The content is solely the responsibility of the authors.
Conflict of interest
None







