Introduction
The assembly rules for temporal and environmental gradients in biological communities are strongly influenced by evolutionary and ecological forces, which can also affect the composition of parasite communities (Morand et al., Reference Morand, Simkova, Matejusova, Plaisance, Verneau and Desdevises2002; Warburton et al., Reference Warburton, Van Der Mescht, Khokhlova, Krasnov and Vonhof2018). Host–parasite relationships can be described based on complexity since, although hosts can be considered as habitats, they are not passive receivers of their parasites (Warburton et al., Reference Warburton, Van Der Mescht, Khokhlova, Krasnov and Vonhof2018), raising the issue of which factors should be considered in order to better understand helminth composition in their hosts.
Some studies have shown that phylogenetic relatedness in fish and lizard communities was the most important variable for explaining parasitic community composition (Muñoz et al., Reference Munõz, Grutter and Cribb2005; Brito et al., Reference Brito, Corso, Almeida, Ferreira, Almeida, Anjos, Mesquita and Vasconcellos2014). These results illustrate that hosts which are phylogenetically closer are more likely to share the same set of parasites, which may be related to niche constraints (Lima et al., Reference Lima, Giacomini, Takemoto, Agostinho and Bini2012). Such patterns are shaped by coevolution, a complex process that mutually matches both host and parasite life cycles (Marcogliese, Reference Marcogliese2004).
When species share the same resources, competition can lead to segregation or exclusion (Gause, Reference Gause1932; Winemiller & Pianka, Reference Winemiller and Pianka1990); thus, coexistence is only possible when the differential use of resources occurs (Vitt, Reference Vitt1981). Anurans commonly share resources due to phylogenetic limitations (Inger, Reference Inger1969; Eterovick & Sazima, Reference Eterovick and Sazima2000; Toledo et al., Reference Toledo, Zina and Haddad2003; Prado & Pombal, Reference Prado and Pombal2005). Protázio et al. (Reference Protázio, Albuquerque, Falkenberg and Mesquita2014) demonstrated the phylogenetic conservatism of spatial niches in an anuran taxocoenosis in the Caatinga biome, northeastern Brazil, dividing Hylidae and Leptodactylidae into arboreal and semiaquatic species. This pattern demonstrates that phylogenetic conservatism plays an important role in the actual functions displayed by the species in a taxocoenosis (Webb et al., Reference Webb, Ackerly, McPeek and Donoghue2002; Cooper et al., Reference Cooper, Jetz and Freckleton2010; Protázio et al., Reference Protázio, Albuquerque, Falkenberg and Mesquita2014). Therefore, we could expect that host traits would be reflected in parasitic species’ compositions.
Anurans use a great variety of microhabitats all over the world, influencing the assembly patterns of their parasitic communities (Poulin & Morand, Reference Poulin and Morand2004). Aho (Reference Aho, Esch, Bush and Aho1990) and Bush et al. (Reference Bush, Aho and Kennedy1990) also highlighted the influence of aquatic environments on intensity of infection. According to Poulin (Reference Poulin1995), parasite community compositions are a result of interactions between the evolutionary history and ecological characteristics of the hosts. Sympatric and phylogenetically closer hosts with similar ecologies, are expected to have more similar parasite communities compared to allopatric and phylogenetically distant hosts (Muñoz et al., Reference Munõz, Grutter and Cribb2005).
In the Caatinga biome, studies on helminth fauna associated with anurans are still scarce, comprising a few recent studies (e.g. Teles et al., Reference Teles, Cabral, Araujo Filho, Dias, Ávila and Almeida2014, Reference Teles, Sousa, Teixeira, Silva, Oliveira, Silva and Ávila2015, Reference Teles, Brito, Araújo Filho, Ribeiro, Teixeira, Mesquita and Almeida2018; Alcantara et al., Reference Alcantara, Ferreira-Silva, Silva, Lins, Ávila, Morais and Silva2018; Madelaire et al., Reference Madelaire, Franceschini, Morais, Gomes and Silva2020). Madelaire et al. (Reference Madelaire, Franceschini, Morais, Gomes and Silva2020) evaluated the influence of seasonality on parasite community composition in three anuran species (Rhinella diptycha, Rhinella granulosa and Pleurodema diplolister), however no influence was detected. These authors also observed similarities between the parasite fauna of the studied hosts; however, their analyses did not elucidate the roles of host ecology and phylogeny on parasite composition. Therefore, there is a need to analyse the relative effect of ecological and/or historic factors on the assembly patterns of parasite communities. Thus, the main aim of this study was to describe the composition of endoparasites found in an anuran assemblage in the Caatinga biome and report their ecological data (e.g. abundance, prevalence and intensity of infection). Our study also aimed to verify the influence of host size, body mass, body volume and sex on parasite abundance, as well as the relative influence of host ecological and historical factors and microhabitat use on parasite community composition.
Material and methods
Study area
Anurans were collected in the municipality of Granjeiro, Ceará State, northeastern Brazil (06°53′S, 39°13′W). This area has a warm tropical and semi-arid climate, with an average rainfall of 1236.6 mm per year and a temperature of 24–26°C. The local vegetation is part of the Caatinga biome, which comprises dense shrub vegetation and thorny deciduous forest (Instituto de Pesquisa e Estratégia Econômica do Ceará, 2015).
Host sampling and microhabitat
Different anuran species were collected during two field trips performed in February and March 2018. The primary sampling method was active searching near temporary ponds, lakes and waterlogged areas, performed during the night from 18:00 to 22:00 h (Crump & Scott, Reference Crump, Scott, Heyer, Donnelly, Mcdiarmid, Hayek and Foster1994). Substrate type was recorded for every collected individual, such as exposed soil, perched on shrub, perched on grass, on a macrophyte, on rocks, on a fallen tree trunk, on leaf-litter, at the water's edge and in the water. We computed microhabitat niche breadths (B) using the inverse of Simpson's (Reference Simpson1949) diversity index:
where p is the proportion of microhabitat category i and n is the number of categories.
The anurans were euthanized with a lethal dose of lidocaine hydrochloride 2%. The collection and use of frogs in the present study was authorized by the Instituto Chico Mendes de Conservação da Biodiversidade ICMBio (62017-1) and by the ethics committee of Universidade Regional do Cariri (00202/2018.2). The anurans were weighed and snout–vent length (SVL), body height and body width were measured with a digital caliper (accuracy ± 0.5 mm). To estimate body volume for each specimen, the volume formula of a parallelepiped was used, multiplying the SVL by body height and width. The specimens were fixed using 10% formalin and conserved in 70% ethanol. Voucher specimens were deposited in the Herpetological Collection of the Universidade Regional do Cariri.
Parasite sampling
The anuran specimens were dissected under a stereomicroscope, sexed and the respiratory, gastrointestinal and body cavities were surveyed for endoparasites. Parasites were mounted on slides with lactophenol and analysed under a light microscope (Zeiss, Imager M2). Parasite abundance (total number of parasites, regardless of species, in a single infected host), prevalence (total number of infected hosts divided by the total number of hosts in the sample × 100) and mean intensity of infection (total number of parasites found in a sample divided by the number of hosts infected with that parasite) were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). All means appear as ± 1 standard deviation.
Statistical analyses
To investigate whether parasite abundance was related to SVL, body mass, body volume or host sex, two analyses using generalized linear mixed models (GLMMs) were performed. In the first model, SVL, body mass and body volume were included as fixed effects, and anuran species (this category allowed us to remove the ontogenetic effect of each anuran species, as well as the effect of anuran abundance) and sex (this category allowed us to remove the effect that host sex may exert on this model) were included as random effects. In the second model, the sex of the host was included as a fixed effect, while anuran species was included as a random effect. In both models, parasite abundance was tested with a Poisson distribution and log link function (Wilson & Grenfell, Reference Wilson and Grenfell1997). GLMMs were performed using the ‘lme4’ package (Bates et al., Reference Bates, Mächler, Bolker and Walker2014) of R software.
To better study potential influences of ecological/historical factors and host microhabitat use on endoparasite composition, a phylogenetic principal component analysis (pPCA) was performed (Jombart et al., Reference Jombart, Dray and Bilgrau2017) using the Adephylo package (Jombart et al., Reference Jombart, Dray and Bilgrau2017) in R software (R Core Team, 2018). For this analysis, species with n < 5 individuals were excluded (Proceratophrys aridus and Leptodactylus troglodytes). The pPCA is a multivariate method that tests phylogenetic autocorrelation (Gittleman & Kot, Reference Gittleman and Kot1990). To perform this test, two matrices (X and W) were built. The X matrix containing p quantitative traits measured (endoparasite prevalence and amphibian microhabitat niche breadth) on n taxa, and a matrix W disposed the phylogenetic matrix with the sampled host species (with the respective phylogenetic distances in the cells).
The aim of this analysis is to find combinations of life history traits that exhibit a high level of variance and exhibit global or local structures (Jombart et al., Reference Jombart, Pavoine, Devillard and Pontier2010). Global structures result in patterns of trait similarity between related taxa which, according to ecological niche theory, indicates the prevalence of historical factors in assemblage structure (Winemiller & Pianka, Reference Winemiller and Pianka1990). In the pPCA the presence of global structures is represented by positive eigenvalues (Jombart et al., Reference Jombart, Pavoine, Devillard and Pontier2010). On the other hand, local structures are consequences of relatively recent events that result in the divergence of evolutionary strategies between closely related taxa, indicating the greater importance of ecological factors in assemblage structure (Winemiller & Pianka, Reference Winemiller and Pianka1990). In the pPCA the presence of local structures is represented by negative eigenvalues (Jombart et al., Reference Jombart, Pavoine, Devillard and Pontier2010). To perform this analysis, we used one of the most recent phylogenetic distances for anurans (fig. 1; Pyron & Wiens, Reference Pyron and Wiens2011). Taxa occurring in this geographical delimitation which were not included in the respective phylogeny were replaced with close relatives, assuming that adequacy does not influence the results, since more marked evolutionary changes must occur in the most basal nodes of the tree (Roelants et al., Reference Roelants, Gower, Wilkinson, Loader, Biju, Guillaume, Moriau and Bossuyt2007).

Fig. 1. Phylogenetic tree of the studied anuran assemblage, obtained from Pyron & Wiens (Reference Pyron and Wiens2011).
Results
A total of 288 individuals from 13 anuran species and five families (table 1) were collected, of which 110 were parasitized (4447 specimens of parasites), with an overall prevalence of approximately 38% and a mean infection intensity of 40 ± 38.6 parasites. The observed nematodes belong to seven different families and were identified as: Aplectana membranosa Miranda, 1924 (n = 1196); Cosmocerca sp. Travassos, 1925 (n = 56); Raillietnema spectans Gomes, 1964 (n = 119); Rhabdias fuelleborni Travassos, 1926 (n = 998); Oswaldocruzia mazzai Travassos, 1935 (n = 541); Schrankiana sp. Strand, 1942 (n = 4); and larvae of Physaloptera sp. (Pinto et al., 1994). Additionally, the digenean species, Glypthelmins pseudium Mañé-Garzón & Holcman-Spector, 1967 (n = 4) and a non-identified cestode (n = 70) were found. The prevalence and mean intensity of infection values were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997), as shown in table 1.
Table 1. Endoparasites recorded in individuals of an anuran assemblage from Granjeiro, Ceará state, north-eastern Brazil.

Abbreviations: P, prevalence; MII, mean intensity of infection; IS, infection sites; SI, small intestine; LI, large intestine; S, stomach; L, lungs.
The GLMMs indicated that SVL (Z = 1.34; R 2 = 0.99; P = 0.17), body mass (Z = 1.39; R 2 = 0.996; P = 0.16), body volume (Z = 1.258; R 2 = 0.996; P = 0.2) and host sex (Z = −0.35; R 2 = 0.99; P = 0.73) did not alter parasite abundance.
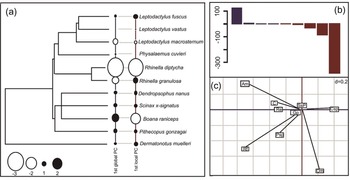
Both global and local phylogenetic structures were found by pPCA in the endoparasite and microhabitat data. For the former, the local structure (recent/ecological factors) was identified as having a greater influence on the organization of the infection patterns observed in amphibians, since the negative eigenvalues (−384.7) were much greater than the positive eigenvalues (125.2) (fig. 2B). Regarding the first global component, the frog species R. diptycha and R. granulosa, had the most negative scores compared to the rest of the tree (taxa with positive scores or close to zero), while the species Boana raniceps had the least similar life history values (fig. 2A). Among the other species, Leptodactylus macrosternum was also distinguished by its negative score. The loadings of the analyses (fig. 2C) showed that the endoparasite species A. membranosa and R. fuelleborni, exerted greater influence on the global negative axis, where the frog species R. diptycha was primarily associated with R. fuelleborni. The unidentified species of Cestoda exerted an intermediate influence in relation to the negative axis, presenting a historical association mainly with R. granulosa. On the other hand, the global positive axis was mainly influenced by the nematode O. mazzai, presenting a close association with the frog species B. raniceps.

Fig. 2. Phylogenetic principal component analysis for the endoparasites of an anuran assemblage from the Caatinga. On the left is the phylogenetic tree built for the assemblage with the 1st global principal component (PC) and 1st local PC. Negative and positive scores were indicated for the white and black circles, respectively. Circle size is proportional to the values of the scores. On the right are the loadings of the first historical (blue) and ecological (red) components. Endoparasites studied: Aplectana membranosa (Am), Cosmocerca sp. (Csp), Physaloptera sp. (Psp), Schrankiana sp. (Ssp), Rhabdias fuelleborni (Rf), Raillitnema spectans (Re), Oswaldocruzia mazzai (Om), Glypthelmins pseudium (Gp) and Cestoda (C).
Regarding the first local component, the frog species R. diptycha and B. raniceps presented the highest negative scores, while R. granulosa, Dendropsophus nanus and Dernatonotus muelleri presented opposite ecological values (low or intermediately positive scores) (fig. 2A). The loadings of the analyses (fig. 2C) showed that the nematodes O. mazzai, R. fuelleborni and Physaloptera sp., exerted the greatest influence on the local negative axis (closer to the axis and far from zero), where the former two species showed a close association with the frog species R. diptycha and the latter was associated with B. raniceps. The positive local axis was moderately influenced by the taxa Schrankiana sp. and Cestoda, presenting ecological associations with Scinax x-signatus and R. granulosa, respectively.
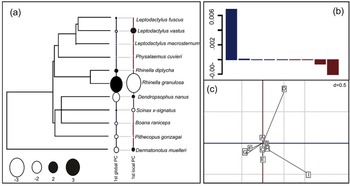
For microhabitat use, the global structure (historical factors/phylogeny) was identified as having a greater influence on the spatial organization (microhabitat) of the studied amphibian assemblage, since the positive eigenvalues (0.006) were greater than the negative eigenvalues (−0.002) (fig. 3B). Regarding the first global component, the species D. nanus and D. muelleri had intermediately negative scores as opposed to the life history value of the species R. granulosa, which presented the highest positive score in the tree (fig. 3A).

Fig. 3. Phylogenetic principal component analysis for anuran microhabitat use in the Caatinga. On the left is the phylogenetic tree built for the analysed anuran assemblage, with the 1st global principal component (PC) and 1st local PC. Negative and positive scores were indicated for the white and black circles, respectively. The size of the circle is proportional to values of the scores. On the right are the loadings of the first historical (blue) and ecological (red) components. Microhabitat categories: A – on leaf litter; B – at the water's edge; C – on a fallen tree trunk; D – on the rocks; E – in a water body; F – on a macrophyte; G – perched on shrub; H – perched on grass; and I – exposed soil.
The loadings of the analyses (fig. 3C) showed that the microhabitats ‘on a macrophyte’ (F) and ‘perched on shrub’ (G) exerted greater influence on the global negative axis composition, where the species D. nanus was the most historically affected by these two categories of spatial use. In contrast, the global positive axis was mainly influenced by the category ‘exposed soil’ (I), where the species R. granulosa was the most historically affected by this category of spatial use.
Regarding the first local component, the frog species R. granulosa presented the highest negative score. In contrast, the species S. x-signatus had a low local positive score (fig. 3A). The loadings of the analyses (fig. 3C) showed that the use of the microhabitat ‘exposed soil’ (I) exerted a greater influence on the local negative axis composition, where the species R. granulosa was the main species affected by this category of spatial use. In addition, the positive local axis was moderately influenced by the category ‘on leaf litter’ (A), where D. nanus and D. muelleri were the main species affected by this category of spatial use.
Discussion
The present study recorded nine helminth species. The observed species richness was lower than previously recorded in other anuran communities studied in South America (Bursey et al., Reference Bursey, Goldberg and Parmelee2001; Toledo et al., Reference Toledo, Schwartz, Nomura, Aguiar, Velota, Silva and Anjos2018). Most of the parasites found in this study were nematodes. The higher nematode prevalence seems to be a common trait in parasite communities associated with anurans (Aho, Reference Aho, Esch, Bush and Aho1990; Campião et al., Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Silva2014).
The parasites recorded herein showed low specificity, occurring in two or more host species (except the species G. pseudium), which may be related to sampling season, since sampling was only carried out during the rainy season at the beginning of the year. Our data corroborate Poulin et al. (Reference Poulin, Krasnov, Morand, Morand, Krasnov and Poulin2006), who attributed low species specificity to small mammalian parasites due to fluctuations in host populations, where the instability of the resource (host population) tends to produce a community of generalist parasites.
In terms of the biotic factors related to the studied anurans, parasite abundance was not influenced by any of the tested variables (SVL, body mass, body volume and sex). These findings differ from other studies on Brazilian anurans, which found a significant influence of host size, body mass and sex on parasite abundance (e.g. Santos & Amato, Reference Santos and Amato2010; Santos et al., Reference Santos, Amato and Borges-Martins2013; Toledo et al., Reference Toledo, Schwartz, Nomura, Aguiar, Velota, Silva and Anjos2018). These contrasting results highlight the need for further studies on the parasites of anuran communities in the Caatinga biome in northeastern Brazil. We highly recommend that future studies approach this topic using the same analytical method utilized in the present study. GLMMs can study the effect of a factor without the interference of other variables and/or pseudoreplication (Bates et al., Reference Bates, Mächler, Bolker and Walker2014).
According to Vieira et al. (Reference Vieira, Santana and Arzabe2009), the mating strategy of anurans in the Caatinga biome is adapted to rainfall patterns in the area, which occurs over the course of a few months. As a result, anurans must aggregate in both time and space, increasing their chances of becoming infected and sharing the same pool of parasite species. This situation also contributes to the use of similar microhabitats by individuals of different species, which can be seen in the case of D. nanus and D. muelleri. According to our results, both species are ecologically influenced by the category ‘on leaf litter’, although in this study, they did not share infections. Furthermore, in a study on lizards in the Caatinga, Brito et al. (Reference Brito, Corso, Almeida, Ferreira, Almeida, Anjos, Mesquita and Vasconcellos2014) showed that the use of microhabitats can influence the composition of endoparasites associated with these hosts, which may represent a pattern for this biome.
In general, anurans are associated with two types of environments (aquatic and terrestrial), allowing a great diversity of parasites to settle in these animals (Chandra & Gupta, Reference Chandra and Gupta2007). The way in which hosts explore their habitats can also explain the richness and diversity of associated parasites (Poulin & Morand, Reference Poulin and Morand2004). The microhabitats used by hosts is an important factor for determining the composition of parasite communities. The specialization of parasites for the same microhabitat as their hosts, leads to an increased likelihood of encounters between the parasites and their hosts, facilitating infection (Kerr & Bull, Reference Kerr and Bull2006).
In the present study, hosts that used the same microhabitats had similar parasite compositions; for example, Cosmocerca sp. was found only in S. x-signatus and R. diptycha, which explored rock microhabitats. Bufonids were found mainly on exposed soil and shared five parasite taxa (A. membranosa, Physaloptera sp. larvae, O. mazzai, R. fuelleborni and R. spectans). Anurans of the Hylidae family, which are usually arboreal species, were found in various microhabitats and followed the pattern of helminthic infection of terrestrial species (Bolek & Coggins, Reference Bolek and Coggins2003). Most of the helminth species found in Hylidae (R. fuelleborni and Cosmocerca sp.) were direct cycle and active infection species (Anderson, Reference Anderson2000). Therefore, similar microhabitat use by related species is directly reflected in endoparasite composition.
Additionally, the present study corroborates Campião et al. (Reference Campião, Ribas, Morais, Silva and Tavares2015) and Toledo et al. (Reference Toledo, Schwartz, Nomura, Aguiar, Velota, Silva and Anjos2018), who found that ecological (contemporary) factors are determinant for the structuring of parasitic communities associated with amphibians. In such cases, ecological adjustment events, such as host switching, parasite dispersal and colonization of new habitats (new types of hosts) can generate inconsistencies regarding host phylogeny (Nuismer & Thompson, Reference Nuismer and Thompson2006). Thus, these parasite life history traits are explained by the frequent alternation between strains of related and unrelated hosts (Krasnov & Shenbrot, Reference Krasnov and Shenbrot2002; Zietara & Lumme, Reference Zietara and Lumme2002; Johnson et al., Reference Johnson, Weckstein, Meyer and Clayton2011). Therefore, by showing a smaller effect of host phylogeny in relation to ecology, our results may be consistent with these views.
Host ecological traits may act as selective barriers against parasites and therefore, each ecological variable may affect parasite species in different ways, thus shaping their communities (Holmes, Reference Holmes1987). Anuran parasites tend to be generalists (Aho, Reference Aho, Esch, Bush and Aho1990) and are exposed to similar ecological conditions. In the present study, the parasite A. membranosa was present in ten of the 13 host species analysed. This result is consistent with the hypothesis that the anurans of the Caatinga biome are exposed to ecological pressures similar to those previously reported for other species in temporary ponds from other arid environments, resulting in similar mating systems and patterns of parasitism (Sullivan, Reference Sullivan1989). Therefore, anuran reproductive behaviour facilitates the contact of potential hosts with the same infective larvae. Furthermore, as anurans from the Caatinga biome have similar diets (Protázio et al., Reference Protázio, Albuquerque, Falkenberg and Mesquita2015), they may have ingested the same parasites through intermediate hosts, thus strengthening our hypothesis about Physaloptera sp. (indirect life cycle) infecting most species in the area.
Finally, another factor that may explain the greater ecological influence on the parasite community organization studied here is time of community formation. According to Brooks & Mclennan (Reference Brooks, Mclennan, RE and D1993) and Losos (1996), communities with different ages may reflect historical adjustments in their organization since the longer the establishment time, the longer the coexistence between the competing taxa and the greater the possibility of resource segregation adjustments, thereby allowing greater structuring, including phylogenetic structuring. As the Caatinga is an environment that presents a high level of climatic unpredictability and constantly suffers from high anthropic interference intensity (Rito et al., Reference Rito, Arroyo-Rodríguez, Queiroz, Leal and Tabarelli2017), it is expected that ecological values exert greater influence on the organization of communities contained in these places.
Acknowledgement
The authors thank Professor Dr Jivanildo Pinheiro Miranda for the English review of the first draft of this manuscript.
Financial support
We thank the Fundação cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) for the fellowship to Nayane Kelly S. Sampaio and Janaina Marques do Nascimento; and research fellowship to Samuel Cardozo Ribeiro (BP4-0172-00223.01.00/20), the Conselho Nacional de Pesquisa e Apoio ao Desenvolvimento Científico e Tecnológico (CNPq) for a research fellowship to Waltécio de Oliveira Almeida; CNPq and the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) for a research fellowship to Adonias Aphoena Martins Teixeira (PDCTR/FAPEMA/CNPq 301692/2021-2). This study was partially financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance code 001.
Conflicts of interest
None.
Ethical standards
All authors gave their consent to participation in the study. The Instituto Chico Mendes de Conservação da Biodiversidade provided the licence to collect the animals and the ethics committee of the Universidade Regional do Cariri approved this study.