INTRODUCTION
Elasmobranchs have been exploited in many parts of the world as part of both the target and by-catch of the tuna, trawl and longline fishery (Anderson, Reference Anderson, Pratt, Gruber and Taniuchi1990). The rapid expansion of these activities has led to the collapse of some shark populations in a short period of time (Anderson, Reference Anderson, Pratt, Gruber and Taniuchi1990), causing important changes in the natural renewal rates of these stocks, which will now require decades to return to their previous levels (Anderson, Reference Anderson, Pratt, Gruber and Taniuchi1990). Moreover, since sharks are apex predators in marine ecosystems, they play an important role in regulating prey populations at lower trophic levels (i.e. fish, invertebrates, reptiles, mammals and birds) (Ellis et al., Reference Ellis, Pawson and Shackley1996).
Studies on the trophic ecology, diet composition and trophic level of sharks shed light on their life histories, roles in marine ecosystems and species distributions as well as energy flows, and the impact of predation by different species (Cortés, Reference Cortés1999). Information regarding important feeding and breeding areas identified by such studies are used in conjunction with other biological studies to develop appropriate strategies for the conservation and management of shark species (Galván-Magaña et al., Reference Galván-Magaña, Nienhuis and Klimley1989).
This information is important as it allows us to make inferences regarding the predator–prey relationship, including prey abundance, distribution, and preferences, as well as possible ontogenetic changes in diet. Further, understanding quantitatively the feeding ecology of the shark species is a very important step to constructing a complex food web (Navia et al., Reference Navia, Cortés and Mejía-Falla2010; Bornatowski et al., Reference Bornatowski, Braga, Abilhoa and Corrêa2014a) and ecosystem models for evaluating the function of each species within an ecosystem, and predicting possible changes through fishing effects (Stevens et al., Reference Stevens, Bonfil, Dulvy and Walker2000). Additionally, studies of feeding ecology are important not only for identifying the relative frequency of the particular prey in a shark's diet, but also for revealing the importance of species (sharks and batoids) as a link between the higher and lower levels of the food chain (Bornatowski et al., Reference Bornatowski, Navia, Braga, Abilhoa and Corrêa2014b).
Carcharhinidae is the second largest family of sharks of commercial importance in Ecuador. The silky shark C. falciformis (Müller & Henle, 1839) is the third most important species for Ecuador's fisheries. The species is distributed in tropical and subtropical waters throughout the Eastern Pacific from Baja California to Peru (Compagno, Reference Compagno1984; Robertson & Allen, Reference Robertson and Allen2002), displaying epipelagic habits and feeding on a variety of prey, particularly bony fishes, cephalopods, and, to a lesser extent, crustaceans (Fischer et al., Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995). Another species targeted by Ecuadorian fisheries is the blacktip shark Carcharhinus limbatus (Müller & Henle, 1839), found only infrequently in landings. The species inhabits the tropical and subtropical waters of the Eastern Pacific from San Diego, California to Peru, including the Revillagigedo and Galapagos Islands (Compagno, Reference Compagno1984; Robertson & Allen, Reference Robertson and Allen2002). This species lives mainly in coastal and oceanic surface waters and is a fast swimmer, allowing it to feed on shoaling fish, rays and squid (Cervigón et al., Reference Cervigón, Cipriani, Fischer, Garibaldi, Hendrickx, Lemus, Márquez, Poutiers, Robaina and Rodriquez1992; Fischer et al., Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995). Similarly, the whitenose shark Nasolamia velox (Gilbert, 1898) is also caught by local fisheries. We know little of this species; however, it is distributed from Baja California to Peru (Compagno, Reference Compagno1984), and considered endemic to the Eastern Tropical Pacific (Robertson & Allen, Reference Robertson and Allen2002), preferring coastal habitats where it feeds on fishes, cephalopods and crustaceans (Compagno, Reference Compagno1984).
Fishery is one of the most important economic activities in Ecuador and often includes the capture of sharks. However there is a lack of studies on the basic biology of sharks in Ecuador, and only a few management studies, including the National Plan for the Conservation of Sharks (MICIP, 2006). Some recent studies have focused on shark dietary habits (Estupiñán-Montaño et al., Reference Estupiñán-Montaño, Cedeño-Figueroa and Galván-Magaña2009; Polo-Silva et al., Reference Polo-Silva, Rendón and Galván-Magaña2009, Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013; Loor-Andrade et al., Reference Loor-Andrade, Galván-Magaña, Elorriaga-Verplancken, Polo-Silva and Delgado-Huertas2015) and reproduction (Romero-Caicedo et al., Reference Romero-Caicedo, Galván-Magaña and Martínez-Ortiz2014). However, to date no studies have examined the biology of silky sharks C. falciformis, blacktip sharks C. limbatus and whitenose sharks N. velox. Thus, the goal of this paper was to investigate the diet and trophic positions of these three shark species, to generate baseline information to improve our knowledge and serve as a starting point for further research on sharks in the country, and thus contribute to scientific knowledge on these species.
MATERIALS AND METHODS
We collected stomachs of 69 Carcharhinus falciformis (43 females, 26 males) from January to December 2004; 44 C. limbatus (four females, 40 males) and 24 Nasolamia velox (17 females, seven males) from August 2003 to March 2004, caught in Ecuadorian waters and landed in the port of Manta (Ecuador). The study area extended from 02°N to 02°S and from the coast to 84°W. For each shark, the total length (TL) was recorded and the digestive tract was removed by dissection. Stomach contents were removed and screened through a 1.5 mm sieve. Prey were identified to the lowest possible taxon considering the state of digestion and subsequently placed in plastic bags and preserved on ice for transportation to the laboratory.
For the taxonomic identification, we consulted different identification keys; for fishes we used those by Clothier (Reference Clothier1950), Rubio (Reference Rubio1988), Fischer et al. (Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995), Chirichigno (Reference Chirichigno1998) and García-Godos (Reference García-Godos2001); whereas to identify cephalopods, we used Wolff (Reference Wolff1982, Reference Wolff1984) and Clarke (Reference Clarke1986). Due to the advanced state of digestion, cephalopods were identified by their mandibular apparatus and crustaceans were classified based on their exoskeletons following Fischer et al. (Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995).
We quantified the stomach contents numerically (N), gravimetrically (W), and in terms of the frequency of occurrence (FO) (Hyslop, Reference Hyslop1980). We also used Pinkas et al.’s (Reference Pinkas, Oliphant and Iverson1971) index of relative importance (IRI), which incorporates the three measurements in the following formula: IRI = (%W + %P) × %FO. Cortés (Reference Cortés1997) subsequently transformed this formula in order to obtain values as percentages and facilitate comparison:
Similarly, we also determined the breadth of the trophic niche using Levin's standardized index (Krebs, Reference Krebs1989):
where n is the number of prey items and P ij is the proportion of the diet of predator i composed of prey j. This index ranges from 0 to 1; values <0.6 indicated specialist predators that consume only certain types of prey, while values ≥0.6 indicated the diets of opportunistic predators that use resources indiscriminately (Labropoulou & Eleftheriou, Reference Labropoulou and Eleftheriou1997).
We also used the Morisita–Horn index to assess the degree of trophic overlap (Smith & Zaret, Reference Smith and Zaret1982):
 $$C{\rm \lambda} = 2 \displaystyle{{\sum\limits_{i = 1}^n {(P_{xi} *P_{yi} )}} \over {\left( {\sum\limits_{i = 1}^n {P_{xi}^2 +} \sum\limits_{i = 1}^n {P_{yi}^2}} \right)}}$$
$$C{\rm \lambda} = 2 \displaystyle{{\sum\limits_{i = 1}^n {(P_{xi} *P_{yi} )}} \over {\left( {\sum\limits_{i = 1}^n {P_{xi}^2 +} \sum\limits_{i = 1}^n {P_{yi}^2}} \right)}}$$where Cλ is the Morisita-Horn index between species x and y, P xi is the proportion of prey i relative to the total prey consumed by predator x, P yi is the proportion of prey i relative to the total prey consumed by y, and n is the total number of prey. Values for this index range from 0 to 1; those closest to zero indicate dietary differences, while values closer to one indicate similarities in the prey consumed (Langton, Reference Langton1982).
In addition, we also assessed the trophic overlap using the ‘mh’ function in the ‘divo’ package of R software, applying bootstraping (nboot = 1000) and setting the confidence level at 95%; this function generates a matrix of the overlap between variables and is represented by a dendrogram. Finally, to evaluate the uncertainty of our classification, we used the ‘pvclust’ package to calculate the P-value quantiles using bootstrapping (bootstrap = 1000). The approximately unbiased (AU) P-value is calculated via multi-scale bootstrapping, while the bootstrap probability (BP) P-value is calculated using standard bootstrapping. The AU is the best approximation of the P-value; AU values >95% strongly support the information (R Core Team, 2014).
To determine the average trophic level of the different prey items identified in the stomachs analysed we used the following formula proposed by Cortés (Reference Cortés1999):
 $$I_{{\rm TR}} = 1 + \left( {\mathop \sum \limits_{\,j = 1}^n P_j \times I_{{\rm T}{\rm R}_j}} \right)$$
$$I_{{\rm TR}} = 1 + \left( {\mathop \sum \limits_{\,j = 1}^n P_j \times I_{{\rm T}{\rm R}_j}} \right)$$where I TRj is the trophic level of each prey taxa j and P j is the proportion of each of the categories of prey j in the predator's diet based on %N (Cortés, Reference Cortés1999). We obtained the trophic levels for different prey species from Froese & Pauly (Reference Froese and Pauly2015) (www.fishbase.org); when no data were available, we used the average trophic level for the corresponding group: cartilaginous fishes (3.65), cephalopods (3.2), teleosts (3.24) and crustaceans (2.52) (Cortés, Reference Cortés1999). All calculations were carried out using the R software (R Core Team, 2014).
RESULTS
Carcharhinus falciformis
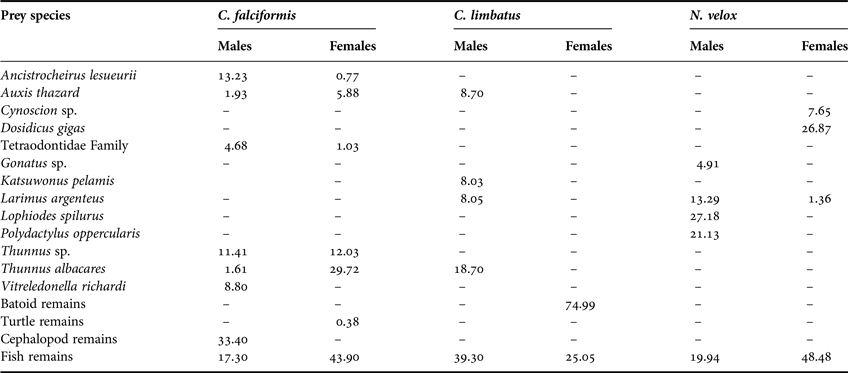
The C. falciformis individuals measured from 95 to 310 cm total length (TL) (mean ± SD = 174.1 ± 35.7 cm TL). Of the 69 (43 female, 26 males) stomachs analysed, 83% (59 stomachs) contained food. We were able to identify 19 dietary components to the lowest taxon: 12 teleosts and eight cephalopods, as well as the remains of fishes, cephalopods and turtles. Based on the %IRI, teleosts contributed most to the C. falciformis diet (Table 1). The most important prey were the Scombridae fishes Thunnus albacares (%IRI = 22.4%), Thunnus sp. (12.9%) and Auxis thazard (4.77%) (Table 1, Figure 1). The trophic spectrum of both females and males consisted of teleosts and cephalopods; females also consumed turtles (Table 2). The most important prey species for males were the cephalopods Ancistrocheirus lesueurii (13.2%) and Vitreledonella richardi (8.8%), while females preferred the fishes T. albacares (29.7%), Thunnus sp. (12.0%) and A. thazard (5.88%) (Table 2).

Fig. 1. Trophic spectrums for C. falciformis, C. limbatus and N. velox in Ecuadorian waters showing the most important prey based on the index of relative importance (%IRI).
Table 1. Trophic spectrum for C. falciformis, C. limbatus, and N. velox in the Ecuadorian Pacific expressed numerically (%N) and gravimetrically (%W) as well as in terms of the frequency of occurrence (%FO) and the index of relative importance (%IRI).

*From: www.fishbase.org (2015), Cortés (Reference Cortés1999), Pauly et al. (Reference Pauly, Trites, Capuli and Christensen1998), Hobson & Welch (Reference Hobson and Welch1992).
Table 2. Trophic spectrum by sex in C. falciformis, C. limbatus and N. velox in Ecuadorian waters, expressed in Index of Relative Importance (%IRI).
Carcharhinus limbatus
The C. limbatus specimens measured from 132 to 224 cm TL (188.7 ± 15.9 cm TL). Of the 44 (four females, 40 males) stomachs analysed, 19 (43.2%) had stomach contents, including 12 identifiable dietary components (10 teleosts, one elasmobranch and one crustacean) and the remains of cephalopods, fishes and batoids. Based on the %IRI, teleosts were the most important group followed by elasmobranchs, crustaceans and cephalopods (Table 1); the fishes T. albacares (27.34%), Exocoetus monocirrhuns (13.29%), A. thazard (13.18%), Katsuwonus pelamis (12.25%) and members of the Ophichthidae family (6.14%) were the most important to C. limbatus diet (Table 1, Figure 1). The small sample size for females (N = 4) impeded the trophic analysis based on sex. Considering each sex separately, 12 prey species were consumed by males (10 teleosts, one batoid and one crustacean), of which the most important prey were: T. albacares (%IRI = 18.7%), A. thazard (8.7%), Larimus argenteus (8.1%) and K. pelamis (8%) (Table 2). Of the four females analysed, only two had stomach contents, which included the remains of teleosts (25.1%) and batoids (75%) (Table 2).
Nasolamia velox
A total of 24 (17 females, seven males) specimens measured between 67 and 192 cm TL (151 ± 31.1 cm TL) were analysed, of which 12 (50%) had stomach contents; we identified 17 dietary components as well as the remains of unidentified organisms. Based on the %IRI, the N. velox diet was composed of teleosts, cephalopods and crustaceans (Table 1); the most important prey were the cephalopod Dosidicus gigas (22.46%), L. argenteus (7.22%), Cynoscion sp. (5.77%) and Lophiodes spilurus (4.4%) (Table 1, Figure 1). The male diet was dominated by teleosts and cephalopods, with the most important prey being the fishes L. spilurus (27.18%), Polydactylus opercularis (21.13%) and L. argenteus (13.29%) (Table 2). In contrast, the female diet also included crustaceans, of which D. gigas (%IRI = 26.87%), members of the Ophidiidae family (2.7%) and Oxyporhamphus micropterus (2.67%) were the most important (Table 2).
Trophic niche, trophic overlap and trophic level
The trophic niches calculated for Carcharhinus falciformis (Bi = 0.57), C. limbatus (Bi = 0.40) and Nasolamia velox (Bi = 0.34) indicate that all three are specialist predators. The trophic niche for male and female of C. falciformis was 0.65 and 0.43, respectively. For C. limbatus and N. velox, this analysis was not performed due to low number of samples of each sex. We use the trophic overlap Morisita–Horn index (Cλ <0.5), indicating low food competition between these three predators (Table 3, Figure 2). The trophic levels calculated for C. falciformis (4.57), C. limbatus (4.28) and N. velox (4.25) suggest they are tertiary carnivores.

Fig. 2. Trophic overlap between C. falciformis, C. limbatus and N. velox in the Ecuadorian Pacific based on the Morisita–Horn index. AU = p-valor multi-scale (1000 replicates).
Table 3. Trophic overlap between C. falciformis, C. limbatus and N. velox in the Ecuadorian Pacific based on the Morisita-Horn index (Cλ).

DISCUSSION
Carcharhinus falciformis
The trophic spectrum of the Carcharhinus falciformis in the present study is consistent with observations made elsewhere in the world. In Colombia, the main prey include members of the Scombridae and Coryphaenidae families, the coastal cephalopod Lolligo sp., and a small percentage of crustaceans (Euphylax robustus) and turtles (Chelonia mydas) (Acevedo, Reference Acevedo1996).
Barranco (Reference Barranco2008) studied the C. falciformis diet at two locations in Mexico, noting that their main prey included the crustacean Portunus xantusii affinis, the pelagic cephalopod Argonauta sp. and the epipelagic fish Euthynnus lineatus. Cabrera-Chávez-Costa et al. (Reference Cabrera-Chávez-Costa, Galván-Magaña and Escobar-Sánchez2010) recorded that silky shark predate mainly on crustacean Pleuroncondes planipes (Baja California Sur, Mexico), the cephalopod D. gigas and the pelagic-coastal fish Scomber japonicus. Duffy et al. (Reference Duffy, Olson, Lennert-Cody, Galván-Magaña, Bocanegra-Castillo and Kuhnert2015) examined the stomach contents of C. falciformis in the Eastern Pacific Ocean (EPO), finding that: (1) this species’ diet varies based on the abundance of different prey, (2) the species displays few ontogenetic changes, (3) they are piscivorous consumers, with over 50% of their prey belonging to Scombridae family (K. pelamis, T. albacares, Thunnus sp. and Auxis sp.) and (4) they consume a variety of prey items, suggesting that they are opportunistic predators.
Although our study was based on a small number (69) of stomachs, our observations are similar to those reported by Duffy et al. (Reference Duffy, Olson, Lennert-Cody, Galván-Magaña, Bocanegra-Castillo and Kuhnert2015). In our study, the most important prey species were fish from the Scombridae family (Thunnus sp.), making them piscivorous. It is clear that both off the coast of Ecuador as well as throughout the EPO, this species prefers fish; however, the trophic spectrum of this species in other parts of the world indicated a more varied diet, including prey from benthic (some crustaceans) and oceanic-coastal (fish and turtles) habitats. This pattern is likely related to differences in size, sex and sexual maturity; however, Duffy et al. (Reference Duffy, Olson, Lennert-Cody, Galván-Magaña, Bocanegra-Castillo and Kuhnert2015) found no differences in diet based on size in the EPO and too little is known about the biology of this species in the Ecuadorian Pacific to confirm this suggestion.
We found changes in the diet of C. falciformis comparing different studies, these changes would be because juveniles of this species are more frequent in areas near the coast, where they consume abundant and easy (e.g. epipelagic crustaceans) prey to save energy during capture; while adults are in oceanic waters feeding on big prey such as tuna, which supply more energy. The C. falciformis in this study prefer to consume prey of oceanic waters (e.g. tuna) because the shark fleet in Ecuador performs their catch in oceanic areas. The studies used to compare the diet in this shark species include catches by small boats close to coastal areas or big boats (e.g. tuna purse seiner), which are used in oceanic waters. This would explain the different prey items consumed by this shark in different areas in the Eastern Pacific Ocean.
Carcharhinus limbatus
We found that the shark species’ diet in Ecuadorian waters includes prey from the same groups or with similar characteristics to those observed previously by Castro (Reference Castro1996), Tavares & Provenzano (Reference Tavares and Provenzano2000), Barry (Reference Barry2002) and Tavares (Reference Tavares2008), who report that teleosts are the most important prey for this piscivorous predator. Moreover, Castro (Reference Castro1996) also reports that both sharks and rays are included in their diet. This supports our findings, which included one longtail stingray D. longa and the remains of batoids.
Castro (Reference Castro1996) and Barry (Reference Barry2002) have noted that small numbers of crustaceans are included in the C. limbatus diet; we also identified one crustacean, Portunus sp., although based on a small sample. Gaitán-Espitia & López-Peña (Reference Gaitán-Espitia and López-Peña2008) identified the remains of fish vertebrae and cephalopod beaks in the stomachs of juvenile C. limbatus.
In the south-eastern USA, Castro (Reference Castro1996) reported that the Atlantic menhaden Brevoortia tyrannus was the most abundant prey; other prey species included the elasmobranchs Rhinoptera banasus, Rhizoprionodon terraenovae and Sphyrna tiburo, as well as some shrimp and small teleosts. In contrast, Barry (Reference Barry2002) mentioned that off the coast of Louisiana, USA, the most important prey were Brevoortia patronus and Micropogonias undulatus. Meanwhile in Los Roques Archipelago, Venezuela, Tavares & Provenzano (Reference Tavares and Provenzano2000) only reported the presence of teleost fishes, of which the following were the most important: Opisthonema oglinum, Gerres cinereus, Albula vulpes and Haemulon sciurus.
Similarly, Tavares (Reference Tavares2008) noted that the main prey consumed by C. limbatus in the Los Roques Archipelago, Venezuela, included Eucinostomus argenteus, O. oglinum and G. cinereus; suggesting a shift over time in this predator's alimentary preferences in the area. In our study, the main prey consumed by C. limbatus in Ecuadorian waters included the fishes T. albacares, E. monocirrhus, A. thazard, K. pelamis and members of the Ophichthidae family. This is not consistent with the results of other studies, and may be related to prey diversity and availability in the different geographic areas examined as well as the influence of the age-class of the specimens examined. Finally, both the present study and previous research on the C. limbatus diet indicate that, regardless of geographic area, their diet is based on high consumption of fish from both coastal and oceanic areas including prey from pelagic, and sometimes even benthic habitats.
Nasolamia velox
In Ecuadorian waters, N. velox feed on various groups of organisms, including fish, shellfish, and crustaceans, with a preference for fish, suggesting they are piscivorous, similar to Compagno (Reference Compagno1984). They feed on coastal habitats and consume prey from the seabed (benthic and demersal species) with 47% of the 19 prey species identified coming from benthic environments, 16% from both coastal and mesopelagic environments, 11% from demersal coastal habitats, and 5% from oceanic and oceanic-coastal areas (Table 1). Nasolamia velox is common in shallow coastal areas (15–24 m, sometimes to 192 m) (Compagno, Reference Compagno1984). This habitat and the presence of fishes from the Sciaenidae (e.g. Cynoscion sp., L. argenteus), Lophiidae (L. spilurus) and Polynemidae (P. opercularis) families, which inhabit coastal zones in sandy and muddy habitats (Robertson & Allen, Reference Robertson and Allen2002), suggest that whitenose shark feed in this habitats.
Trophic niche, trophic overlap and trophic level
Based our results, we consider C. falciformis to be a specialist predator; this is consistent with Barranco (Reference Barranco2008), Cabrera-Chávez-Costa et al. (Reference Cabrera-Chávez-Costa, Galván-Magaña and Escobar-Sánchez2010) and Duffy et al. (Reference Duffy, Olson, Lennert-Cody, Galván-Magaña, Bocanegra-Castillo and Kuhnert2015), who consider C. falciformis a specialist predator, because although consuming many prey species, some prey are more important in their diet. Duffy et al. (Reference Duffy, Olson, Lennert-Cody, Galván-Magaña, Bocanegra-Castillo and Kuhnert2015) report that this shark species has a preference for fishes of the Scombridae family (T. albacares and Thunnus sp.). Although our study area was small and we analysed few (69) stomachs, our results are similar to those obtained by Duffy et al. (Reference Duffy, Olson, Lennert-Cody, Galván-Magaña, Bocanegra-Castillo and Kuhnert2015) who examined 786 stomachs. Silky shark feeding patterns indicate that this species has a broad trophic niche, suggesting that they make use of a variety of available resources. In contrast, C. limbatus (Bi = 0.40) and N. velox (Bi = 0.33) have a reduced trophic niche. It is worth noting that these are approximations of the niche breadth for the latter two species because we lack information on their diets; the present study is the first to examine the diets of C. limbatus and N. velox in Ecuador.
While C. falciformis, C. limbatus and N. velox are all present in the Ecuadorian Pacific, our results suggest low interaction between them (Table 3) due to the distribution of resources in the area and differences in the habitat preferences of these shark species; C. falciformis prefers oceanic habitats, C. limbatus frequents oceanic-coastal habitats and also feeds on prey from the water column and seabed, and N. velox is a coastal species that consumes benthic prey. Thus, these species avoid potential competition for food even though our calculations place C. falciformis (4.57), C. limbatus (4.28) and N. velox (4.25) in the same trophic level (i.e. tertiary consumers).
Very few studies have examined the trophic positions of these sharks. Of the few studies that have been undertaken, Cortés (Reference Cortés1999) estimated trophic positions for both C. falciformis (4.2) and C. limbatus (4.2), which are similar to those reported here. Other studies relying on different techniques have produced results similar to ours. For example, in two studies involving the stable isotopes analysis of δ15N, Galindo (Reference Galindo2014) assigned C. falciformis in a trophic position between 3.3 and 3.8, while Yunkai et al. (Reference Yunkai, Gong, Chenn, Dai and Zhu2014) placed this species between 3.4 and 5.3. Other authors have identified C. falciformis as secondary (Mearns et al., Reference Mearns, Young, Olson and Schafer1981) or tertiary consumers (Cortés, Reference Cortés1999) based on a variety of techniques. Trophic level estimates for N. velox make no mention of trophic position, illustrating the lack of information regarding the species.
The information presented here serves as a strong base for increasing our understanding of the trophic ecology of the different species of sharks found in Ecuadorian waters. Future studies should focus on examining the diets of these shark species using complementary techniques (e.g. stable isotope analysis, etc.). In order to improve our understanding of their role in the ecosystem, other studies of cartilaginous fishes are needed, including assessing alimentary ontogeny, sexual segregation of feeding areas, inter- and intra-specific competition, and estimating their trophic levels.
ACKNOWLEDGEMENTS
We thank D. Castañeda, A. Sandoval, A. Baigorrí, J. Méndez, J. Figueroa, and the fish butchers of Tarqui Beach in Manta, Ecuador.
FINANCIAL SUPPORT
FMG thanks the Instituto Politécnico Nacional (IPN; National Polytechnic Institute) for fellowships provided through the Estímulo al Desempeño de los Investigadores (EDI; Performance Incentives) and the Comisión de Operación y Fomento de Actividades Académicas (COFAA; Commission for the Advancement of Academic Activities).







