Introduction
Diabetes is one of the most prevalent chronic conditions worldwide and a public health priority in many countries (Tamayo et al., Reference Sperl-Hillen and O’Connor2014; International Diabetes Federation, Reference Heianza, Arase, Fujihara, Tsuji, Saito, Hsieh, Kodama, Shimano, Yamada, Hara and Sone2015). In Europe, an estimated 9.8 million people suffer from diabetes; type 2 diabetes is responsible for 90% of cases. People with type 2 diabetes are at high risk for developing complications, such as cardiovascular disease and kidney failure, which in turn lead to increased health-care costs (Tamayo et al., Reference Sperl-Hillen and O’Connor2014; International Diabetes Federation, Reference Heianza, Arase, Fujihara, Tsuji, Saito, Hsieh, Kodama, Shimano, Yamada, Hara and Sone2015). To prevent diabetes-related co-morbidities and complications, and lower medical care expenditure for patients with type 2 diabetes, it is important to implement effective and efficient management strategies. An example of such a strategy is the implementation of integrated care. It aims to improve patient care and experience through improved coordination (Shaw et al., Reference Scheen2011).
The implementation of integrated care programs is widespread in North America, Europe, and other parts of the world (Kodner, Reference Khattab, Khader, Al-Khawaldeh and Ajlouni2009; Shaw et al., Reference Scheen2011). However, most integrated care programs are not tailored to patients’ needs and preferences, but rather highly standardized according to evidence-based guidelines for specific diseases, such as diabetes. Findings from recent studies suggest that not all patients benefit equally from such a standardized approach (Rothe et al., Reference Robinson, Baron, Cooper and Janson2008; Pimouguet et al., Reference Ostgren, Lindblad, Ranstam, Melander and Rastam2011; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). These studies report that patients with poorly controlled diabetes benefit mostly from intensive, provider-driven disease management, whereas patients with adequate glucose levels might maintain these levels independent of the type of care they receive.
In 2012, the European Association for the Study of Diabetes and the American Diabetes Association recommended a more patient-centered approach for the management of type 2 diabetes (Inzucchi et al., 2012). In a patient-centered approach, care is tailored according to individual patient needs and preferences (Commitee on Quality of Health Care in America; Institute of Medicine, 2001; Inzucchi et al., 2012; American geriatrics society expert panel on person-centered care, 2016; Coulourides Kogan et al., Reference Coulourides Kogan, Wilber and Mosqueda2016). It draws on the concept of ‘mass customization’, where goods and services are delivered with enough variety and customization that nearly everyone finds exactly what they want (Tseng and Hu, Reference Tseng and Hu2014). Dividing the population based on health-care needs creates groups that are more homogenous than the population as a whole. Hence, care offered to these groups will be more tailored to the patients’ needs, while acknowledging that a certain amount of heterogeneity within the subgroups will remain.
There is increasing consensus that a patient-centered approach could improve the management of type 2 diabetes (Inzucchi et al., 2012). However, to date, it is unclear what the best method is for establishing patient-centered care (Epstein and Street, Reference El-Kebbi, Cook, Ziemer, Miller, Gallina and Phillips2011). Since intensive, provider-driven disease management is not beneficial to every type 2 diabetes patient, several studies have pointed toward patient characteristics – for example, number of co-morbidities, disease duration or attitude – as possible effect modifiers of treatment (Hasnain-Wynia and Baker, Reference Groeneveld, Petri, Hermans and Springer2006; Inzucchi et al., 2012; Riddle and Karl, Reference Quinn, Shardell, Terrin, Barr, Park, Shaikh, Guralnik and Gruber-Baldini2012; Scheen, Reference Rothman, DeWalt, Malone, Bryant, Shintani, Crigler, Weinberger and Pignone2016). These effect modifiers could be used to identify patients with different care needs and preferences, and subsequently serve as input to tailor treatment (Goldberger and Buxton, Reference Fonseca2013; Constand et al., Reference Constand, MacDermid, Dal Bello-Haas and Law2014) . However, it is unclear which effect modifiers should guide a more patient-centered approach. Therefore, the aim of this systematic review was to identify which patient effect modifiers influence the outcomes of integrated care programs for type 2 diabetes in primary care. These effect modifiers can help to segment the chronically ill population into subgroups with similar health-care needs for whom, based on insight into their needs and preferences, a range of matching care and support options can be developed.
This review is the first part of the research project entitled ‘PROFiling patients’ healthcare needs to support Integrated, person-centered models for Long-term disease management (PROFIle)’ (Elissen et al., Reference Elissen, Hertroijs, Schaper, Vrijhoef and Ruwaard2016). The aim of this four-year Dutch project is explicitly not to develop another disease-specific approach, but we use type 2 diabetes as starting point to develop, validate and test so-called ‘patient profiles’ as an instrument to support more patient-centered chronic care management in practice.
Methods
Data sources and searches
A systematic literature search according to PRISMA guidelines (Moher et al., Reference McCulloch, Price, Hindmarsh and Wagner2009) was performed on PubMed, CINAHL and EMBASE databases in January 2015. Included were English- or Dutch-language randomized controlled trials (RCT), prospective and retrospective cohort- and cross-sectional studies which: (1) focused on integrated care (defined below); (2) included adult patients (⩾18 years) with type 2 diabetes; (3) were set in primary care; (4) measured effects on 1 or more measures of diabetes management [hemoglobin A1c (HbA1c), low-density lipoprotein cholesterol (LDL-c) and systolic blood pressure (SBP)], and/or health-care utilization as outcome variables; and (5) included sub-analyses with patient characteristics as independent variables. In line with previous research, integrated care was defined as interventions combining two or more components of the well-known Chronic Care Model (CCM) (Busetto et al., Reference Busetto, Luijkx, Elissen and Vrijhoef2016). The CCM stresses the need for a more proactive health-care system by focusing on four components: self-management support (eg, patient education), decision support (eg, evidence-based guidelines), delivery system design (eg, care process) and clinical information systems (eg, electronic registries) (McCulloch et al., Reference Luijks, De Grauw, Bor, Van Weel, Lagro-Janssen, Biermans and Schermer1998; Coulter et al., Reference Coulter, Entwistle, Eccles, Ryan, Shepperd and Perera2015). Since the CCM was developed in 1998, only studies published in or after 1998 were included (Austin et al., Reference Austin, Wagner, Hindmarsh and Davis2000). The search strategy included targeted terms related to diabetes, integrated care, CCM components, care outcomes and subgroup analyses based on patient characteristics. The complete search terms and search string can be found in Table 1. The snowball method was used to search for other relevant studies.
Table 1 Search terms and search string

CCM=Chronic Care Model.
Study selection
Potentially relevant studies were retrieved from the electronic databases based on the inclusion criteria in three screening rounds. First, titles and abstracts were screened. The first 50 titles and abstracts were screened independently by two reviewers (D.H. and A.E.). More than 90% agreement was reached. Therefore, the remainder of the titles and abstracts were screened by 1 reviewer (D.H.). Second, the first 20 full texts were screened independently by two reviewers (D.H. and A.E.). Again, more than 90% agreement was reached and therefore, each reviewer independently screened half of the full texts. Third, the reference lists of the included studies were screened to obtain additional studies. Steps 1 and 2 of the study selection process were then repeated.
Data extraction and quality assessment
Descriptive data on studies were extracted by 1 reviewer (D.H.) between August and October 2015. Studies were coded for author names, year of publication, country, study design, length of follow-up, population size, age, percentage of males and CCM components. In case of uncertainties, a group discussion was held with two other authors (A.E. and M.B.).
The Effective Public Health Practice Project Quality Assessment Tool (EPHPP) was used to assess the quality of the included studies (Armijo-Olivo et al., Reference Armijo-Olivo, Stiles, Hagen, Biondo and Cummings2012). This tool was chosen because it allows the assessment of different study designs. The studies were rated based on six domains: (1) selection bias; (2) study design; (3) confounders; (4) blinding; (5) data collection; and (6) withdrawals and dropouts. Each domain was rated as ‘strong,’ ‘moderate’ or ‘weak’. A global rating was given based on the number of weak components.
Two reviewers (D.H. and M.B.) independently performed the quality assessment for each study. Disagreements were resolved via discussion conform EPHPP guidelines.
Data synthesis and analysis
The included studies were categorized according to: (1) the reported outcome(s) of interest (HbA1c, LDL-c, SBP and/or health-care utilization); and (2) the type of patient characteristic(s) investigated in subgroup analyses. Characteristics were classified as person-related (predisposing), context-related (enabling) or health-related (illness level) characteristics according to Andersen and Newman’s (Reference Andersen and Newman1973) Behavioral Model of Health Service Use. The model provides a theoretical framework for viewing health services utilization, taking into account both societal and individual characteristics. The model was chosen, because the individual characteristics can inform tailored care by, for example, helping determine the best intensity of care for the individual patient. Relationships between outcomes and characteristics were depicted as ‘+’ for significant positive relationships, as ‘−‘for significant negative relationships and as ‘o’ for non-significant relationships.
Results
Search results
In total, 1374 studies were identified through electronic databases and by checking the references of the included studies. Figure 1 shows the flow diagram of the study selection. Most studies were excluded because none relevant outcomes were reported (n=453), and/or type of care was not integrated (n=257). After the title, abstract and full text screening, 27 studies were included (Groeneveld et al., Reference Goldberger and Buxton2001; Ostgren et al., Reference Nielsen, de Fine Olivarius, Gannik, Hindsberger and Hollnagel2002; El-Kebbi et al., 2003; Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; Rothman et al., Reference Rothman, Malone, Bryant, Horlen and Pignone2004; Uitewaal et al., Reference Uitewaal, Bruijnzeels, Bernsen, Voorham, Hoes and Thomas2004; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; Uitewaal et al., Reference Uitewaal, Voorham, Bruijnzeels, Berghout, Bernsen, Trienekens, Hoes and Thomas2005; De Alba Garcia et al., Reference De Alba Garcia, Dallo, Salcedo Rocha, Rodriguez, Perez, Baer and Weller2006; Nielsen et al., Reference Nam, Chesla, Stotts, Kroon and Janson2006; Taweepolcharoen et al., Reference Tamayo, Rosenbauer, Wild, Spijkerman, Baan, Forouhi, Herder and Rathmann2006; Trief et al., Reference Trief, Morin, Izquierdo, Teresi, Eimicke, Goland, Starren, Shea and Weinstock2006; Wahba and Chang, Reference Wahba and Chang2007; Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009; De Fine Olivarius et al., Reference De Fine Olivarius, Siersma and Hansen2009; Robinson et al., Reference Riddle and Karl2009; Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011; Cardenas-Valladolid et al., Reference Cardenas-Valladolid, Salinero-Fort, Gomez-Campelo, De Burgos-Lunar, Abanades-Herranz, Arnal-Selfa and Andres2012; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012; Liu et al., Reference LeBlanc, Rosales, Kachroo, Mukherjee, Funk and Nichols2013; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013; LeBlanc et al., Reference Kodner2015; Luijks et al., Reference Liu, Li, Sha, Wang and He2015; Moreira et al., Reference Mold, While and Forbes2015; Quinn et al., Reference Quah, Liu, Luo, How and Tay2016).
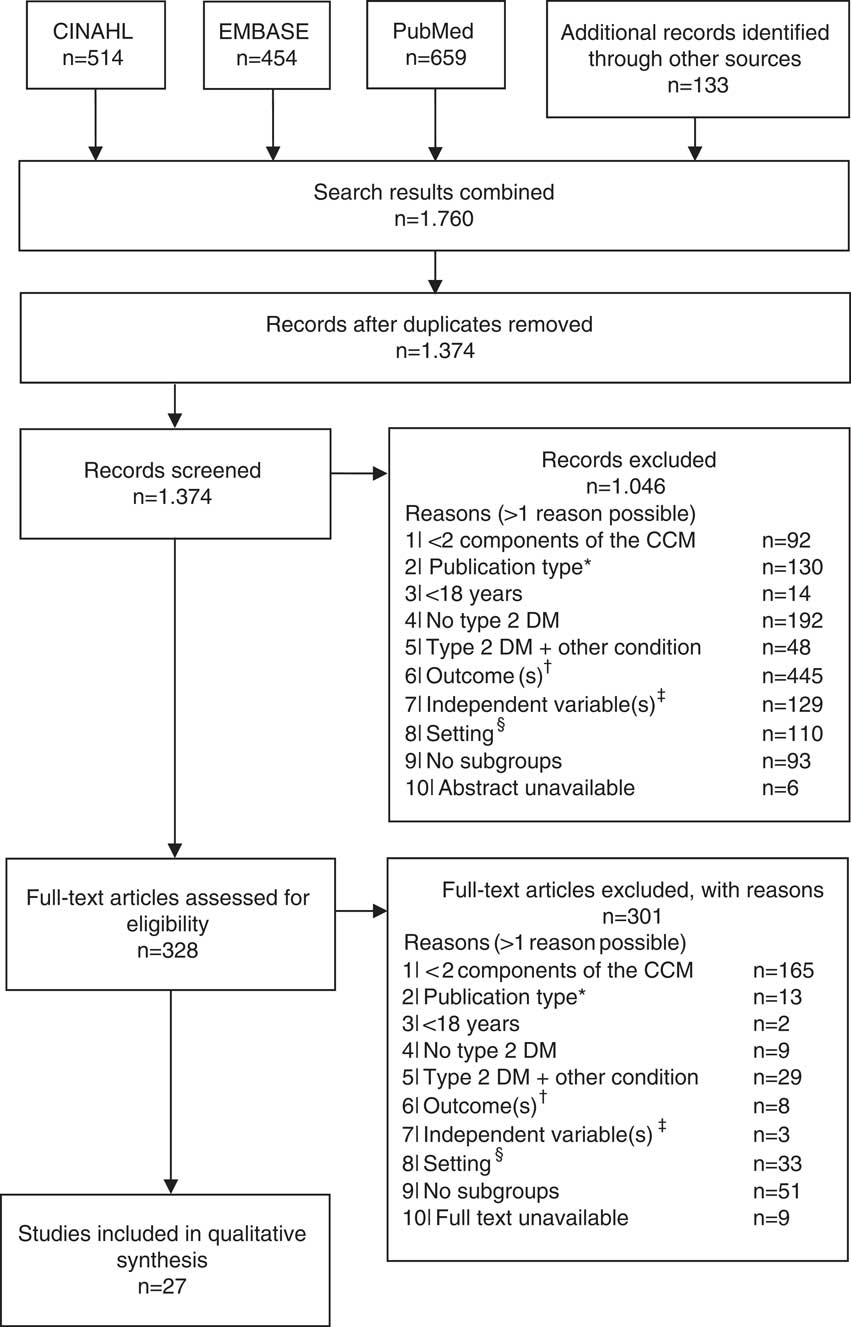
Figure 1 Flow diagram of the study selection. *Qualitative, or mixed-method studies; †any outcome other than hemoglobin A1c, low-density lipoprotein cholesterol, blood pressure or health-care utilization; ‡independent variable is not a person-, context- or health-related patient characteristic (eg, health-care provider characteristics); §setting is not a primary care setting (eg, hospital). CCM=Chronic Care Model; DM=diabetes mellitus.
Quality assessment
The methodological quality of the included studies can be found in Supplementary Table 1. The domains with the most ‘weak’ ratings were confounders (n=10), blinding (n=9) and selection bias (n=9). Almost all studies (n=25) scored high on the domain data collection. The overall study quality was strong for four studies, moderate for 11 studies and low for 12 studies. Most studies with low quality had a cross-sectional study design and did not report on or adjust for possible confounders.
Study and sample characteristics
Of the included studies, nine (33.3%) were retrospective cohort studies, seven (25.9%) cross-sectional studies, seven (25.9%) (randomized) controlled studies and four (14.8%) prospective cohort studies. Table 2 shows that the median follow-up duration for retrospective cohort, prospective cohort and randomized controlled studies (n=20) was 15 months (range 6–112). The median sample size consisted of 376 individuals (range 80–105 056) with an average age of 60.0 years (range 50.5–70.9); the percentage of male subjects ranged from 31.3 to 68.0.
Table 2 Study and sample characteristics
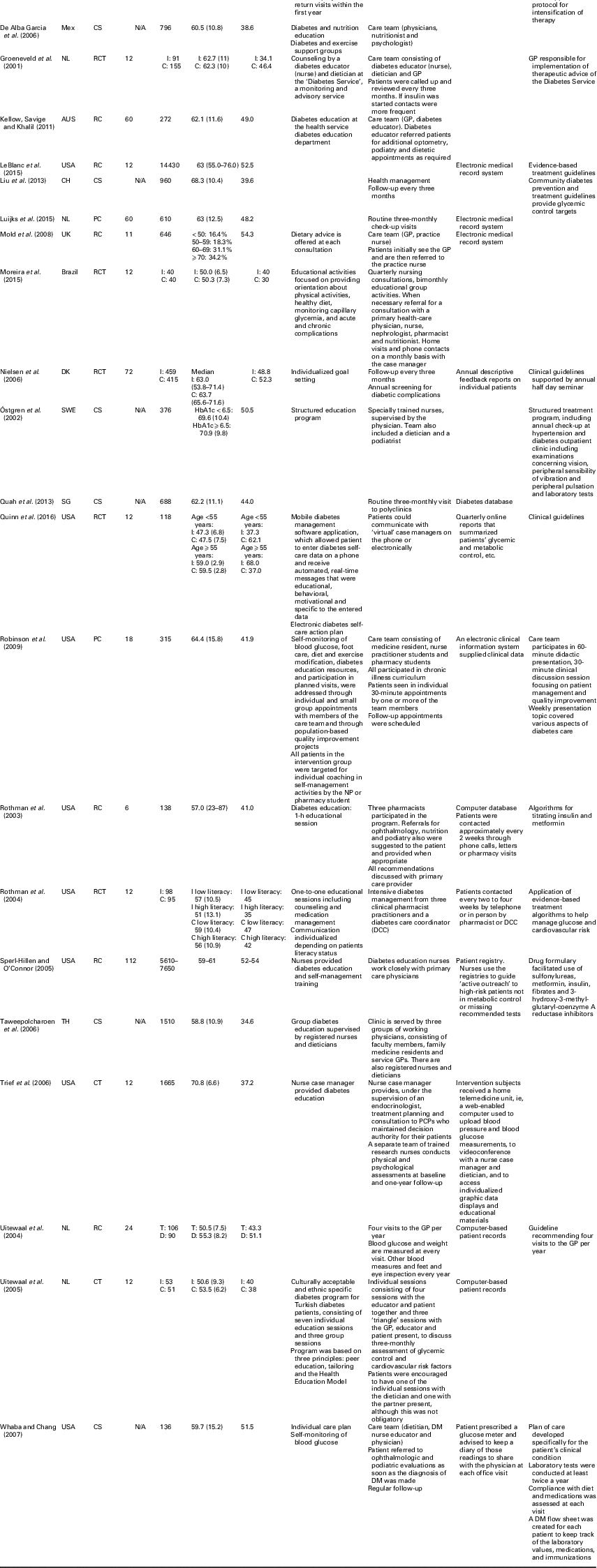
CCM=chronic care model; Jor=Jordan; CS=cross-sectional; N/A=not applicable; RC=retrospective cohort; ES=Spain; PC=prospective cohort; DK=Denmark; NL=the Netherlands; Mex=Mexico; RCT=randomized controlled trials; AUS=Australia; CH=China; SWE=Sweden; HbA1c=hemoglobin A1c; SG=Singapore; TH=Thailand; CT=controlled trial; PCP=prospective cohort physician; T=Turkish; D=Dutch; DM=diabetes mellitus
Table 2 also provides an overview of the CCM components implemented in each study. Eight studies included all four components of the CCM model. The CCM component delivery system design was included in most studies (n=25), followed by self-management support (n=20). Of the studies that included the components delivery system design, most introduced a care team (n=13), followed by regular follow-up visits (n=8). Self-management support was mostly realized through individual educational sessions on diabetes, health and nutrition (n=14).
Outcome variables
HbA1c
In total, 18 uncontrolled studies – including prospective, retrospective and cross-sectional cohort designs – measured the effects of integrated care programs on HbA1c. In addition, seven studies compared the influence of patient characteristics on the effectiveness of integrated diabetes care programs between intervention and control groups. In total, 51 patient characteristics were assessed as potential effect modifiers of the relationship between integrated care and HbA1c. The results will be presented according to study design. For RCTs all characteristics assessed by this study design will be discussed. Due to the high number of characteristics assessed by the cross-sectional, retrospective and prospective cohort studies, only characteristics assessed by three or more studies will be presented.
(Randomized) controlled trials: Five RCTs and two controlled trials (CTs) compared the influence of patient characteristics on the effectiveness of integrated diabetes care programs on the HbA1c level between intervention and control groups (Table 3). In total, eight patient characteristics were evaluated as potential modifiers.
Table 3 Subgroup intervention effects on hemoglobin A1c (HbA1c)
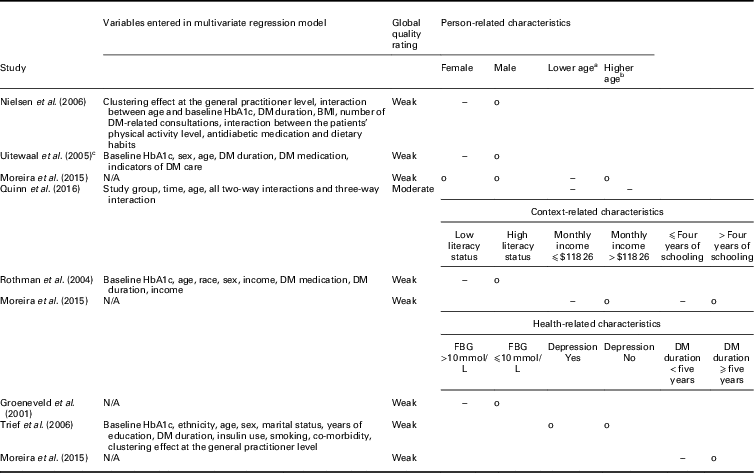
DM=diabetes mellitus; BMI=body mass index; N/A: not applicable; FBG=fasting blood glucose.
a Lower age: ⩽52 years (Moreira et al., Reference Mold, While and Forbes2015), <55 years (Quinn et al., Reference Quah, Liu, Luo, How and Tay2016).
b Higher age: >52 years (Moreira et al., Reference Mold, While and Forbes2015), ⩾55 years (Quinn et al., Reference Quah, Liu, Luo, How and Tay2016).
c Intervention and control groups only consisted of patients with a baseline HbA1c >7%.
o: No significant relationship between the characteristic with HbA1c for people in the intervention group compared to usual care; −: significant negative relationship between the characteristic with HbA1c for patients in the intervention group compared to usual care.
Sex and age were the person-related characteristics evaluated as potential effect modifiers. Three studies assessed sex as a potential modifier, of which two found that women in the intervention group had statistically significant lower HbA1c values at follow-up compared to women in the control group (Uitewaal et al., Reference Uitewaal, Voorham, Bruijnzeels, Berghout, Bernsen, Trienekens, Hoes and Thomas2005; Nielsen et al., Reference Nam, Chesla, Stotts, Kroon and Janson2006). For men, no statistically significant difference was found. The third study did not find a statistically significant relationship (Moreira et al., Reference Mold, While and Forbes2015). Age was assessed by two studies. Both found that younger patients receiving integrated diabetes care had statistically significantly lower HbA1c values at follow-up compared to patients receiving usual care (Moreira et al., Reference Mold, While and Forbes2015; Quinn et al., Reference Quah, Liu, Luo, How and Tay2016).
Three health-related characteristics were evaluated as potential effect modifiers of the relationship between integrated diabetes care programs and HbA1c: literacy status, income and number schooling years. Literacy status was assessed by one study (Rothman et al., Reference Rothman, Malone, Bryant, Horlen and Pignone2004), which found that patients in the intervention group with low literacy status (⩽6th grade) had statistically significant lower HbA1c values at follow-up compared to patients with low literacy status receiving usual care. Monthly income and number of schooling years were also each assessed by one study. Patients with lower monthly income (⩽$118.26) and ⩽four years of schooling at baseline receiving integrated diabetes care had significantly lower HbA1c values at follow-up compared to patient receiving usual care (Moreira et al., Reference Mold, While and Forbes2015).
Three health-related characteristics were evaluated as potential effect modifiers of the relationship between integrated diabetes care programs and HbA1c: fasting blood glucose (FBG), depression and diabetes mellitus (DM) duration. Each characteristic was assessed by one study. Patients with high FBG (>10 mmol/L) at baseline receiving integrated diabetes care had significantly lower HbA1c levels at follow-up compared to patients receiving usual care (Groeneveld et al., Reference Goldberger and Buxton2001). For patients with a FBG ⩽10 mmol/L no significant difference was found in HbA1c levels at follow-up between the intervention and control groups. Depression was not an effect modifier of the association between integrated diabetes care programs and HbA1c (Trief et al., Reference Trief, Morin, Izquierdo, Teresi, Eimicke, Goland, Starren, Shea and Weinstock2006). Patients with a DM duration <five years receiving integrated diabetes care had significantly lower HbA1c levels at follow-up compared to patients receiving usual care (Moreira et al., Reference Mold, While and Forbes2015).
No RCTs assessed context-related characteristics as potential effect modifiers of the relationship between integrated diabetes care programs and HbA1c.
Prospective and retrospective cohort studies: In total, 11 prospective and retrospective cohort studies measured the effects of integrated diabetes care programs on HbA1c (Tables 4 and 5). Three studies compared the change in HbA1c between levels of patient characteristics (Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). The other eight studies compared HbA1c levels at follow-up between levels of patient characteristics (El-Kebbi et al., 2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; De Fine Olivarius et al., Reference De Fine Olivarius, Siersma and Hansen2009; Robinson et al., Reference Riddle and Karl2009; Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011; Cardenas-Valladolid et al., Reference Cardenas-Valladolid, Salinero-Fort, Gomez-Campelo, De Burgos-Lunar, Abanades-Herranz, Arnal-Selfa and Andres2012; LeBlanc et al., Reference Kodner2015).
Table 4 Relationship between hemoglobin A1c (HbA1c) and person-related and context-related characteristics
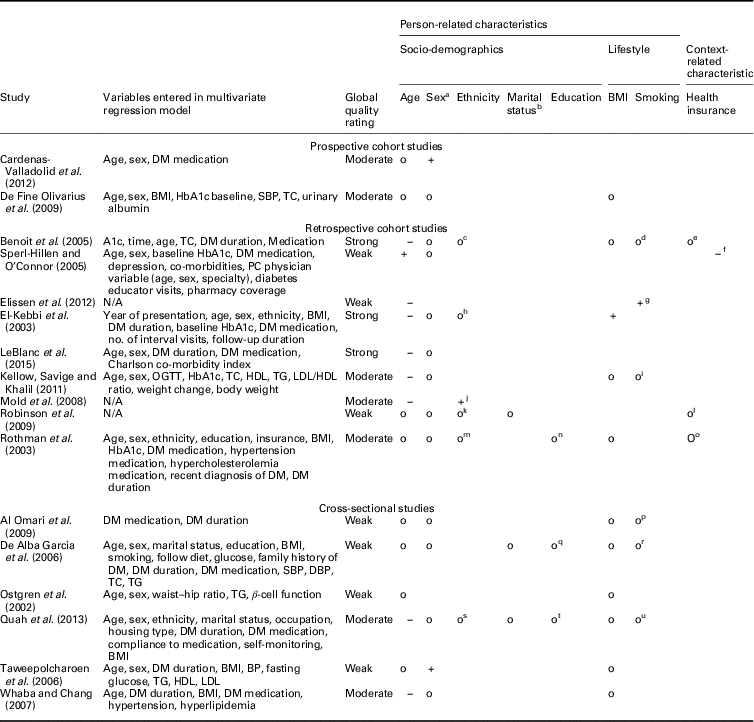
BMI=body mass index; DM=diabetes mellitus; SBP=systolic blood pressure; TC=total cholesterol; PC=prospective cohort; N/A=not applicable; OGTT=oral glucose tolerance test; HDL=high-density lipoprotein; LDL=low-density lipoprotein; TG=triglycerides; BP=blood pressure.
a 0=male, 1=female.
b 0=not married, 1=married.
c 0=Hispanic, black and white, 1=Asian.
d 0=current smoker, 1=past smoker, 2=never smoker.
e 0=insured, 1=County Medical Services, 3= uninsured.
f 0=pharmacy coverage, 1=no pharmacy coverage.
g 0=current smoker, 1=none smoker/previous smoker.
h 0=others, 1=African American.
i 0=non-smoker, 1= current smoker.
j 0=white, 1=black Caribbean/African.
k 0=white, 1=Asian, 2=black, 3=other.
l 0=insured, 1=uninsured.
m 0=black, 1=others.
n 0=less than high school, 1=high school or higher.
o 0=Medicaid or pharmacy assistance programs, 1= no Medicaid or pharmacy assistance program.
p 0=current smoker, 1=past and none smoker.
q 0=none, 1=incomplete primary, 2=completed primary, 3=primary.
r 0=smoker, 1=none smoker.
s 0=Chinese, 1=Malay, 2=Indian, 3=others.
t 0=no formal education, 1=formal education.
u 0=none smoker, 1=past smoker, 2=current smoker.
+: positive significant relationship; o- non-significant relationship; −: significant negative relationship.
Table 5 Relationship between hemoglobin A1c (HbA1c) and health-related characteristics
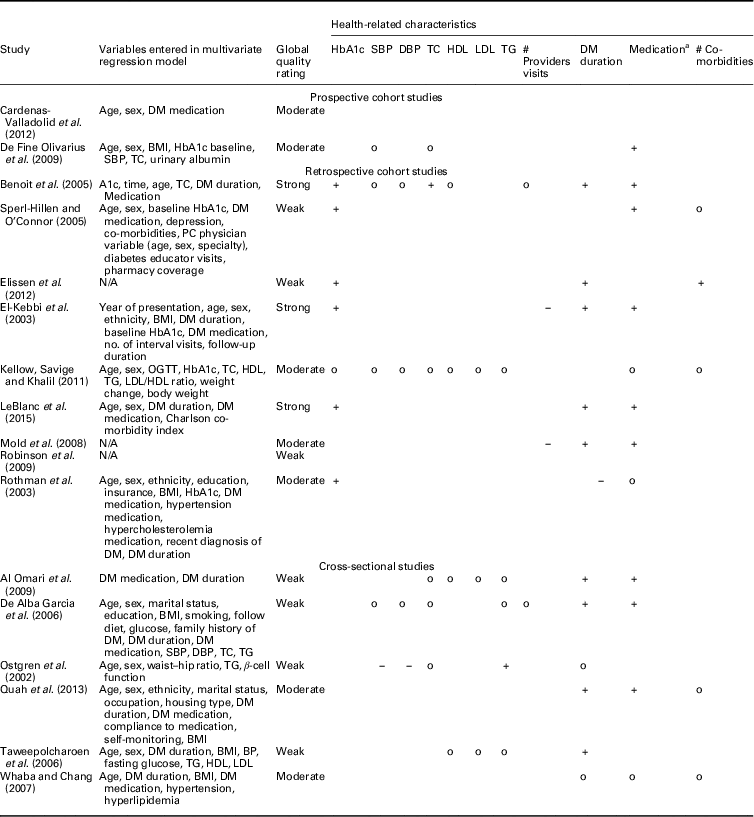
SBP=systolic blood pressure; DBP=diastolic blood pressure; TC=total cholesterol; HDL=high-density lipoprotein; LDL=low-density lipoprotein; TG=triglycerides; DM=diabetes mellitus; PC=primary care; OGTT=oral glucose tolerance test; N/A=not applicable; BMI=body mass index; BP=blood pressure.
+: positive significant relationship; o: non-significant relationship; −: significant negative relationship.
a 0=no insulin, 1=insulin.
Most examined person-related characteristics were age (n=11) and sex (n=9). In seven studies the effect of integrated diabetes care programs on HbA1c differed significantly across ranges of age: younger patients had higher HbA1c levels at follow-up compared to older patients (n=5) and experienced greater change from baseline in HbA1c (n=2) (El-Kebbi et al., 2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012; LeBlanc et al., Reference Kodner2015). As to the latter, the direction of the measured change in HbA1c differed: one study found a significant improvement (Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005) and the other a significant increase (Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012) in HbA1c. Age was not a significant effect modifier in the other four studies (Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; De Fine Olivarius et al., Reference De Fine Olivarius, Siersma and Hansen2009; Robinson et al., Reference Riddle and Karl2009; Cardenas-Valladolid et al., Reference Cardenas-Valladolid, Salinero-Fort, Gomez-Campelo, De Burgos-Lunar, Abanades-Herranz, Arnal-Selfa and Andres2012). The effect of integrated care on HbA1c did not differ between men and women in eight studies (El-Kebbi et al., 2003; Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; De Fine Olivarius et al., Reference De Fine Olivarius, Siersma and Hansen2009; Robinson et al., Reference Riddle and Karl2009; Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011; LeBlanc et al., Reference Kodner2015). In one study females had significantly higher HbA1c levels at follow-up compared to males (Cardenas-Valladolid et al., Reference Cardenas-Valladolid, Salinero-Fort, Gomez-Campelo, De Burgos-Lunar, Abanades-Herranz, Arnal-Selfa and Andres2012).
Most examined health-related characteristics were medication use (n=8), baseline HbA1c (n=7) and duration of type 2 diabetes (n=6). The effect of integrated diabetes care programs on HbA1c was different for people on insulin therapy. These patients had higher HbA1c levels at follow-up compared with patients on diet and/or oral therapy in five studies(El-Kebbi et al., 2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; De Fine Olivarius et al., Reference De Fine Olivarius, Siersma and Hansen2009; LeBlanc et al., Reference Kodner2015) and less desirable changes in HbA1c from baseline (Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005). In two studies the relationship between integrated diabetes care programs and HbA1c did not differ between types of medication (Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011). In the studies assessing baseline HbA1c, patients with higher baseline HbA1c levels had higher HbA1c levels at follow-up (n=3) (El-Kebbi et al., 2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; LeBlanc et al., Reference Kodner2015), but did have greater improvements in HbA1c from baseline (n=3) (Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012) compared to patients with lower baseline HbA1C levels. In one study baseline HbA1c was not a significant effect modifier (Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011). The effect of integrated diabetes care programs on HbA1c differed significantly across ranges of diabetes duration in five studies. Patients with longer diabetes duration had significantly higher HbA1c levels at follow-up compared to patients with shorter diabetes duration (n=5) (El-Kebbi et al., 2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012; LeBlanc et al., Reference Kodner2015). In one study a significant opposite effect was found (Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003).
Health insurance status was assessed by four studies. It did not seem to significantly modify the observed effect of integrated care on HbA1c in three studies (Rothman et al., Reference Rothe, Muller, Schwarz, Seifert, Kunath, Koch, Bergmann, Julius, Bornstein, Hanefeld and Schulze2003; Benoit et al., Reference Benoit, Fleming, Philis-Tsimikas and Ji2005; Robinson et al., Reference Riddle and Karl2009). Patients with no health insurance coverage had less desirable changes in HbA1c than those with health insurance coverage (Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005). No other context-related characteristics were examined by the included studies.
Cross-sectional studies: In total, six cross-sectional studies measured the modifying effect of patient characteristics on the relationship between integrated diabetes care programs and HbA1c (Tables 4 and 5).
Most examined person-related characteristics were age (n=6), body mass index (BMI) (n=6) and sex (n=5). Four studies of integrated care programs found non-significant associations between age and HbA1c (Ostgren et al., Reference Nielsen, de Fine Olivarius, Gannik, Hindsberger and Hollnagel2002; De Alba Garcia et al., Reference De Alba Garcia, Dallo, Salcedo Rocha, Rodriguez, Perez, Baer and Weller2006; Taweepolcharoen et al., Reference Tamayo, Rosenbauer, Wild, Spijkerman, Baan, Forouhi, Herder and Rathmann2006; Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009). In two studies significant associations were found: in these studies, younger patients had higher HbA1c levels (Wahba and Chang, Reference Wahba and Chang2007; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013). The effect of integrated diabetes care programs on HbA1c did not significantly differ between levels of BMI in all studies (Ostgren et al., Reference Nielsen, de Fine Olivarius, Gannik, Hindsberger and Hollnagel2002; De Alba Garcia et al., Reference De Alba Garcia, Dallo, Salcedo Rocha, Rodriguez, Perez, Baer and Weller2006; Taweepolcharoen et al., Reference Tamayo, Rosenbauer, Wild, Spijkerman, Baan, Forouhi, Herder and Rathmann2006; Wahba and Chang, Reference Wahba and Chang2007; Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013). The effect on HbA1c did also not differ between men and women in four studies (De Alba Garcia et al., Reference De Alba Garcia, Dallo, Salcedo Rocha, Rodriguez, Perez, Baer and Weller2006; Wahba and Chang, Reference Wahba and Chang2007; Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013). In one study females had significantly higher HbA1c levels compared to males (Taweepolcharoen et al., Reference Tamayo, Rosenbauer, Wild, Spijkerman, Baan, Forouhi, Herder and Rathmann2006).
Most examined health-related characteristics were duration of type 2 diabetes (n=6) and medication use (n=4). The effect of integrated care programs on HbA1c differed significantly across ranges of diabetes duration in four studies (De Alba Garcia et al., Reference De Alba Garcia, Dallo, Salcedo Rocha, Rodriguez, Perez, Baer and Weller2006; Taweepolcharoen et al., Reference Tamayo, Rosenbauer, Wild, Spijkerman, Baan, Forouhi, Herder and Rathmann2006; Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013). Patients with longer diabetes duration had higher HbA1c levels compared to patients with shorter diabetes duration in these studies. In two studies diabetes duration was not a significant effect modifier (Ostgren et al., Reference Nielsen, de Fine Olivarius, Gannik, Hindsberger and Hollnagel2002; Wahba and Chang, Reference Wahba and Chang2007). The effect of integrated care programs on HbA1c was also different for people on insulin therapy. These patients had higher HbA1c concentrations compared with patients on diet and/or oral therapy in three studies (De Alba Garcia et al., Reference De Alba Garcia, Dallo, Salcedo Rocha, Rodriguez, Perez, Baer and Weller2006; Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013). In one study type of medication was not a significant effect modifier (Wahba and Chang, Reference Wahba and Chang2007).
No context-related characteristics were assessed by three or more studies.
LDL-c
Three prospective and retrospective cohort studies measured the effect of integrated diabetes care programs on LDL-c. The RCTs and cross-sectional studies included in this review did not measure this effect. In total, 11 patient characteristics were assessed by the studies. Only those results that were assessed by at least two studies will be discussed.
Prospective and retrospective cohort studies: The person-related characteristic age was examined by three studies (Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; Robinson et al., Reference Riddle and Karl2009; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). The relationship between age and LDL-c was inconsistent: a negative and positive as well as a non-significant relationship were found.
The modifying effect of baseline LDL-c on the relationship between integrated diabetes care programs and changes in LDL-c over baseline was assessed by two studies (Sperl-Hillen and O’Connor, Reference Shaw, Rosen and Rumbold2005; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). Both found that patients with higher baseline LDL-c had greater LDL-c improvements.
No context-related characteristics were assessed by the included studies.
SBP
Four retrospective and prospective cohort studies measured the effect of integrated diabetes care programs on SBP. In total, nine patient characteristics were assessed by the studies. Only those results that were assessed by at least two studies will be discussed.
Retrospective cohort and prospective cohort studies: Age was measured by three studies (Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; Robinson et al., Reference Riddle and Karl2009; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). These studies found that higher age was associated with higher SBP at follow-up (Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; Robinson et al., Reference Riddle and Karl2009) and greater improvement (Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). The modifying effect of ethnicity on integrated care programs and SBP was measured by two studies (Mold et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2008; Robinson et al., Reference Riddle and Karl2009). The effect was unclear, as results were inconsistent between these studies. Four other characteristics were assessed, one context-related and three health-related characteristics, by one study each.
Health-care utilization
Health-care utilization was assessed by three studies: one RCT (Nielsen et al., Reference Nam, Chesla, Stotts, Kroon and Janson2006), one retrospective cohort study (Uitewaal et al., Reference Uitewaal, Bruijnzeels, Bernsen, Voorham, Hoes and Thomas2004) and one cross-sectional study (Liu et al., Reference LeBlanc, Rosales, Kachroo, Mukherjee, Funk and Nichols2013). Together they measured the modifying effect of integrated care programs and health-care utilization for five person-related characteristics, one context-related characteristic and one health-related characteristic. Most examined characteristic was sex, which was measured by two studies (Nielsen et al., Reference Nam, Chesla, Stotts, Kroon and Janson2006; Liu et al., Reference LeBlanc, Rosales, Kachroo, Mukherjee, Funk and Nichols2013). Nielsen et al. (Reference Nam, Chesla, Stotts, Kroon and Janson2006) found that females in the intervention group had statistically significant more GP consultations per year compared to females in the control group (Nielsen et al., Reference Nam, Chesla, Stotts, Kroon and Janson2006). For males, no difference was found. Liu et al. found that the effect of integrated diabetes care programs on health-care utilization was different between males and females (Liu et al., Reference LeBlanc, Rosales, Kachroo, Mukherjee, Funk and Nichols2013). Females had higher utilization of community health centers compared to male.
Discussion
This paper presents a literature review on relevant patient characteristics for guiding tailored integrated type 2 diabetes care in primary care. HbA1c was considered an outcome in 93% of the 27 studies identified. Many different patient characteristics were investigated by these studies. Findings indicate that the effect of integrated primary care programs on HbA1c differs significantly according to a number of person and health-related characteristics. Younger age, longer disease duration, higher baseline HbA1c and insulin therapy were associated with higher HbA1c levels. Health insurance status, living situation and income were the only context-related characteristics in the included studies and were not frequently assessed.
Compared to HbA1c, LDL-c, SBP and health-care utilization were included far less. It was found that higher baseline LDL-c lead to greater LDL-c improvement. Patients with higher age had higher SBP levels at follow-up as well as greater improvements in SBP compared to younger patients. The relationship between integrated care and health-care utilization seemed to be modified by sex: women had more consultations per year compared to men.
Several factors might explain the elevated HbA1c levels in a subset of patients with type 2 diabetes. Younger patients tend be more non-adherent to oral medication therapy and experience less profound diabetes-related health problems than older patients (Pyatak et al., Reference Pimouguet, Le Goff, Thiebaut, Dartigues and Helmer2014; Tunceli et al., Reference Tunceli, Iglay, Zhao, Brodovicz and Radican2015). The latter might cause them to believe that a proactive attitude toward their disease is less important. Moreover, younger patients and/or those with longer disease duration undergo a more rapid decline in β cell function and pancreatic insulin secretion, resulting in the need for a more complex and intensive drug therapy (Al Omari et al., Reference Al Omari, Khader, Dauod, Al-Akour, Khassawneh, Al-Ashker and Al-Shdifat2009; Fonseca, Reference Epstein and Street2009; Khattab et al., Reference Kellow, Savige and Khalil2010; Kellow et al., Reference Jotkowitz, Rabinowitz, Raskin Segal, Weitzman, Epstein and Porath2011). Higher HbA1c levels for patients on insulin therapy compared to patients on diet and/or oral therapy could be due to a delayed start or low intensity of insulin therapy (Abraira et al., Reference Abraira, Colwell, Nuttall, Sawin, Nagel, Comstock, Emanuele, Levin, Henderson and Lee1995; El-Kebbi et al., 2003; Mosenzon and Raz, Reference Moreira, Mantovani Mde and Soriano2013). Furthermore, maintaining glycemic control, while minimizing hypoglycemia and sticking to a diet might be difficult (Jin et al., Reference James, Oparil, Carter, Cushman, Dennison-Himmelfarb, Handler, Lackland, Lefevre, Mackenzie, Ogedegbe, Smith, Svetkey, Taler, Townsend, Wright, Narva and Ortiz2008; Quah et al., Reference Pyatak, Florindez, Peters and Weigensberg2013).
High HbA1c at baseline also seemed to be predictive of later HbA1c. First, type 2 diabetes is a heterogeneous disease in both pathogenesis and clinical manifestation (Inzucchi et al., 2012), thus a high HbA1c at baseline and at follow-up could be due to decreased insulin sensitivity, secretion and β-cell dysfunction (Heianza et al., Reference Hasnain-Wynia and Baker2012). Second, unhealthy lifestyle habits, such as low physical activity, and a diet rich in carbohydrates have been associated with less glycemic control (Mozaffarian et al., Reference Mosenzon and Raz2009; Inzucchi et al., 2012). Changing these lifestyle factors is easier said than done, making it difficult for patients to improve their glycemic control.
Several factors could explain the differences in levels of LDL-c, SBP and health-care utilization between levels of patient characteristics. Prescription of statins usually follows when LDL-c level is 2.5 mmol/L or higher, possibly leading to greater improvements in LDL-c for those patients with high baseline LDL-c levels (The Dutch college of general practitioners, Reference Taweepolcharoen, Sumrithe, Kunentrasai and Phraisuwanna2011). The higher SBP levels at follow-up for older patients may be due to less stringent treatment targets (van Hateren et al., Reference van Hateren, Landman, Kleefstra, Houweling, Van Der Meer and Bilo2012; James et al., Reference Inzucchi, Bergenstal, Buse, Diamant, Ferrannini, Nauck, Peters, Tsapas, Wender and Matthews2014). The greater health-care utilization by women compared to men might be explained by the difference in perception of illness between men and women. According to some studies, it is more culturally and socially accepted for women to be ill than it is for men (De Visser et al., Reference De Visser, Smith and McDonnell2009).
Overall, our results indicate the need to implement integrated diabetes care programs specifically tailored to the needs, values and preferences of younger patients and to those on insulin therapy, with longer disease duration and/or higher HbA1c levels and older patients with high SBP levels. These effect modifiers can help to provide the right care to the right person at the right time. At this moment, not every patient with these characteristics receives such care. Current practice might therefore not be suitable for all patients. Lack of motivation, family support and feeling burned-out from managing diabetes are reported barriers to optimal self-management (Browne et al., Reference Browne, Scibilia and Speight2013). To tackle these barriers, diabetes treatment programs should take them into account by, for example, providing shared decision making and simple and specific instructions and advice, involving family members and offering online consultations or evening primary care opening hours. In addition to patients who find it difficult to keep their diabetes under control, there is a large group of patients who does manage to control their diabetes (Rothe et al., Reference Robinson, Baron, Cooper and Janson2008; Elissen et al., Reference Elissen, Duimel-Peeters, Spreeuwenberg, Spreeuwenberg and Vrijhoef2012). For these patients, fewer visits to primary care might have similar outcomes and thus should be taken into consideration by both the GP and the patient. Allowing care givers to provide care based on patient characteristics constitutes a promising approach for achieving the so-called ‘Triple Aim’ by: (1) improving patient experience, by including patients’ care needs, preferences, and abilities in treatment decisions; (2) improving population health and quality of life, by supporting tailored diabetes care; and (3) reducing the per capita cost of diabetes care, by reducing the over-, under- and misuse of health-care services (Berwick et al., Reference Berwick, Nolan and Whittington2008).
This review has several limitations that should be taken into account. First, given the scarceness of studies assessing the differences in the effect of integrated diabetes care programs on diabetes control measures by levels of patient characteristics, it was decided to include RCTs, prospective and retrospective cohort studies. However, this introduced significant heterogeneity and made it impossible to conduct a meta-analysis. Second, quality of the studies was weak for most studies. This was mainly due to the cross-sectional study design of more than one-third of the studies and the use of less robust statistical methods. Fortunately, it is unlikely that these studies altered the results, as their findings were similar to those of the other, more robust studies. Third, very few context- and person-related characteristics were analyzed. Studies performed in a non-integrated diabetes care setting, found that context-related characteristics, such as socio-economic status and social network, are associated with measures of diabetes control and are likely to be strong predictors of diabetes control (Jotkowitz et al., Reference Jin, Sklar, Min Sen Oh and Chuen Li2006; Nam et al., Reference Mozaffarian, Kamineni, Carnethon, Djousse, Mukamal and Siscovick2011). Person-related characteristics, such as low mastery and low self-efficacy, have been related to negative health outcomes (Bosma et al., Reference Bosma, Theunissen, Verdonk and Feron2014; Elissen et al., Reference Elissen, Hertroijs, Schaper, Bosma, Dagnelie, Henry, Van Der Kallen, Koster, Schram, Stehouwer, Schouten, Berendschot and Ruwaard2017). Traditionally, researchers and care providers have looked at diabetes from a mostly biomedical viewpoint, which might explain the relatively scarce collection of context- and person-related characteristics in routinely collected individual patient data (Hasnain-Wynia and Baker, Reference Groeneveld, Petri, Hermans and Springer2006).
The current review provides a good understanding of which characteristics can help to identify patients with different health-care needs and preferences. However, to implement an effective integrated type 2 diabetes tailored care program, it is necessary to know which context- and person-related characteristics are important to identify patients. Furthermore, implementation of an effective tailored diabetes care program is only possible by taking into account the care preferences of patients and caregivers. In the next phase of the PROFILe project (Elissen et al., Reference Elissen, Hertroijs, Schaper, Vrijhoef and Ruwaard2016), data rich in non-health-related characteristics will be analyzed to assess which of these are predictors of diabetes control measures and a discrete choice experiment will be conducted to gain knowledge on patients’ care preferences as a first step toward patient-centered diabetes care.
Acknowledgments
None.
Financial Support
This PROFILe project was supported by a grant from Novo Nordisk Netherlands (no grant number). The sponsor had no role in study design, in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of interest
None.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S146342361800004X









