Introduction
Tetracycline (TC), an effective and inexpensive broad-spectrum antibiotic, has been widely used for human beings and animals to prevent or treat bacteria-caused infections. TC was reported to be the second most used antibiotic worldwide in the year 2020 (Leichtweis et al., Reference Leichtweis, Vieira, Welter, Silvestri, Dotto and Carissimi2022). However, the majority (70–95%) of TC in animals is excreted into the environment (Qiao et al., Reference Qiao, Li, Duan and He2020). TC and its derivatives are environmentally persistent and readily accumulate in aquatic environments, thereby negatively affecting the ecosystem and human health (Xiao et al., Reference Xiao, Wu, Wang, Chen and Liu2021; Xu et al., Reference Xu, Zhang, Xiong, Zhu, Liao and Jiang2021). Therefore, the demand is increasing for efficient, economical, and eco-friendly removal of TC from contaminated waters.
Various technologies have been developed for TC removal, such as adsorption, membrane filtration, and chemical/photochemical degradation (Gopal et al., Reference Gopal, Alex, Chandrasekaran and Mukherjee2020; Leichtweis et al., Reference Leichtweis, Vieira, Welter, Silvestri, Dotto and Carissimi2022). The adsorption method is most widely accepted due to its advantages of easy operation, low consumption, and re-usability. Many adsorbents have been designed, of which nanomaterials usually exhibit excellent performance due to their surface activity and large specific surface area arising from their unique nanostructures (Xiong et al., Reference Xiong, Zeng, Yang, Zhou, Zhang, Cheng, Liu, Hu, Wan, Zhou, Xu and Li2018; Zhang et al., Reference Zhang, Li, Shi, Guo, He, Cao, Hu, Cui and Liu2018; Qiao et al., Reference Qiao, Li, Duan and He2020; Wang et al., Reference Wang, Gao, Cao, Dan and Yang2021). However, the manufacture of nanomaterials often consumes abundant chemical reagents and produces secondary pollution, thus limiting their practical applications.
As a kind of natural nanomaterial, clay minerals have been widely considered to be sustainable adsorbents for water-pollution control (Peng et al., Reference Peng, Liu, Zheng and Zhou2015; Feng et al., Reference Feng, Hung, Yang and Liu2019; Yang et al., Reference Yang, Cai, Chen, Cao, Liu and Liu2021; Ewis et al., Reference Ewis, Ba-Abbad, Benamor and El-Naas2022). Some studies have conducted the TC adsorption on various clay minerals, such as illite (Chang et al., Reference Chang, Li, Jean, Jiang, Wang and Lin2012), kaolinite (Bansal, Reference Bansal2013), halloysite (Zhang et al., Reference Zhang, Wang, Su, Liu and Du2021), and montmorillonite (Parolo et al., Reference Parolo, Savini, Vallés, Baschini and Avena2008; Wu et al., Reference Wu, Zhao, Jing, Shao, Liu, Lv, Hu, Zhang, Meng and Liu2019; Maged et al., Reference Maged, Iqbal, Kharbish, Ismael and Bhatnagar2020). Allophane (1–2SiO2·Al2O3·5–6H2O) is a natural nanosized clay mineral and commonly shows some physicochemical properties similar to nanomaterials due to its unique hollow spherical structure. Allophane consists of 3.5–5.0 nm diameter hollow spherules, of which the spherule wall that contains several defect pores (0.35–0.5 nm) is composed of a curved gibbsite-like sheet with orthosilicate/oligomeric silicate groups attached to its inside. As a result, allophane has plenty of surface hydroxyl groups and a large specific surface area (200–500 m2g–1, theoretically up to >1000 m2g–1) (Huang et al., Reference Huang, Lowe, Churchman, Schipper, Cursons, Zhang, Chen and Cooper2016; Wang et al., Reference Wang, Du, Yuan, Liu, Song, Zhou, Deng and Liu2020), thereby exhibiting large adsorption capacities to polar organic matter (e.g. DNA, fatty acids, norfloxacin, ciprofloxacin) (Nishikiori et al., Reference Nishikiori, Kobayashi, Kubota, Tanaka and Fujii2010; Huang et al., Reference Huang, Lowe, Churchman, Schipper, Cursons, Zhang, Chen and Cooper2016; Ma et al., Reference Ma, Wei, Zhao, Wang, Zhang, Liu and Yuan2023a; Ma et al., Reference Ma, Zhao, Wei, Wang, Liu and Yuan2023b). Accordingly, allophane presumably should show a strong affinity towards TC, but the actual adsorption of TC to it is not fully understood.
The goal of the present study, therefore, was to evaluate the performance of, and mechanisms for, the adsorption of TC to hollow, spherical allophane, and to highlight the nanostructural advantage of allophane over two other clay minerals with different nanostructures, namely 1:1 layered nanotubular halloysite and 2:1 layered montmorillonite. Results from this study are expected to expand the application of allophane in the control of environmental pollution and to provide fundamental knowledge for the development of efficient and environmentally friendly TC adsorbent materials based on clay minerals.
Materials and methods
Materials and chemicals
To avoid the interference of impurities, allophane was obtained via a synthetic method as reported previously (Wang et al., Reference Wang, Du, Yuan, Liu, Song, Zhou, Deng and Liu2020). Because the drying process might cause serious and irreversible aggregation of allophane nanoparticles (Du et al., Reference Du, Thill, Yuan, Wang, Liu, Gobeaux, Deng and Song2020), an allophane suspension (mass concentration of ~5 mg mL–1) without drying was used for adsorption. Halloysite and montmorillonite powders with high purity were sourced from mine deposits in Shanxi Province and Inner Mongolia, China, respectively; both clay minerals were purified by a conventional sedimentation method and then passed through a 200 mesh sieve. The chemical composition of the clay minerals (Table 1) was determined by an X-ray fluorescence spectrometer (XRF) (Shimadzu XRF-1800). The cation exchange capacities (CEC) of allophane, halloysite, and montmorillonite were 85, 31, and 108 mmol g–1, respectively, determined by the hexaamminecobalt trichloride method (Zhu et al., Reference Zhu, Zhu, Xu and Ruan2007).
Table 1. Chemical composition of three clay minerals determined by XRF (wt.%)

Tetracycline, NaCl, KCl, CaCl2, MgCl2, NaOH, Na2CO3, HCl, and potassium hydrogen phthalate of AR grade were purchased from Shanghai Maclin Biochemical Technology Co., Ltd, China. Solutions were prepared using deionized water (18.25 MΩ cm). TC is known to show three pKa values (3.3, 7.7, 9.7) due to its tricarbonyl amide (C-1; C-2; C-3), phenol diketone (C-5; C-7; C-9), and dimethylamine (C-18) groups (Fig. 1). It appears mainly in cationic form at pH ≤3.3, zwitterionic form at pH 3.3–7.7, and anionic form at pH >7.7 (Kulshrestha et al., Reference Kulshrestha, Giese and Aga2004).
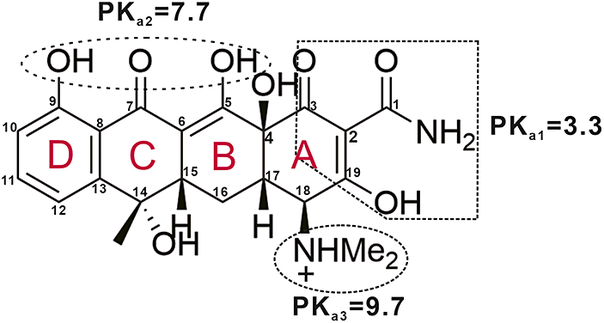
Figure 1. Planar chemical structure of the tetracycline molecule and its pKa values (Kulshrestha et al., Reference Kulshrestha, Giese and Aga2004).
Adsorption experiments
The adsorption behavior of TC on the clay minerals was investigated through batch adsorption experiments. Five milligrams of the clay mineral was mixed with 50 mL of TC solution (100 mg L–1) in a 100 mL Erlenmeyer flask followed by shaking on a thermostatic shaker at a speed of 200 rpm at 25°C. The suspension pH was kept at 7 (adjusting with 0.1 M HCl or NaOH solution, <0.1 mL per addition). After adsorption, suspensions were centrifuged at 10,000×g for 10 min, and the supernatants were collected to measure the TC concentration using a PC TU-1810 UV-Vis spectrophotometer at 275 nm. The adsorption capacity (q e, mg g–1) was calculated as follows:
where C 0 and C e (mg L–1) are the TC concentrations before and after adsorption, respectively; V (mL) is the volume of TC solution, and m (g) is the mass of adsorbent.
Measurements were taken at various time intervals ranging from 1 to 1440 min and the resulting kinetic data were fitted to the pseudo-first order (Eqn 2) and pseudo-second order (Eqn 3) models:
where q e and q t (mg g-1) represent the adsorption capacities at equilibrium and adsorption time t (min); and k1 (min–1) and k2 (g mg–1 min–1) are the kinetic constants for the above-mentioned kinetic models, respectively.
For adsorption isotherms, the C 0 of TC solution was set in the range of 10–100 mg L–1 with an interval of 10 mg L–1. The pH of the suspension was kept at ~7. After adsorption for 720 min, the C e of TC in supernatants was measured and the adsorption capacities (q e) were calculated. Isothermal adsorption data were described by the Langmuir (Eqn 4) and Freundlich (Eqn 5) models, respectively:
where KL (L mg-1) and KF ((mmol g–1)(L mmol–1)1/n ) are the equilibrium constants of Langmuir and Freundlich models, respectively; Q m (mg g–1) represents the Langmuir adsorption capacity; and n is the variable of adsorption efficiency.
To investigate the effects of pH on adsorption capacity, TC solutions (100 mg L–1) with an initial pH of 3–10 were used. The pH of the suspension was adjusted every 3 h using 0.1 M HCl or NaOH solution (<20 μL per addition) to maintain the initial pH. To reinforce the selective adsorption of TC, 1 mM and 10 mM of cations (Na+, K+, Ca2+, and Mg2+) were chosen for competitive adsorption, which are three and 30 times as high as that of TC, respectively. In addition, to evaluate the re-use performance of the clay minerals, the regeneration capacity was investigated. Upon saturated adsorption of TC at pH 7.0, the adsorbents were regenerated using NaOH solution (pH 11.5). The adsorption-regeneration experiments were repeated five times, and the extent of adsorption was calculated according to Eqn 1.
Characterization methods
Transmission electron microscopy (TEM) images (at 200 kV) of the clay minerals were obtained using a FEI Talos F200S electron microscope. The mineral adsorbent was diluted and uniformly dispersed with deionized water, and one to two drops of suspension was added to the ultra-thin carbon film supported by copper mesh and then dried naturally.
Fourier-transform infrared spectroscopy (FTIR) spectra were recorded on a Bruker Vertex 70 infrared spectrometer (Manheim, Germany). The sample (0.9 mg) was mixed with KBr (~90 mg) and then pressed into a pellet. X-ray diffraction (XRD) patterns were obtained using a Bruker D8 Advance diffractometer (Manheim, Germany). The scanning rate was set at 3°2θ min–1 in the range of 3–80°2θ.
Zeta potential and isoelectric point (pHiep) were measured on a Zetasizer Nano ZS90 (Malvern Instruments). An appropriate amount of sample was dispersed in 10 mmol L–1 KCl solution (solid–liquid ratio of 200 mg L–1) followed by sonication for 5 min to disperse the sample. The prepared suspension was left to stand for 5 min to allow the coarse particles to settle and to obtain a stable suspension. With 0.1 mol L–1 KOH and HNO3, the pH range was adjusted to 3–10. Each pH point was measured three times. The size distribution of allophane nanoparticles in suspension was also determined using the Zetasizer Nano ZS90; before measurements, allophane suspension was diluted with deionized water at a concentration of ~200 mg L–1.
The surface site density (D s) and point of zero net proton charge (pHpzc) analyses were conducted at 25°C by acid–base titration using a Metrohm 916 Ti-Touch titrator. HCl (0.1 mol L–1) and NaOH (0.1 mol L–1) solutions were used as titrants with concentrations calibrated by Na2CO3 and potassium hydrogen phthalate, respectively. For titration, 150 mg of the clay mineral was dispersed into 50 mL of deionized water in a 100 mL titration cup. The pH of the suspension was adjusted to ~3 by HCl solution. After 10 min of equilibration, the suspension was slowly back-titrated to pH 11, using an end-point titration (EP) method with NaOH. Each step was stabilized until the pH drift was <0.005 pH unit per minute. Deionized water without samples (blank) was also titrated to deduct the effects of background factors.
N2-physisorption was carried out on a Micromeritics ASAP2020 system at liquid-N2 temperature (–196°C). Before measurement, the sample was degassed at 200°C for 12 h to remove the physically adsorbed water. The specific surface area (S BET) was calculated by the Brunauer–Emmett–Teller (BET) method; the pore size distribution was determined using density functional theory (DFT); the total pore volume (V total) was derived from the N2 uptake amount at a relative pressure of 0.95, and micropore volume (V micro) was evaluated via the t-plot method (Thommes et al., Reference Thommes, Kaneko, Neimark, Olivier, Rodriguez-Reinoso, Rouquerol and Sing2015).
Results and Discussion
Characteristics of allophane, halloysite, and montmorillonite
TEM results
The TEM images (Fig. 2) revealed directly that allophane, halloysite, and montmorillonite are different in nanostructure dimensions. The allophane showed a hollow sphere diameter of ~5 nm (Fig. 2a), which is in good agreement with the morphological characteristics of natural allophane (Kaufhold et al., Reference Kaufhold, Dohrmann, Abidin, Henmi, Matsue, Eichinger, Kaufhold and Jahn2010). Some aggregates of allophane nanoparticles were also observed, showing a size of submicron to micron scales. Consistent with the above observations, the size distribution of allophane particles also revealed a size range of several nanometers to microns with an average size of 234±9 nm (inset in Fig. 2a). Halloysite showed a nanotubular morphology with a length of 300–700 nm, an inner diameter of 30–100 nm, and an outer diameter of 50–150 nm (Fig. 2b). It is well known that halloysite is a kind of kaolin group mineral with a multiwall nanotube structure, of which the unit layers consist of one silica tetrahedral outer sheet and one alumina octahedral inner sheet, i.e. a 1:1 layered nanotubular clay mineral (Yuan et al., Reference Yuan, Tan and Annabi-Bergaya2015). Montmorillonite showed a two-dimensional layer morphology with a size of several micrometers (Fig. 2c), consistent with its typical two-dimensional layered structure. The layer units followed the stacking sequence of tetrahedral-octahedral-tetrahedral sheets, i.e. a 2:1 layered clay mineral (Golubeva, Reference Golubeva2016).

Figure 2. TEM images of (a) allophane (inset shows the size distribution and the yellow arrows indicate the single hollow spherical nanoparticle), (b) halloysite, and (c) montmorillonite.
XRD pattern analysis
The XRD patterns (Fig. 3) of allophane, halloysite, and montmorillonite revealed the differences in their skeleton structure. Allophane consisted of a major reflection at ~3.4 Å (26°2θ) with two weak reflections at ~2.3 Å (40°2θ) and ~1.4 Å (66.5°2θ), which are typical characteristics of allophane (Levard et al., Reference Levard, Doelsch, Basile-Doelsch, Abidin, Miche, Masion, Rose, Borschneck and Bottero2012). The first reflection is assigned to the constructive interference between adjacent silica tetrahedra, while the latter two reflections arise from the skeleton structure ((OH)3Al2O3SiOH, named imogolite-like local structure, ImoLS). Allophane and imogolite are chemically similar and both have imogolite local structure (ImoLS), i.e. fragments formed by the substitution of hydroxyl groups on one side of what appears to be an alumina trihydrate sheet by an isolated protosilicate group leading to its curling. Therefore, allophane and imogolite can be regarded as nanomineral polytypes consisting of the ImoLS units (Wang et al., Reference Wang, Zhang, Liu, Yuan, Li, Du, Zhao, Yu and Wang2024). One broad reflection at ~11 Å was also observed, which should arise from the long-range order associated with structural water in allophane (Du et al., Reference Du, Yuan, Liu, Wang, Song and Guo2018). The XRD pattern of halloysite showed three main reflections at 7.2 Å (12.3°2θ), 4.4 Å (20.1°2θ), and 3.6 Å (24.8°2θ), corresponding to the (001), (100), and (002) reflections of 7 Å nanotubular halloysite, respectively (Gray-Wannell et al., Reference Gray-Wannell, Holliman, Greenwell, Delbos and Hillier2020). The first reflection in the montmorillonite XRD pattern, corresponding to the d 001 basal spacing, was observed at 12.5 Å (7.02°2θ), suggesting a Na-montmorillonite (Deng et al., Reference Deng, Yuan, Liu, Annabi-Bergaya, Zhou, Chen and Liu2017). Beyond that, two reflections located at ~3.3 Å (26.6°2θ) and ~3.0 Å (29.4°2θ) are ascribed to quartz and calcite, respectively (Hayati-Ashtiani, Reference Hayati-Ashtiani2012). Accordingly, the CaO component as discussed above in relation to XRF data is supposed to arise from the impurity of calcite.
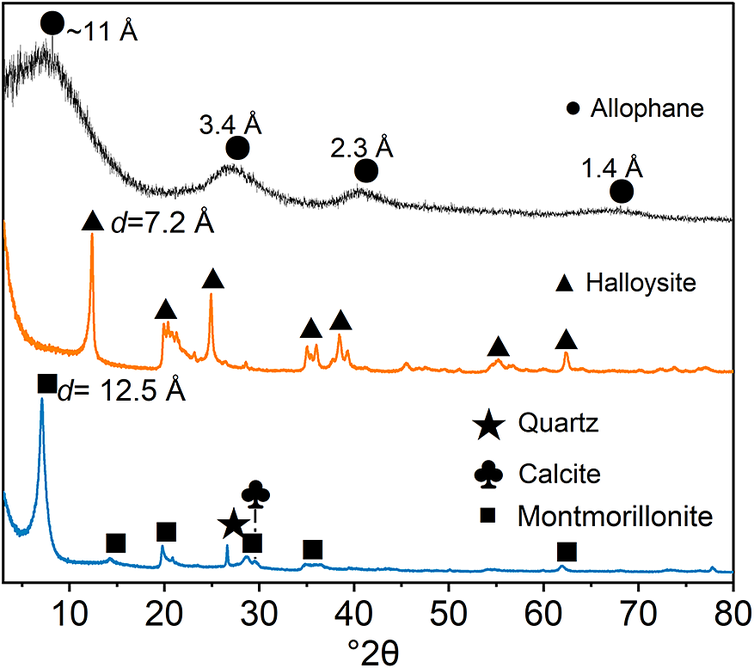
Figure 3. XRD patterns of allophane, halloysite, and montmorillonite.
FTIR spectra analysis
The FTIR spectra of allophane, halloysite, and montmorillonite (Fig. 4) also revealed the distinct differences in their structures. The main characteristic bands of allophane were observed as reported previously (Wang et al., Reference Wang, Du, Yuan, Liu, Song, Zhou, Deng and Liu2020). The broad band at ~3506 cm–1 is ascribed to the -OH stretching vibration in allophane and physically adsorbed water, while the band at 1641 cm–1 is ascribed to the H-O-H bending vibration of physically adsorbed water. In the 1200–400 cm–1 region, the fingerprint bands of hollow spherical allophane were observed, wherein the primary bands at 967 and 581 cm–1 arose from the Si-O-(Al) stretching vibration and the Al octahedra in ImoLS units, respectively (Levard et al., Reference Levard, Doelsch, Basile-Doelsch, Abidin, Miche, Masion, Rose, Borschneck and Bottero2012). For halloysite, the 3695 and 3620 cm–1 bands belong to the inner-surface and inner Al-OH groups, respectively, and the 913 cm–1 band is related to the bending vibration of inner Al-OH groups (Christoforidis et al., Reference Christoforidis, Melchionna, Montini, Papoulis, Stathatos, Zafeiratos, Kordouli and Fornasiero2016). The skeleton structural bands of halloysite were also observed, e.g. 1097, 1033, 540, and 470 cm–1 (Yuan et al., Reference Yuan, Southon, Liu, Green, Hook, Antill and Kepert2008). In the spectrum of montmorillonite, the 3628 cm–1 band belongs to the stretching vibration of structural hydroxyl groups, while the 3445 and 1636 cm–1 bands correspond to the H-O-H stretching and bending vibrations of interlayer water, respectively. The skeleton structural features were observed in the 1200–400 cm–1 region as reported previously (He et al., Reference He, Ray and Zhu2004). In addition, the band at ~1435 cm–1 is attributed to the carbonate impurities such as calcite as mentioned above (Lu et al., Reference Lu, Tan, Yu, Ren and Chen2016).
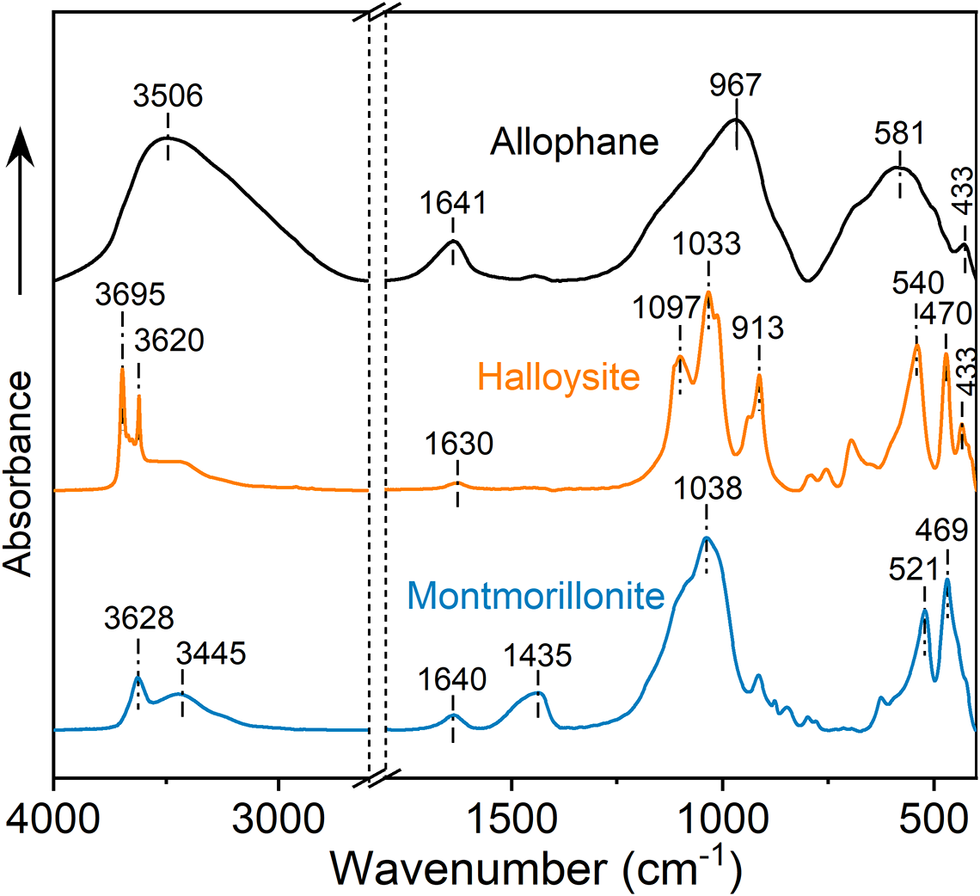
Figure 4. FT-IR spectra of allophane, halloysite, and montmorillonite.
N2 physisorption analysis
The N2 adsorption-desorption isotherms and derived power spectral density (PSD) curves of allophane, halloysite, and montmorillonite (Fig. 5), together with the derived textural parameters (Table 2), suggested that these clay minerals have different nanopore structures. According to the IUPAC notations (Thommes et al., Reference Thommes, Kaneko, Neimark, Olivier, Rodriguez-Reinoso, Rouquerol and Sing2015), the N2 adsorption-desorption isotherm of allophane (Fig. 5a) is classified as Type-I. The adsorption penetration curve of allophane increased significantly at low relative pressure, and no apparent hysteresis loop was observed, indicating the dominance of micropores in allophane (Cychosz et al., Reference Cychosz, Guillet-Nicolas, García-Martínez and Thommes2017). Indeed, allophane showed a majority of 1–2 nm micropores with a small proportion of 2–3 nm mesopores (Fig. 5b), giving an average pore size of ~1.83 nm (Table 2). The isotherms of halloysite and montmorillonite (Fig. 5c,e) were similar to each other and exhibited Type-II isotherms with type-H3 hysteresis, implying the dominant roles of mesopores and macropores (Thommes et al., Reference Thommes, Kaneko, Neimark, Olivier, Rodriguez-Reinoso, Rouquerol and Sing2015), consistent with their PSD curves (Fig. 5d,f). Correspondingly, the average pore sizes of both minerals were 15.65 and 12.86 nm, respectively, which are much larger than those of allophane. As is well known, the nanopores of the halloysite mainly consisted of mesopores and macropores, which are often evaluated by using the BJH method (suitable for nanopores with a diameter of >2 nm) (Liu et al., Reference Liu, Guo, Zou, Du and Jia2008; Song et al., Reference Song, Yuan, Du, Deng, Wei, Liu, Zhong and Zhou2020). In the present work, the DFT method, which is all-powerful and suitable for evaluation of micropores and macropores (Thommes et al., Reference Thommes, Kaneko, Neimark, Olivier, Rodriguez-Reinoso, Rouquerol and Sing2015), was used to evaluate the pore-size distribution of halloysite for a better comparison study. Although some differences were observed in the pore-size distribution curves between current work and previous reports (Liu et al., Reference Liu, Guo, Zou, Du and Jia2008) due to the employment of different fitting models, all of the pore-distribution curves showed the predominant position of mesopores and macropores in halloysite, without micropores (<2 nm).

Figure 5. N2 adsorption-desorption isotherms of (a) allophane, (c) halloysite, and (e) montmorillonite; pore-size distributions of (b) allophane, (d) halloysite, and (f) montmorillonite.
Table 2. Textural parameters, pHpzc, and surface site densities of samples

d avg = average pore width; pHPZC = point of zero net proton charge; H s = total surface proton concentration; D s = surface site density. The values were calculated by Eqn 12 using S BET and H s.
Note that the interlayers of montmorillonite were filled with guests (e.g. water, cations) (Macht et al., Reference Macht, Eusterhues, Pronk and Totsche2011) and that N2 adsorption is kinetically limited in pores of <0.5 nm in diameter (Pereira et al., Reference Pereira, Arbestain, Kelliher, Theng, McNally, Macias and Guitian2019). As a consequence, the S BET of montmorillonite (60 m2 g–1) was considered to be much lower than its real value. By comparison, the S BET and nanopores of halloysite can be detected by N2 adsorption-desorption, due to its inner diameter being ~30–100 nm (Fig. 2b). In addition, the V total (0.14 cm3 g–1) and S BET (301 m2 g–1) of allophane (freeze-dried powder) (Table 2) were considered to be much less than its theoretical values (Abidin et al., Reference Abidin, Matsue and Henmi2006), implying that allophane nanoparticles aggregated to a significant extent during the drying process. Overall, allophane has a much larger S BET value than halloysite and montmorillonite, resulting from its relatively large proportion of micropores. Therefore, nano hollow spherical allophane was expected to exhibit better N2 adsorption performance than halloysite and montmorillonite.
Surface charge property
The zeta potential curves and acid–base titration data of allophane, halloysite, and montmorillonite (Fig. 6) were obtained to reveal their surface-charge properties. With the increase in pH, the surfaces of these minerals became more negative. The pHiep of allophane was ~9 and had a positively charged surface over a wide pH range, while halloysite and montmorillonite showed negatively charged surfaces over the measured pH range (3–10); these results are consistent with previously reported data (Cui et al., Reference Cui, Zhang and Han2020; Melnikov et al., Reference Melnikov, Reshetina, Novikov, Cherednichenko, Stavitskaya, Stytsenko, Vinokurov, Huang and Glotov2023; Wang et al., Reference Wang, Zhang, Liu, Yuan, Li, Du, Zhao, Yu and Wang2024). Because the deprotonation of Si-OH groups via ionization is easier than that of Al-OH under higher pH conditions (Arancibia-Miranda et al., Reference Arancibia-Miranda, Silva-Yumi and Escudey2015), allophane with abundant Al-OH groups on its outer surface should logically have a higher pHpzc than either halloysite or montmorillonite. The outer surface of halloysite is a relatively less reactive Si-OH group with a negative charge, whereas the outer surface of allophane is perhaps a relatively more reactive Al-OH group with a positive charge. Moreover, the tricarbonyl system and the phenol diketone portion of the TC molecule readily isolate some of the protons from water, and the tetracycline exists as either a monovalent anion or a divalent anion. As a result, allophane is favorable for capturing negatively charged species, while halloysite and montmorillonite are more likely to attract positively charged species.
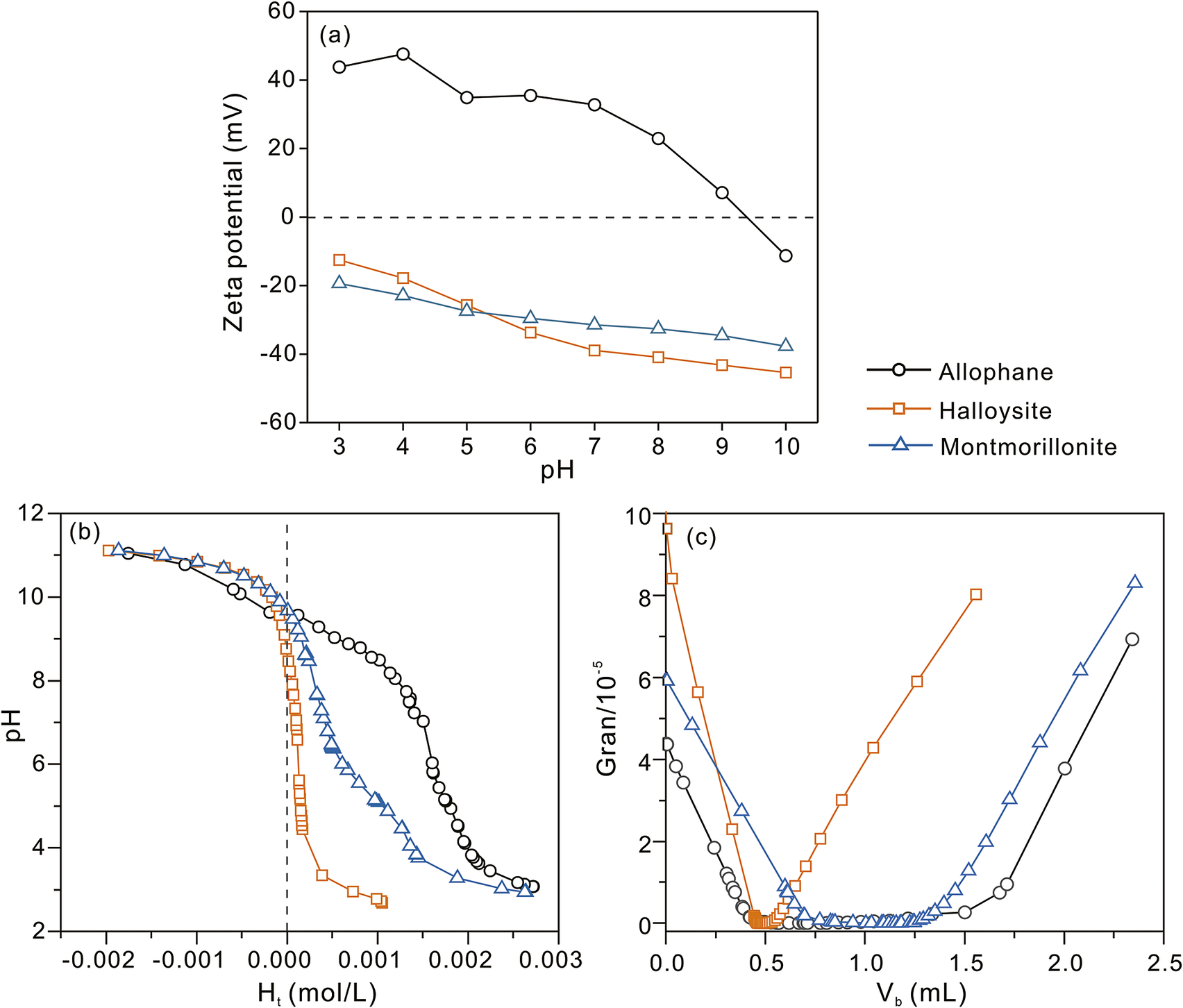
Figure 6. (a) Zeta potential curves, (b) acid–base titration curves in deionized water, and (c) Gran plots of titration data to obtain V eb1 and V eb2 (see text for notation) of the clay minerals.
Surface hydroxyl groups are essential to surface reactions occurring at the mineral–water interface, which can be determined by acid–base titration. The acid–base titration results of allophane, halloysite, and montmorillonite (Fig. 6b,c) were obtained, from which some parameters (Table 2) were also derived. H t represents the total proton concentration and is defined as (Nie et al., Reference Nie, Pan, Zhang and Pan2013):
where [H+] and [OH−] represent the concentrations of proton and hydroxide ions in solution, respectively; and [≡SOH2+] and [≡SO–] represent the protonated and deprotonated species of surface hydroxyl groups on clay minerals (Eqns 7 and 8), respectively. The H t can be calculated from the difference between protons added during the titration and protons remaining in solution (Eqn 9):
where C a and C b (mol L–1) are the calibrated concentrations of HCl and NaOH solutions; and V 0 (L), V a (L), and V b (L) are the volumes of initial deionized water, consumed HCl solution, and consumed NaOH solution, respectively.
The pHpzc refers to the pH value where the total net proton charge on the mineral surface is zero, which is useful for predicting the electrostatic attraction of charged species in aqueous solution. The pHpzc of allophane (~9.6; Fig. 6b and Table 2) was higher than those (5–8) of natural allophane samples (Diez et al., Reference Diez, Quiroz, Ureta-Zañartu, Vidal, Mora, Gallardo and Navia2005), which may result from the impurities in natural allophane. The pHpzc values of halloysite and montmorillonite were ~7.8 and ~9.8, consistent with those reported previously (Tombacz and Szekeres, Reference Tombacz and Szekeres2004). Note that the pHpzc values of these clay minerals were higher than their pHiep values, which can be attributed to the isomorphic substitutions in their structures, e.g. the replacement of Al3+ by Mg2+ in octahedral sites and Si4+ by Al3+ in tetrahedral sites (Yucelen et al., Reference Yucelen, Choudhury, Leisen, Nair and Beckham2012; Golubeva, Reference Golubeva2016; Yu et al., Reference Yu, Xu, Tan, Fang, Roden and Wan2020). Nanoscale pore confinement may also cause an increase in the pHpzc of halloysite, due to the deviation of surface chemistry of aluminum groups in the nanopores (Yu et al., Reference Yu, Xu, Tan, Fang, Roden and Wan2020).
The surface site density of clay minerals can be determined by the Gran equations (Eqns 10 and 11) (Yu et al., Reference Yu, Xu, Tan, Fang, Roden and Wan2020):
where V 0, V a, and V b are the same as those in Eqn 9; K w is the ionic product of water, which is –13.93 (25°C) here (Bujnakova et al., Reference Bujnakova, Balaz, Zorkovska, Sayagues, Kovac and Timko2013). The value of Gran was plotted vs the added volume of NaOH solution (Fig. 6c), then the curves were fitted with two lines, yielding two intersections with the x-axis at V eb1 and V eb2. The total proton concentration (H s, mol L–1) was calculated as follows (Eqn 12):
and the surface site density (D s, sites nm-2) was calculated by Eqn 13:
where V eb2-blank and V eb1-blank are derived from the Gran function of deionized water (blank); NA is Avogadro’s constant, 6.02×1023 mol–1; S (m2 g–1) is the specific surface area of samples, and in this study the S BET was used; and C s (g L–1) is the sample dosage.
The H s values of the clay minerals (Table 2) followed the order allophane > montmorillonite > halloysite. However, the D s of montmorillonite was much greater than that of allophane, not to mention that of halloysite. This can be explained by the same explanation as given above, i.e. the S BET of montmorillonite is much lower than its true value because only the external specific surface area is measured by N2 molecules, whereas the interlayer space of montmorillonite is accessible for H+ (Cui et al., Reference Cui, Zhang and Han2020), thus causing an extremely high D s value.
Adsorption performances of allophane, halloysite, and montmorillonite
Effects of pH
Adsorption data under various pH conditions showed that the pH had great effects on the adsorption capacity of the clay minerals (Fig. 7). The uptake amount of TC increased at first and then decreased, which should be related to both the surface charge properties of the clay minerals and the forms of TC. Under strong acidic conditions, electrostatic repulsion existed between cationic TC molecules and positive allophane surfaces (Figs 1 and 6a). With the increase of pH, TC molecules became more negative, which promoted its adsorption on positive allophane surfaces (pH<9) but suppressed its adsorption on negative allophane surfaces (pH>9). Consequently, allophane gave a maximal adsorption amount of 796 mg g–1 at pH 9. In the same way, at pH<7.7, the cationic and/or zwitterionic TC species were more favorable to be adsorbed on negatively charged halloysite and montmorillonite at conditions with higher pH, giving maximal adsorption amounts of 83 and 225 mg g–1 at about pH 7 for halloysite and montmorillonite, respectively. Similar results have been reported for the effects of pH on tetracycline adsorption on clay minerals (Liu et al., Reference Liu, Wang, Liu, Liu, Weng, Koopal and Tan2012). These results suggest that electrostatic attraction is the primary driving force.
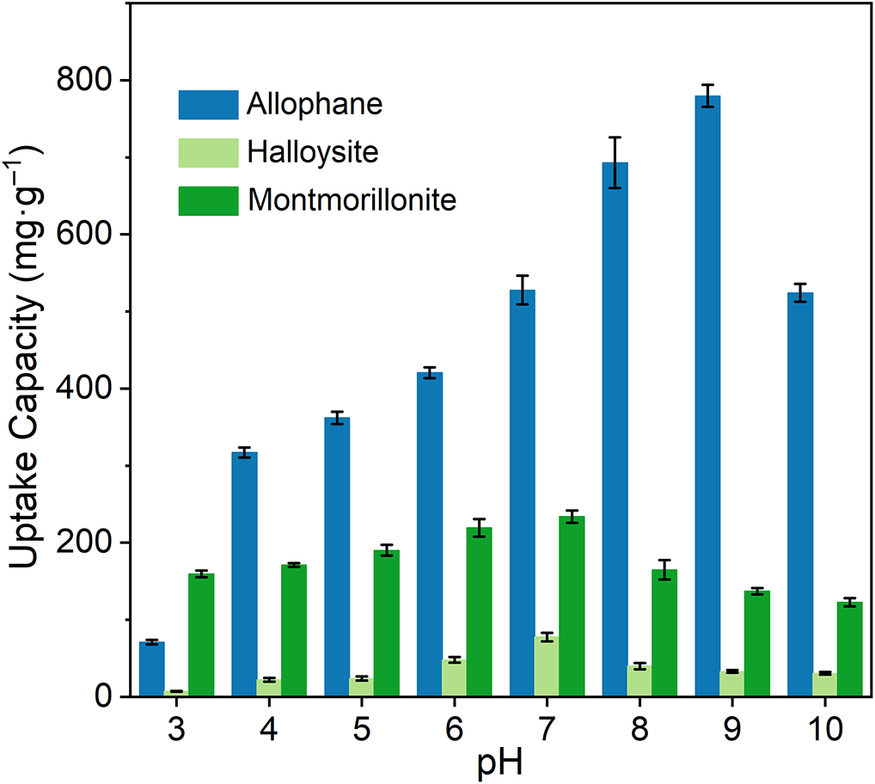
Figure 7. The effect of pH on the adsorption performance of clay minerals (adsorbent dosage, 5 mg; TC, 50 mL and 100 mg L–1).
Influence of co-existing ions and ionic strength
The effects of co-existing cations (Na+/K+/Mg2+/Ca2+) and their strength (1 mM/10 mM) on TC adsorption (Fig. 8) were evaluated. The adsorption capacities of allophane and halloysite were affected little in the presence of Na+ and K+, implying the dominant role of inner-sphere complexation in TC adsorption on both clay minerals (Su et al., Reference Su, Pan, Wan, Zhang and Lv2010). As TC can form inner-sphere complexes with hydrous oxides of Al via ligand exchange (Gu and Karthikeyan, Reference Gu and Karthikeyan2005), such an interaction might also occur between TC and surface Al-OH groups of clay minerals. In addition, TC adsorption on allophane decreased in the presence of Mg2+ and Ca2+. One possible explanation is that divalent Mg2+ and Ca2+ can form metal complexes with TC molecules to reduce the free concentration of TC in the solution (Pulicharla et al., Reference Pulicharla, Hegde, Brar and Surampalli2017). For montmorillonite, the adsorption capacity decreased with increasing cationic strength, suggesting that non-specific adsorption mainly occurred. Moreover, the inhibiting effects of divalent cations (Mg2+ and Ca2+) on adsorption performance were relatively weak, consistent with what has been reported previously (Aristilde et al., Reference Aristilde, Lanson, Miehe-Brendle, Marichal and Charlet2016). It is attributed to the formation of ternary complexes mediated by the bridge role of divalent cations in the interlayer spaces (entering via cation exchange) of montmorillonite.
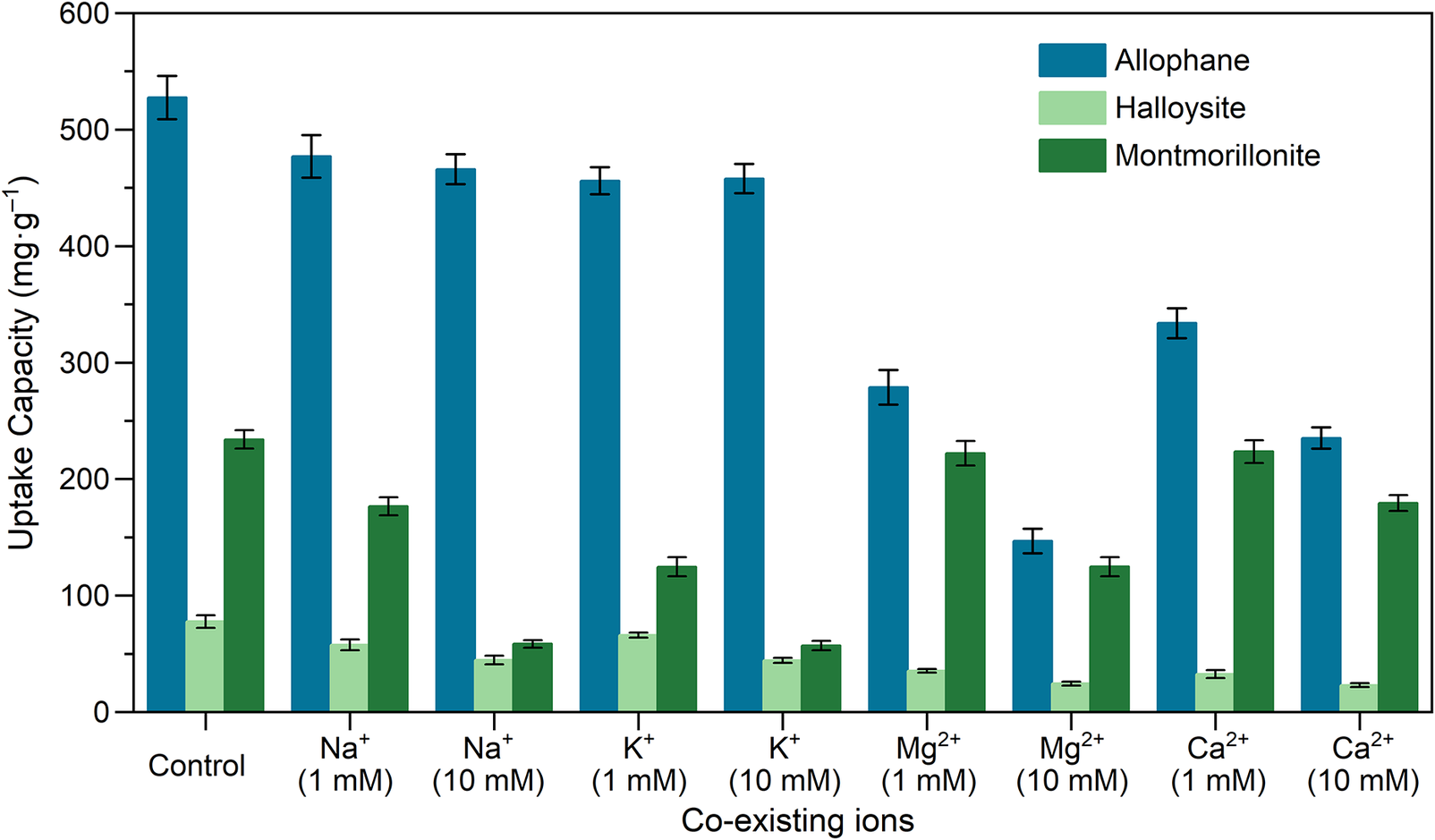
Figure 8. Effects of ion strength on the adsorption properties of clay minerals (adsorbent dosage, 5 mg; TC, 50 mL and 100 mg L–1; equilibrium pH was set at ~7.0).
The values of D s and S BET of allophane were much greater than those of halloysite and montmorillonite (Table 2), and meanwhile the external surfaces of halloysite and montmorillonite had Si-O-Si bonds and Si-O- defect sites, in which the TC species are thought to be physically bonded (Qiao et al., Reference Qiao, Wang, Liao, Zhang and Liu2021). One, therefore, could reasonably conclude that allophane exhibits the best performance for TC adsorption, which strongly suggests that allophane is a promising adsorbent for TC removal.
Adsorption kinetics
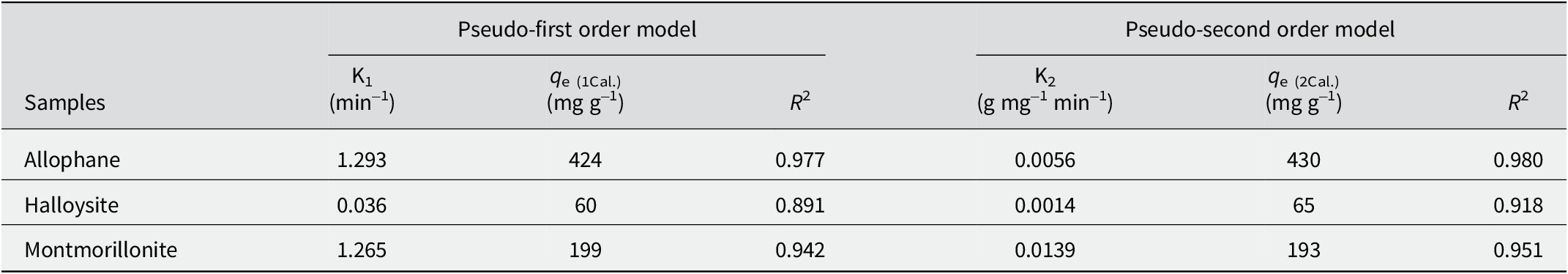
The adsorption kinetics of TC on allophane, halloysite, and montmorillonite (Fig. 9) showed that these adsorption processes were very fast at the beginning (<240 min) and then slowed down until equilibrium, giving an adsorption capacity order of allophane > montmorillonite > halloysite. The kinetic data were fitted by the pseudo-first order and pseudo-second order models (Eqns 2–3), respectively. Results showed that the latter model better described the adsorption process (Fig. 9 and Table 3), suggesting that surface complexation may take a dominant position.
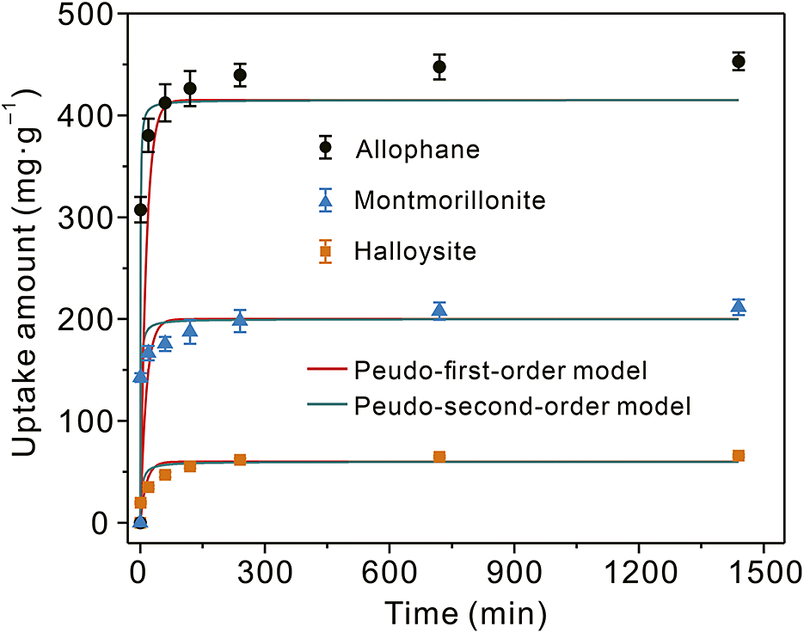
Figure 9. Adsorption kinetics of allophane, halloysite, and montmorillonite (adsorbent dosage, 5 mg; TC, 50 mL and 100 mg L–1; equilibrium pH, ~7.0).
Table 3. Pseudo-first order and pseudo-second order adsorption kinetics parameters
Adsorption isotherms
The adsorption isotherms of TC (Fig. 10) were obtained and showed that the equilibrium uptake capacities (q e) of these minerals increased with the equilibrium concentration (C e). To better understand the adsorption mechanisms, the isothermal data were fitted with the Langmuir and Freundlich models, and the fitting results are summarized in Table 4. Based on the coefficient of regression (R 2) values, the Langmuir model was better for predicting isothermal data than the Freundlich model. These results imply that all adsorption sites are energetically equivalent and that TC adsorption on these clay minerals is more likely to be monolayer adsorption, which depends on the available active sites on the mineral surface (Obradovic, Reference Obradovic2020).

Figure 10. Adsorption isotherms of allophane, halloysite, and montmorillonite (dosage, 5 mg; TC, 50 mL and 100 mg L–1; equilibrium pH ~ 7.0).
Table 4. Langmuir and Freundlich adsorption isotherm fitting parameters

Regeneration and re-usability
The adsorption data of regenerated clay minerals were also obtained (Fig. 11); a dilute NaOH solution with a pH of 11.5 was used to regenerate the adsorbents. After five cycles, the adsorption capacities of all adsorbents were maintained at >75%, implying that these three adsorbents had good re-usability and stability in the regeneration process. Furthermore, although the adsorption efficiency showed an order of halloysite > montmorillonite > allophane, the initial adsorption capacity of allophane is much higher than that of halloysite and montmorillonite. These results highlight the huge potential application of allophane in TC removal.

Figure 11. Regeneration and reusability of allophane, halloysite, and montmorillonite (adsorbent dosage, 5 mg; TC, 50 mL and 100 mg L–1; equilibrium pH ~ 7.0).
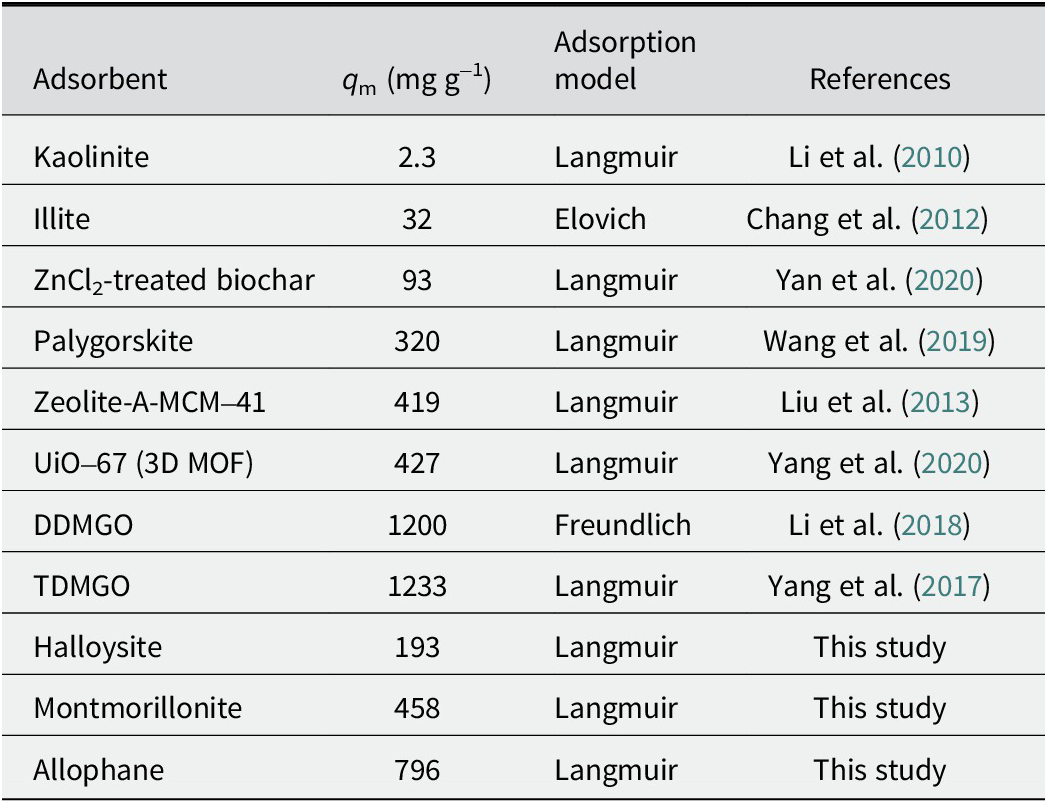
Adsorbent performances for TC uptake from aqueous media
The adsorption effectiveness for antibiotics depends mainly on the adsorbents’ structure and properties, such as specific surface area and surface-interface properties. The adsorption capacities of these clay minerals with different nanostructures in this study were compared with the materials widely used for TC removal (Table 5). Allophane has a greater adsorption capacity for TC than materials reported in most literature. With an increased interest in nanomaterials, eco-friendly and inexpensive clay minerals, especially hollow spherical allophane, would be a preferred candidate for chemically synthesized nanomaterials.
Table 5. Comparison of adsorption capacities of various media for TC
Adsorption mechanisms of TC on three clay minerals
XRD patterns
After TC adsorption, the XRD patterns of TC-adsorbed allophane, halloysite, and montmorillonite (Fig. 12) were recorded. Compared with the spectra of raw minerals, the reflections of allophane and halloysite remained almost constant, demonstrating that TC molecules were adsorbed on the surface sites of both minerals and had no significant effect on their skeleton structures. Given that the reflection at 11 Å (8°2θ) is related to some long-range order of structural water in allophane, its disappearance after adsorption should result from the inner-sphere complexation of TC with allophane Al-OH sites, which can readily expel the water molecules from around the outer surface of the allophane. For montmorillonite, the d 001 value shifted from 12.4 Å to 18.7 Å, which is consistent with the XRD results reported previously, and is attributed to the intercalation of TC in the interlayer spaces (Wu et al., Reference Wu, Zhao, Jing, Shao, Liu, Lv, Hu, Zhang, Meng and Liu2019). Despite that, some interlayer spaces of Na-montmorillonite were not occupied, as indicated by a shoulder reflection at ~13 Å (6.7°2θ).

Figure 12. XRD patterns of allophane, halloysite, and montmorillonite after TC adsorption.
FTIR results of clay minerals after TC adsorption
After TC adsorption, the FTIR spectra of allophane, halloysite, and montmorillonite after TC adsorption (Fig. 13a) were also obtained. Because the fingerprint bands of TC are mainly in the wavenumber range of 1400–1700 cm–1 (Zhang et al., Reference Zhang, Lan, Liu and Qu2015; Li et al., Reference Li, Wang, Zhang, Han and Wei2017), the FTIR spectra in this region were magnified (Fig. 13b). Compared with the spectra of raw minerals (Fig. 4), several bands related to TC molecules appeared in those of adsorption products, together with shifts in some bands, which demonstrated the interactions between TC molecules and clay minerals.

Figure 13. (a) FTIR spectra of allophane, halloysite, and montmorillonite after TC adsorption; (b) enlargement of the section of the FTIR spectra as indicated by the red dashed line in part a.
In the spectrum of allophane-TC, the broad band at ~3472 cm–1 is attributed to the overlapping of the 3506 cm–1 band of allophane and the 3391 cm–1 band of TC, implying a mass of adsorbed TC on allophane. After adsorption, the structural bands of allophane (967 and 578 cm–1) shifted several wavenumbers (to 970 and 568 cm–1, respectively), and meanwhile, the bands assigned to -C=O and -NH2 groups of TC were not observed or overlapped with other bands. These results demonstrate that the -C=O and -NH2 groups of TC were strongly bound with the surface Al-OH groups of allophane, which is in agreement with the inner-sphere adsorption of TC on allophane as discussed above. The strong interactions may also affect the skeleton vibration of the TC molecule greatly, which shifted the 1454 cm–1 band of TC to about 1466 cm–1 after adsorption.
Because the inner Al-OH groups of halloysite cannot be reached by organic molecules (Yuan et al., Reference Yuan, Southon, Liu, Green, Hook, Antill and Kepert2008), its signal intensity (3620 or 913 cm–1) can be used as an internal reference for comparing band intensities. Following that, the relative intensities of 3695 and 1033 cm–1 of halloysite decreased after TC adsorption, suggesting the interactions of TC species with Al-OH and Si-O groups, respectively. Similar results have been reported for oxytetracycline (OTC) adsorption on halloysite (Ramanayaka et al., Reference Ramanayaka, Sarkar, Cooray, Ok and Vithanage2020). The characteristic bands of TC in the 1700–1400 cm–1 region were identified with slight shifts, implying that TC species were mainly adsorbed via outer-sphere complexation that had no significant change in TC structure. As the Si-OH groups on the defect sites of halloysite are deprotonated under weakly acidic to alkaline conditions (Tan et al., Reference Tan, Yuan, Liu, Du, Yuan, Thill and Bergaya2016), it can be speculated that TC species mainly formed inner-sphere complexes with Al-OH groups at inner surfaces but electrostatically adsorbed on the negatively charged external surface.
Concerning montmorillonite-TC, its FTIR spectrum is comparable to that of raw montmorillonite, which is consistent with the results of previous studies (Parolo et al., Reference Parolo, Savini, Vallés, Baschini and Avena2008; Ortiz-Ramos et al., Reference Ortiz-Ramos, Leyva-Ramos, Mendoza-Mendoza and Aragón-Piña2022). The structural bands of TC were also observed; the band at 1620 cm–1 was attributed to the overlap of the TC bands and interlayer water, and the 1500 cm–1 band may arise from the shifted -NH2 amide groups of TC (1517 cm–1). Combined with XRD results, it can be inferred that the intercalation of TC into the interlayer spaces of montmorillonite made no significant change in the skeleton structure of TC and montmorillonite.
TC adsorption mechanisms
As discussed above, the nanostructure of clay minerals has a great effect on their specific surface areas, nanopore structures, and surface charge properties, thereby significantly limiting their adsorption performance and interaction mechanism with TC species. The possible adsorption mechanisms of TC on allophane, halloysite, and montmorillonite are illustrated in Fig. 14. Owing to its unique hollow spherical structure, allophane has the largest specific surface area compared with halloysite and montmorillonite, together with plenty of hydroxyl groups on the surface and near the defect pores. Consequently, allophane exhibited the largest adsorption capacity to TC species (796 mg g–1), which was dominated by ligand exchange with Al-OH groups, forming inner-sphere complexes. Such an inner-sphere complexation is thought to occur at the inner surfaces with many Al-OH groups of halloysite nanotubes, due to the lumen diameter being much larger than the TC molecule. In addition, allophane and halloysite also contain some Si-OH groups at the defect sites and/or structural edges, which appear in deprotonated form (Si-O–) in most pH cases. Therefore, some cationic TC species may also be electrostatically attracted by the negatively charged Si-O– sites. For montmorillonite with an expandable layered structure, cation exchange is the main mechanism for TC adsorption, which could increase the interlayer space but barely change the skeleton structure of the TC species and montmorillonite.

Figure 14. Illustration of main the adsorption mechanisms of TC on three clay minerals with different nanostructures.
Summary and conclusions
In summary, the adsorption of TC on allophane, halloysite, and montmorillonite, which have varying nanostructures, was compared. The nanostructural features and surface physicochemical properties of these clay minerals were characterized systematically via a combination of techniques, i.e. TEM, XRD, FTIR, zeta potential, N2-physisorption, acid–base titration, etc. The adsorption processes of TC on these three minerals were best described by pseudo-second order kinetics and Langmuir models. These clay minerals performed better under neutral to weakly alkaline conditions, and by comparison, the adsorption capacity of allophane reached 796 mg g–1, which was much greater than those of halloysite and montmorillonite. In addition, these minerals still had large adsorption capacities in the presence of Na+/K+/Ca2+/Mg2+ and maintained remarkable efficiencies, exceeding 75% after five recycles. Furthermore, some changes were observed in the surface groups and nanostructures of the clay minerals, and combined with the adsorption behaviors, the adsorption mechanisms were proposed. Driven by electrostatic forces, the inner-sphere complexation of TC with Al-OH groups dominated its adsorption on the external surface of allophane and the halloysite inner surfaces, accompanied by electrostatic attraction between a small amount of cationic TC species and Si-O– sites, while the TC adsorption on montmorillonite was dominated by cation exchange in the interlayer spaces. This study provides a better understanding of the effects of nanostructures of clay minerals on their TC adsorption performances and strongly suggests that allophane is a promising inexpensive adsorbent for the efficient removal of TC from wastewater.
Author contributions
Qiyi Ma: Conceptualization, Writing original draft, Data curation, Formal analysis. Ning Zhao: Validation, Data Curation. Shun Wang: Supervision, Conceptualization, Writing-review & editing, Project administration, Funding acquisition. Baifa Zhang: Methodology, Data Curation. Mengyuan Li: Methodology, Data Curation. Dong Liu: Supervision, Formal analysis. Xiang Zhou: Formal analysis. Maxim Rudmin: Writing-review & editing. Antoine F. Mulaba-Bafubiandi: Writing-review & editing. Peng Yuan: Supervision, Writing-review & editing.
Acknowledgements
None.
Financial support
Support from the National Natural Science Foundation of China (grant no. 52161145405), the Guangdong Basic and Applied Basic Research Foundation (grant no. 2023A1515012770), the National Special Support for High-Level Personnel, the Science and Technology Program of Guangxi, China (grant no. AD 20159079), and the Construction Projects of Ten National Science and Technology Innovation Platforms of Qinghai Province (2024-ZJ-J03) is gratefully acknowledged.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
Data will be made available on request.






















