CLINICIAN'S CAPSULE
What is known about the topic?
Personal mobile device use to record patient data is prevalent throughout hospitals and may conflict with patient privacy.
What did this study ask?
To what extent and purpose do Canadian physicians and residents use personal mobile devices to record patient data in the emergency department?
What did this study find?
Clinicians who use personal mobile devices to record patient data believing it is beneficial to patient care may be unaware of relevant regulations.
Why does this study matter to clinicians?
Personal mobile device recording of patient data is common, so further research is needed to determine patient benefit while maintaining patient confidentiality.
BACKGROUND
Personal mobile devices are prevalent throughout hospitals.Reference Koehler, Vujovic and McMenamin1 Use of personal mobile devices within the clinical setting can improve patient careReference O'Reilly, Nason and Liddy2 including documentation of treatmentReference Lund, Joo, Lewis, Arikan and Grunfeld3 and imaging transfer.Reference Bullard, Rosenberg and Ladde4
Although patients are receptive to personal mobile device use,Reference Sikka, Carlin and Pines5 concerns around privacy and confidentiality have been raised.6 Previous research has shown a lack of awareness of, or adherence to, relevant guidelines, often resulting in breaches of patient confidentiality.Reference Kornhaber, Betihavas and Baber7 However, research on personal mobile device use in the emergency department (ED) is limited.Reference Kornhaber, Betihavas and Baber7 A UK study on photo-documentation found 45.2% of EDs did not have relevant policies in place, and only 7.5% documented consent for use.Reference Bhangoo, Maconochie, Batrick and Henry8
The purpose of this study was to determine the frequency of personal mobile device use to record patient data by emergency physicians (EPs) and emergency medicine residents and to investigate current practices.
METHODS
Online survey instrument and design
The University of British Columbia (UBC) Survey Tool, which is Freedom of Information and Privacy Protection Act (FIPPA) compliant, was used to capture anonymous, confidential data from respondents.
Thirteen questions were captured: demographics, personal mobile device use to record patient data and rationale, consent, documentation format, confidentiality, and limitations (Supplementary Material). Online questions were coded into numerical data or left in an open-text format as applicable. Non-responses were excluded from analysis.
Ethics and approval
The UBC Research Ethics Review Board approved the study, and consent was assumed based on participation after reading the supplied information.
Distribution and sampling
The format followed standard physician survey guidelines.Reference Burns, Duffett and Kho9 From February 27, 2017, to March 23, 2017, the Canadian Association of Emergency Physicians (CAEP) sent three email blasts to consenting CAEP members. Documents were provided in French and English.
Data analysis
Descriptive statistics are reported as frequencies and percentages. Open-text responses were thematically analyzed by reviewers and reported qualitatively.
RESULTS
One thousand eight-hundred CAEP members who agreed to participate in research surveys were contacted. Of these, 415 responded (23.1%). Nine responses were excluded owing to insufficient data, resulting in 406 participants. The majority of EP and resident respondents had formal training in emergency medicine (EM; 80.3% and 78.2%, respectively) and worked in large urban academic hospitals (56.5% and 78.7%, respectively) (Supplementary Material).
Participant use of personal mobile devices to record patient data in the ED is presented in Table 1. Overall, 31.5% (95% confidence interval [CI] 27.0%–36.3%) of EPs and residents surveyed reported using personal mobile devices to record patient data, at least monthly by 78.1% and every shift by 7.0%. The reported use of personal mobile devices to record patient data was most common for communication with consultants (78.1%) and medical education (65.6%). Most reported their personal mobile device is password protected, and they used the native application of their personal mobile device to record patient data (89.8% and 76.6%, respectively).
Table 1. The use of personal mobile devices
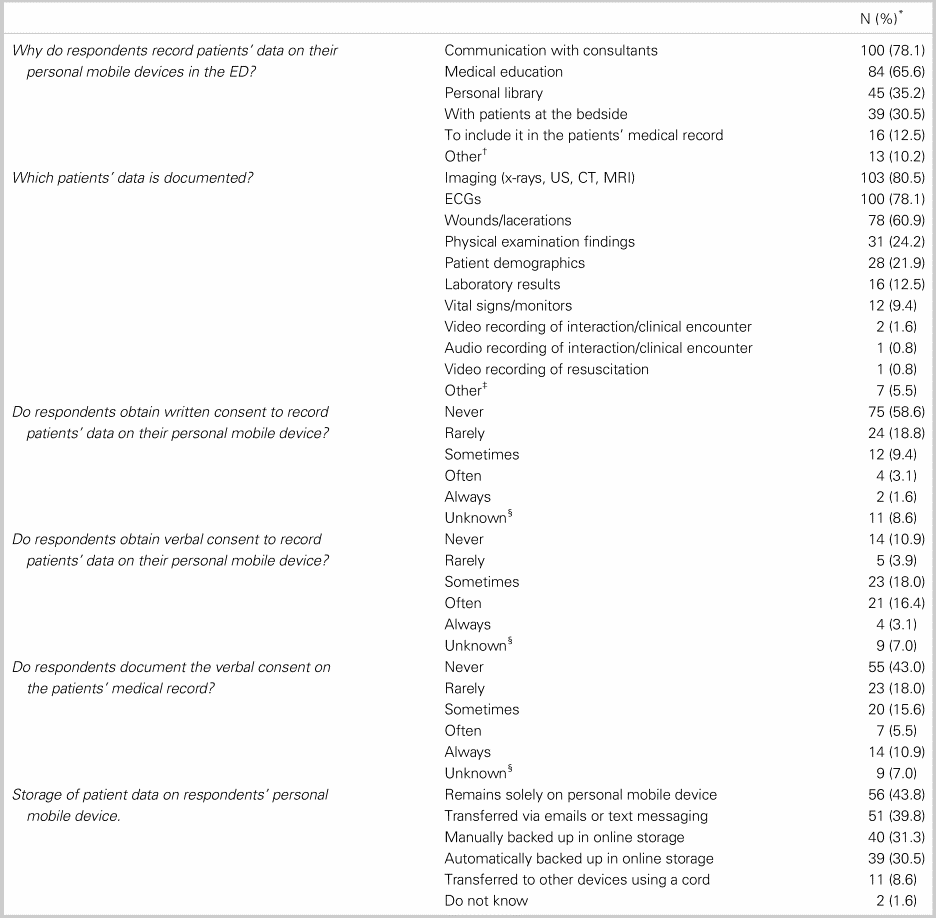
CT = computed tomography; ECG = electrocardiogram; MRI = magnetic resonance imaging; US = ultrasound.
*% = N/128 (Total number of participants who responded to these questions).
†Ultrasound log; research; follow-up; for delayed input in electronic medical record (EMR); and communication with RNs.
‡Census of ED.
§Question unanswered.
Of participants who reported using personal mobile devices to record patient data, 77.8% (95% CI 69.1%–84.3%) indicated that they obtained written consent rarely or never. Participants reported obtaining verbal consent sometimes (18.0%), often (16.4%), or always (43.8%) and documenting this consent rarely (18.0%) or never (43.0%). Fourteen participants (10.9%) reported not obtaining any consent.
Approximately one-third of participants (36.7%) indicated that using personal mobile devices to record patient data improves the care provided by consultants and expedites management (31.3%). In qualitative responses, many suggested personal mobile device use to record patient data improves patient flow by promoting efficient communication with consultants.
Approximately one-third of participants (32.8%) indicated that using personal mobile devices to record patient data aids medical education. Recurrent descriptive themes of responses included using personal mobile device recorded data for general teaching, formal didactic sessions, or presentation of cases.
A minority of participants believed that using personal mobile devices to record patient data did not (9.4%) affect care or have a negative effect (5.2%) on care. Qualitatively, participants described these negative effects as time-consuming, distracting, or threatening privacy and confidentiality.
Over one-half of the participants (53.2%) were unaware of regulations at their institution, and of those who were, 19.7% felt restricted by them. Most participants suggested that changes to regulations or physician practice patterns may allow improved use of personal mobile devices to record patient data.
DISCUSSION
To our knowledge, this is the first study investigating EP and resident use of personal mobile devices for recording patient data in Canadian EDs. It is already known that personal mobile device use is ubiquitous in the medical community.Reference Koehler, Vujovic and McMenamin1,Reference Dexheimer and Borycki10
Most participants indicated that recording patient data with personal mobile devices is beneficial for a number of reasons. Few participants indicated personal mobile device use to record patient data is detrimental, and most concerns were related to privacy and confidentiality. Patient data recorded on personal mobile devices included information that can be difficult to describe or needs specific expertise to interpret. Recording patient data for medical education purposes is frequent and included rare conditions or presentations to which learners may not otherwise be exposed. When recording patient data with a personal mobile device, participants indicated they were unlikely to obtain written consent, whereas seeking verbal consent was more common, but rarely documented. Our findings suggest that the majority of Canadian EPs and residents do take some steps to secure the information on their devices. Consistent with research in other disciplines, over one-half of the participants were unaware of regulations regarding personal mobile device use to record patient data.Reference Kornhaber, Betihavas and Baber7
Canadian EPs and residents should be aware of several privacy issues surrounding personal mobile device use to record patient data in the ED. The Canadian Medical Protective Association (CMPA) advises that consent for recording patient data should always be obtained and documented, and extra care applies with non-encrypted formats.6 The Federal Personal Information Protection and Electronic Documents Act and individual provincial legislation state that recorded patient information should be securely stored and shared within Canada that is not the case with many personal mobile devices.11 This is a dynamic field in which technology may provide some solutions. For example, ShareSmart™ and Hypercare© are recently released Canadian personal mobile device applications that are compliant with privacy legislation.12,13 These applications have encrypted texting platforms and the ability to include consent with documentation.
LIMITATIONS
The major limitation of our study was the low response rate and resulting potential for nonresponse bias. When our demographics were compared with those from a recent comprehensive national study, work environment appeared representative; however, residents and EM-certified physicians appeared over-represented in our sample.14 The resident responses might not completely reflect those in EM as the CAEP database includes non-EM residents. Our low response rate, as well as the over-representation of certain groups, may reduce the generalizability of our results. In addition, the potential for sampling bias exists because of the limitations of contacting physicians. Our study was also limited to a Canadian population, where laws, guidelines, and regulations differ from those of other countries. To ensure anonymity, limited demographic information was obtained that precluded our ability to carry out detailed, categorized analyses. Despite being an anonymous survey, the potential for social desirability bias in responses exists.
CONCLUSIONS
Personal mobile device use by EPs and residents for recording patient data is common in Canadian EDs, both for communication with consultants and medical education purposes. Most Canadian EPs and residents believe personal mobile devices use to record patient data positively influences patient care. Further studies are required to determine if patient care improves with the use of personal mobile devices to record patient data and whether this involves privacy breaches.
Acknowledgements
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2019.29
Competing interests
None.



