Introduction
Single-stranded RNA coronaviruses have pervaded the human microbiome for centuries.Reference Yamamoto, Saito, Tamura, Prawisuda, Mizutani and Yotsuyanagi1 Endemic within human populations,Reference Cereda, Pagani and Romero2 coronaviruses contribute to 15–30 per cent of common colds,Reference Liu, Liang, Fung, Bamford and Zuckerman3 manifesting as rhinorrhoea, sinusitis, fever, cough, tachypnoea and odynophagia.Reference Su, Wong, Shi, Liu, Lai and Zhou4 However, clinical manifestations of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) – responsible for the current coronavirus disease 2019 (Covid-19) pandemic – are broad, ranging from mild cough to acute respiratory disease syndrome and multi-organ failure.Reference Zhou, Yu, Du, Fan, Liu and Liu5 Although mortality rates are falling, the long-term impact of SARS-CoV-2 remains uncertain.Reference Desforges, Gurdasani, Hamdy and Leonardi6
Emulating its relatives, the novel coronavirus spreads via droplet transmission,Reference Li, Guan, Wu, Wang, Zhou and Tong7 forming reservoirs within the upper aerodigestive tract.Reference Liaw, Saadi, Patel and Isildak8 Single-cell RNA analysis suggests the nasal cavity carries significant expression of angiotensin-converting enzyme (ACE2; the cell surface receptor for SARS-CoV-2).Reference Sungnak, Huang, Bécavin, Berg, Queen and Litvinukova9 Such increased viral load inspired the generation of reverse-transcriptase polymerase chain reaction assays using nasopharyngeal swabs.Reference Zou, Ruan, Huang, Liang, Huang and Hong10
Contrary to expectations, Hasanoglu et al.Reference Hasanoglu, Korukluoglu, Asilturk, Cosgun, Kalem and Altas11 found increased SARS-CoV-2 viral load occurs in asymptomatic patients. Additionally, coronaviruses have long been associated with middle-ear pathology,Reference Elkhatieb, Hipskind, Woerner and Hayden12 and consequent inflammation is represented as mastoid and middle-ear effusion on computed tomography (CT) imaging.Reference Brennan and Saadia-Redleaf13 Coronaviruses are implicated in the pathogenesis of acute otitis media.Reference Bulut, Güven, Otlu, Yenişehirli, Aladağ and Eyibilen14,Reference Pitkäranta, Jero, Arruda, Virolainen and Hayden15 Hence, the first isolation of the SARS-CoV-2 virus from middle-ear autopsy specimens in July 2020 was unsurprising.Reference Frazier, Hooper, Mostafa and Stewart16
The long-term implications of the presence of SARS-CoV-2 in the middle ear, mastoid and sinonasal tract are not yet quantified. Case reports suggest SARS-CoV-2 may be linked with sensorineural hearing loss,Reference Koumpa, Forde and Manjaly17–Reference Sriwijitalai and Wiwanitkit22 and its association with anosmia is well documented.Reference Brann, Tsukahara, Weinreb, Lipovsek, Van den Berge and Gong23,Reference Zhang, Shan, Abdollahi and Nace24 However, the radiological implications of SARS-CoV-2 and predictors of mortality remain unclear.
This study aimed to assess the incidence of radiological inflammation within the paranasal sinuses, middle ear and mastoid cavity in patients with confirmed SARS-CoV-2, and to determine whether this has any relationship with all-cause mortality.
Materials and methods
This retrospective cohort study examined radiological findings in 147 consecutive adults with confirmed Covid-19 who underwent CT of the head between 1 March 2020 and 24 June 2020. Patients aged over 18 years with confirmed SARS-CoV-2 infection within 7 days of imaging by reverse-transcriptase polymerase chain reaction testing were included. All CT images were reviewed and reported by a consultant radiologist; patients were excluded from the study if there was any evidence of temporal bone or skull base fracture. We were unable to obtain access to full patient notes for retrospective data collection; therefore, we were unable to exclude patients who had undergone previous otological or rhinological surgery.
Axial and coronal CT images were analysed to determine the extent of paranasal sinus, middle-ear and mastoid opacification. Patients were excluded if there was any evidence of temporal bone or skull base fracture. The Lund–Mackay 3-point scoring system was used to individually categorise opacification of maxillary, frontal, anterior and posterior ethmoidal, osteomeatal complex and sphenoid sinuses bilaterally (Table 1 and Figure 1), with a maximum total score of 24. Similarly, mastoid and middle-ear opacification was graded bilaterally using a 3-point scale (Table 2 and Figure 2), with a maximum total score of 8. Mortality was recorded if it occurred within 30 days of the date of imaging. Our outcomes were compared against pre-pandemic studies examining the prevalence of incidental mastoidReference Mughal, Charlton and Clark25 and sinusReference Nazri, Bux, Tengku-Kamalden, Ng and Sun26 opacification.
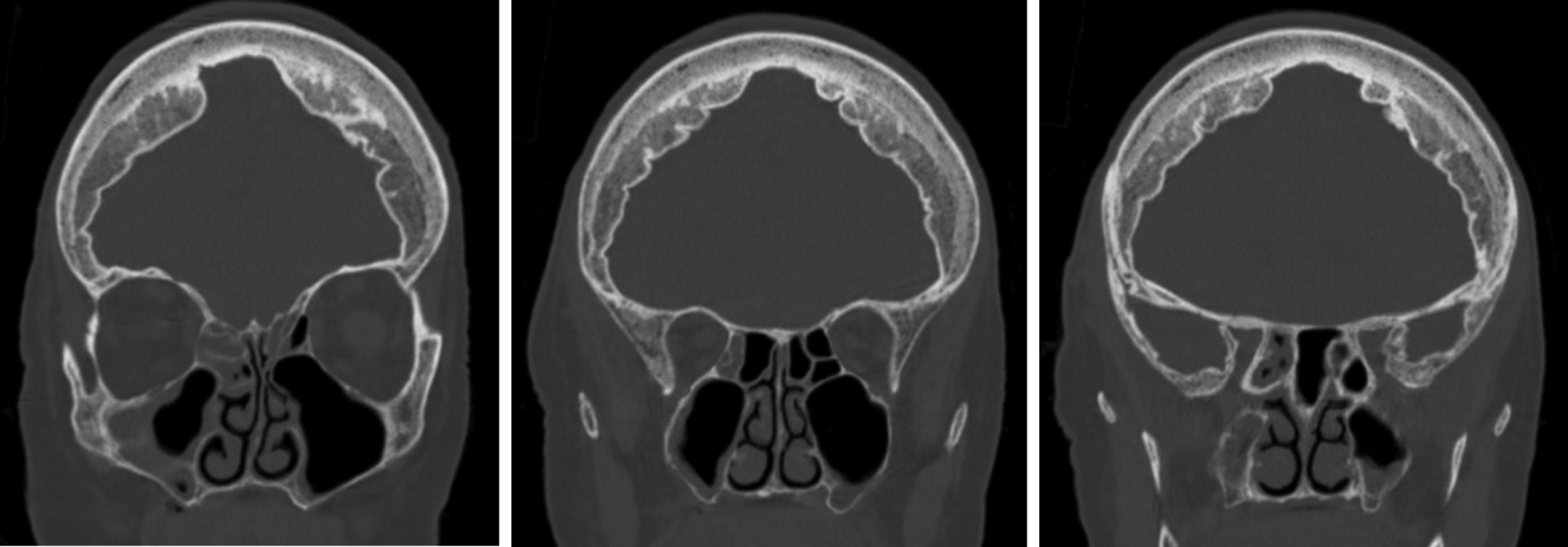
Fig. 1. Example of Lund–Mackay sinus scoring. Non-contrast coronal computed tomography images of the head of a patient with a total Lund–Mackay sinus score of 4: partial opacification of left anterior ethmoid sinus (1 point); partial opacification of right maxillary sinus (1 point); partial opacification of right anterior ethmoid sinus (1 point); and partial opacification of right sphenoid sinus (1 point).
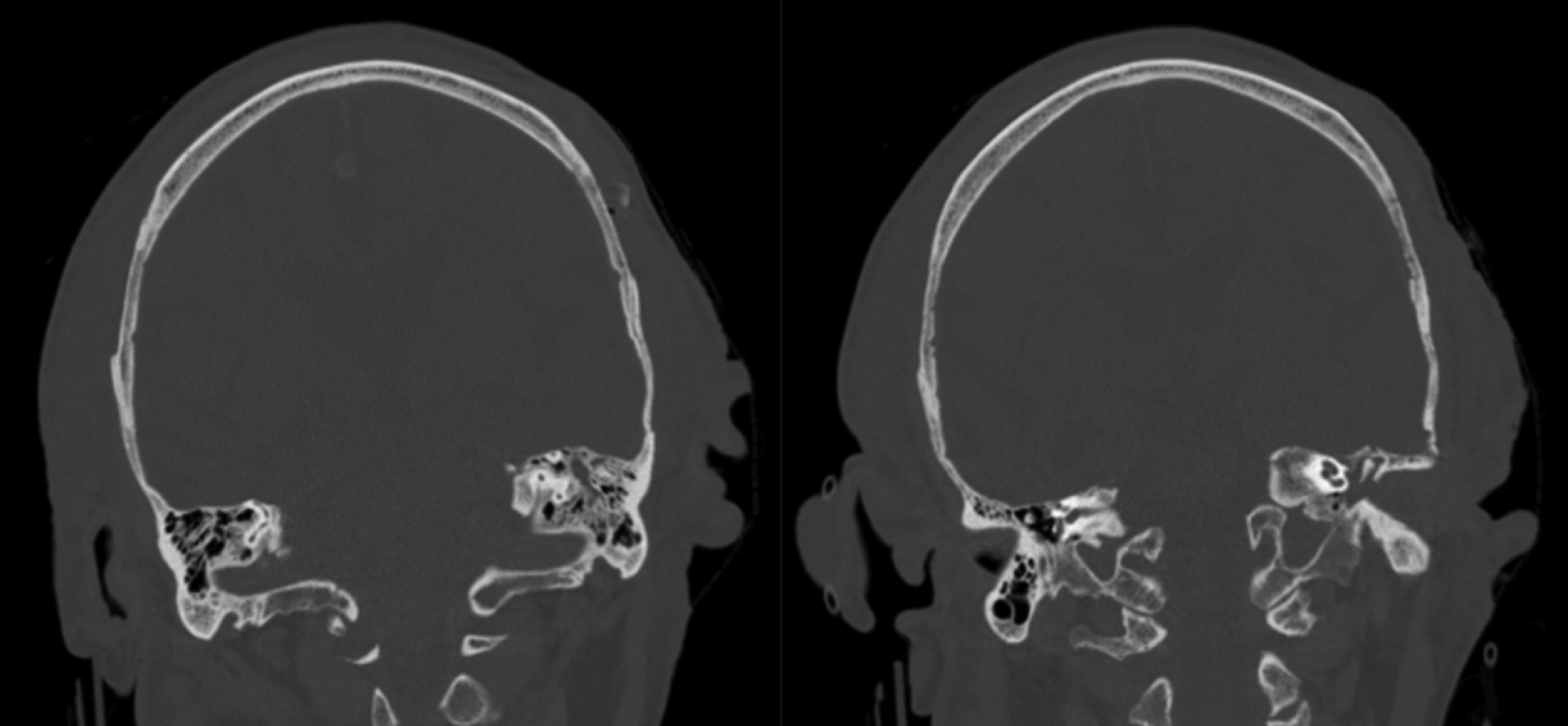
Fig. 2. Example of middle-ear and mastoid opacification scoring. Non-contrast coronal computed tomography images of the head of a patient with a total middle-ear and mastoid opacification score of 3: partial opacification of left mastoid (1 point) and total opacification of left middle ear (2 points).
Table 1. Lund–Mackay sinus scoring

Table 2. Middle-ear and mastoid opacification scoring

A Mann–Whitney U test was used to determine whether there were differences in total Lund–Mackay sinus scores or middle-ear and mastoid opacification scores between patients who had died or survived at 30-days’ follow up. P-values of less than 0.05 were considered a statistically significant result. Statistical analysis was performed using SPSS Statistics software for MacBook, version 28.0.1.0 (IBM, Armonk, New York, USA).
Results
Of 147 patients originally identified, only 83 patients had a positive polymerase chain reaction test result within 7 days of imaging, thereby meeting the criteria for inclusion. Forty-nine patients (59.0 per cent) were male and 34 (41.0 per cent) were female (Table 3). Mean age was 77.3 years (standard deviation = 14.5), with an age range of 19–98 years. Thirty-eight patients (45.8 per cent) died within 30 days of the date of imaging. The most common indications for CT of the head were suspected intracranial haemorrhage (31.3 per cent, n = 26) and suspected cerebrovascular accident (26.5 per cent, n = 22) (Table 4).
Table 3. Patient demographics and incidence of opacification on head CT

*n = 83; †n = 45; ‡n = 38. CT = computed tomography; SD = standard deviation; M = male; F = female
Table 4. Indication for head CT

CT = computed tomography; CVA = cerebrovascular accident; GCS = Glasgow Coma Scale
The incidence of opacification by anatomical subsite is reported in Table 5. Out of 83 patients, 43 had some form of sinus opacification (51.8 per cent); of these 43 patients, 21 had bilateral sinus opacification (48.8 per cent). Twenty of the 83 patients had some middle-ear or mastoid opacification (24.1 per cent); of these 20 patients, 9 had bilateral ear involvement (45 per cent).
Table 5. Incidence of opacification by subsite

Lund–Mackay sinus score
The median total Lund–Mackay sinus score for all included patients was 1.0 (interquartile range = 2.0). A Mann–Whitney U test was performed to determine whether there were differences in the total Lund–Mackay sinus score between patients who were alive at 30 days post imaging and those who had died. Distributions of the Lund–Mackay sinus score between the two groups of patients were similar, as assessed by visual inspection. Median total Lund–Mackay sinus scores for patients who survived (1.0, interquartile range = 2) and those who died (0.5, interquartile range = 1) were not statistically significantly different (U = 747.5, p = 0.294).
Middle-ear and mastoid opacification score
The median middle-ear and mastoid opacification score for all included patients was 0 (interquartile range = 0). A Mann–Whitney U test was performed to determine whether there were differences in the total middle-ear and mastoid opacification score between patients who were alive at 30 days post imaging and those who had died. Distributions of the temporal bone opacification score between the two groups of patients were similar, as assessed by visual inspection. Median temporal bone opacification scores for patients who survived (0, interquartile range = 1.0) and those who died (0, interquartile range = 0) were not statistically significantly different (U = 839, p = 0.845).
Discussion
Of 83 patients eligible for inclusion, 51.8 per cent (n = 43) had evidence of sinus opacification and 24.1 per cent (n = 20) had evidence of middle-ear or mastoid opacification. The median total Lund–Mackay sinus score for all patients was 1.0 (interquartile range = 2.0), and the median middle-ear and mastoid opacification score was 0 (interquartile range = 0). There was no statistically significant difference in sinus or middle-ear and mastoid opacification between patients after stratification based on 30-day all-cause mortality.
Marginally lower rates of sinus and mastoid opacification in patients with Covid-19 are reported in the literature (Table 6);Reference Mughal, Charlton and Clark25–Reference İslamoğlu, Ayhan, Bercin, Kalem, Kayaaslan and Güner30 41.8 per cent of Moonis and colleagues’ cohort of 55 patients had sinus disease, yet only 7 per cent demonstrated mastoid opacification.Reference Moonis, Mitchell, Szeto and Lalwani29 Similarly, İslamoğlu et al. reported much lower levels of mastoid opacification in a cohort of 129 patients, at only 2.32 per cent.Reference İslamoğlu, Ayhan, Bercin, Kalem, Kayaaslan and Güner30
Table 6. Literature review of studies examining incidence of sinus and mastoid and middle-ear opacification

N/A = not applicable
Our results reflect higher levels of sinus opacification in patients with Covid-19 when compared with a 14.8–37 per cent rate of incidental prevalence of sinus opacification on CT prior to the SARS-CoV-2 pandemic.Reference Nazri, Bux, Tengku-Kamalden, Ng and Sun26 However, Naeini et al. analysed CT imaging of patients with anosmia secondary to Covid-19 infection, and found that most patients (83.7 per cent) had a Lund–Mackay score of 0, discrediting a conductive pathophysiology for anosmia.Reference Naeini, Karimi-Galougahi, Raad, Ghorbani, Taraghi and Haseli27 Similarly, a systematic review of radiological imaging in Covid-19 patients with anosmia, by Keshavarz et al., suggested sinus opacification in only 12.5 per cent.Reference Keshavarz, Haseli, Yazdanpanah, Bagheri, Raygani and Karimi-Galougahi28 The pattern of sinus involvement was also variable. Maxillary and ethmoidal sinus involvement predominated in our study, whereas frontal sinus involvement was uncommon. Similarly, ethmoidal, maxillary and sphenoid sinuses were most commonly affected in Moonis and colleagues’ cohort.Reference Moonis, Mitchell, Szeto and Lalwani29
Although the nasopharynx has been demonstrated to host high titres of SARS-CoV-2,Reference Sungnak, Huang, Bécavin, Berg, Queen and Litvinukova9,Reference Zou, Ruan, Huang, Liang, Huang and Hong10 literature suggests this is not reflected in radiological findings.Reference Moonis, Mitchell, Szeto and Lalwani29,Reference İslamoğlu, Ayhan, Bercin, Kalem, Kayaaslan and Güner30 Moonis et al. further demonstrated that olfactory cleft opacification and nasopharyngeal thickness did not correlate with Covid-19 infection.Reference Moonis, Mitchell, Szeto and Lalwani29 Such limited sinonasal involvement does not reflect radiological findings reported for other viral upper respiratory tract infections.Reference Gwaltney, Phillips, Miller and Riker31 Moonis et al. also reported minimal radiological evidence of sinusitis secondary to SARS-CoV-2, which correlated with their clinical findings; less than 10 per cent of their study population reported symptoms of upper respiratory tract infection.Reference Moonis, Mitchell, Szeto and Lalwani29 Systematic reviewsReference Lovato and de Filippis32 and case seriesReference Huang, Wang, Li, Ren, Zhao and Hu33–Reference Zhang, Dong, Cao, Yuan, Yang and Yan35 suggest sinonasal symptoms are atypical in Covid-19 infection, with lower respiratory tract infection and constitutional symptoms predominating. Reports suggest nasal congestion and rhinorrhoea are less common symptoms,Reference Lovato and de Filippis32 in comparison to fever, fatigue, cough and shortness of breath.Reference Huang, Wang, Li, Ren, Zhao and Hu33–Reference Zhang, Dong, Cao, Yuan, Yang and Yan35 Although magnetic resonance imaging modalities were beyond the scope of this paper (as they were not available for the patients), other studies have correlated anosmia with loss of olfactory bulb volume, opacification of the olfactory cleft and a T2-weighted fluid-attenuated inversion recovery (‘FLAIR’) hyperintensity of the olfactory tract.Reference Laurendon, Radulesco, Mugnier, Gérault, Chagnaud and El Ahmadi36–Reference Strauss, Lantos, Heier, Shatzkes and Phillips38
Mastoiditis is a frequent incidental radiological finding on CT of the head, as fluid collects in mastoid air cells whilst the patient lies supine as the scan is performed.Reference Mughal, Charlton and Clark25 Mughal et al. reported that 8.4 per cent of head CT scans suggested incidental mastoid opacification.Reference Mughal, Charlton and Clark25 Acute otitis media is a common sequelae of upper respiratory tract infection,Reference Zou, Ruan, Huang, Liang, Huang and Hong10,Reference İslamoğlu, Ayhan, Bercin, Kalem, Kayaaslan and Güner30 with consequent middle-ear effusion and mastoid opacification identifiable on radiological imaging.Reference Pitkäranta, Jero, Arruda, Virolainen and Hayden15 Thus, Covid-19 was postulated to manifest as ontological involvement. However, İslamoğlu et al. evaluated temporal CT scans of patients with Covid-19 and found no significant middle-ear or mastoid inflammation,Reference İslamoğlu, Ayhan, Bercin, Kalem, Kayaaslan and Güner30 reflecting findings seen in our study, which shows a 24.1 per cent rate of ontological radiological signs.
We recognise that our study has some limitations. A comparator group of coronavirus-negative patients undergoing CT of the head was deemed impractical owing to initially high false negative polymerase chain reaction results.Reference Kucirka, Lauer, Laeyendecker, Boon and Lessler39 Whilst Moonis et al.Reference Moonis, Mitchell, Szeto and Lalwani29 correlated imaging with patient symptoms, contemporaneous assessment of clinical features was renounced owing to the contagious nature of Covid-19 and concomitant pressures on healthcare workers. Similarly, pure tone audiology would have provided an objective assessment of hearing, but was deemed unfeasible for the purposes of this study as the patients were in isolation and, in some cases, critically unwell.
Images were reviewed by otolaryngologists with prior experience in analysing CT scans of the sinuses and temporal bones. Ideally, imaging findings would be independently corroborated by a second reviewer, or reported by a specialist head and neck radiologist.Reference Moonis, Mitchell, Szeto and Lalwani29 Emulating Moonis et al.,Reference Moonis, Mitchell, Szeto and Lalwani29 further analysis could have assessed nasopharyngeal thickness and olfactory recess opacification. Intubated patients or those with nasogastric tubes at the time of imaging were not excluded, as cross-sectional imaging was not always sufficiently caudal. Finally, patients who had previously undergone sinus surgery or required prior otological procedures were not excluded from this study.
Although there was no statistical correlation between sinus opacification or middle-ear and mastoid opacification and mortality, there have been no assessments of long-term sequelae. This is pertinent to ENT, with known long coronavirus effects of anosmiaReference Walker, Pottinger, Scott and Hopkins40 and sensorineural hearing lossReference Koumpa, Forde and Manjaly17 publicised. Additionally, the statistics presented may prove beneficial when explaining radiological imaging to patients in clinic.
• This study assessed paranasal sinus, middle-ear and mastoid radiological inflammation in coronavirus disease 2019 (Covid-19)
• Sinus opacification levels were higher in Covid-19 patients compared with incidental prevalence prior to the pandemic
• High titres of severe acute respiratory syndrome coronavirus-2 have been identified previously in the nasopharynx and mastoid
• Only mild mucosal disease within the sinuses, middle ear and mastoid was identified radiologically
• This study does not demonstrate a statistically significant correlation between sinus or middle-ear or mastoid opacification and 30-day mortality
Variable sinus and mastoid and middle-ear opacification may occur between different coronavirus variants. There may be value in repeating this study in another time cohort, to assess whether there is any variation in results with a different viral genotype, or variation in symptoms of otitis media with effusion, hearing loss, rhinosinusitis or Eustachian tube dysfunction.
Conclusion
Radiological findings on CT imaging in patients with SARS-CoV-2 infection suggest only mild mucosal disease within the paranasal sinuses (51.8 per cent), middle ear and mastoid cavity (24.1 per cent). There is no statistical correlation between sinus or mastoid opacification and mortality.
Competing interests
None declared










