Introduction
The Black-cheeked Lovebird Agapornis nigrigenis is endemic to Zambia and is Africa’s most localised parrot (Warburton Reference Warburton2003; Warburton and Perrin Reference Warburton and Perrin2005a). It inhabits the driest region of Zambia, a large plain bisected by seasonal rivers and streams within the country’s Southern and Western Provinces, where it is associated with mopane Colophospermum mopane woodland, requiring tracts near to permanent water sources and with large trees for nesting and roosting (Dodman Reference Dodman1995a). The species has been classified as “Vulnerable” by the International Union for Conservation of Nature (IUCN) all this century because of a continuing decline in its small population “owing to the gradual desiccation of water bodies within a highly localised range” (BirdLife International 2024). Overall, granivorous birds in southern Africa have been found to be more water dependent than insectivores and omnivores (Smit et al. Reference Smit, Woodborne, Wolf and McKechnie2017). Many parrots with diets dominated by herb and grass seeds are obligate drinkers, needing regular water intake to maintain overall homeostatic water balance (Collar Reference Collar, del Hoyo, Elliott and Sargatal1997; Warburton and Perrin Reference Warburton and Perrin2005b). With a diet dominated by grass seeds, Black-cheeked Lovebirds need to drink at least twice daily and are therefore heavily dependent on surface water throughout the year, but they are highly cautious drinkers and generally will not visit pools disturbed by humans or livestock (Warburton and Perrin Reference Warburton2003, Reference Warburton and Perrin2005b, Reference Warburton and Perrin2005c).
Normal annual rainfall in southern Zambia is 600–900 mm per year but can be as little as 300–450 mm during El Niño periods and as high as 1,200 mm during flooding events (Pierce and Lang Reference Pierce and Lang2008; Rembold et al. Reference Rembold, Kerdiles, Lemoine and Perez-Hoyos2016). There are two distinct seasons within the lovebird’s range: a wet season, usually from November to April, reaching a peak between December and February, and a dry season from May to October, the latter causing serious water shortages lasting from June to December (Libanda et al. Reference Libanda, Nkolola and Musonda2015). Black-cheeked Lovebirds have been recorded using a variety of water sources categorised as: “mopane pools”, formed as natural shallow depressions where water collects within the mopane woodland; “river pools” in riverbeds and drainage channels; “grassland pools” in seasonal floodplains, usually adjoining mopane woodland and “artificial pools”, any anthropogenic water source such as shallow wells, troughs, dams, and reservoirs close to human settlements and livestock grazing areas (Mzumara et al. Reference Mzumara, Perrin and Downs2016; Warburton and Perrin Reference Warburton and Perrin2005b; CGP 2020, personal observation).
The absence of Black-cheeked Lovebirds from large portions of otherwise suitable habitat within their range has been found to be directly related to the absence of water (Dodman et al. Reference Dodman, Katanekwa, Apsinwall and Stjernstedt2000). Moreover, the range itself has been shrinking because of the gradual decline in rainfall in the second half of the last century (Warburton Reference Warburton2003; Warburton and Perrin Reference Warburton and Perrin2005b). With these insights, we sought to identify the spatial patterns in the distribution of pools, the important predictors of their use by lovebirds, and any significant differences in usage across the four pool categories. An understanding of the factors influencing the lovebirds’ spatio-temporal access to water sources is key to the future conservation management of the species.
Methods
Study area
The Black-cheeked Lovebird (BCL) is known to occur from as far north as the Nanzhila plains in the Kafue National Park (KNP) to Sichili in the west, Ngwezi in the east, and the Zambezi River to the south (Figure 1), with a total range estimated to be around 17,500 km² (BirdLife International 2024). The species is reported to exist in two geographically separate subpopulations, as its mopane habitat is bisected by a rocky ridge of miombo (Brachystegia–Julbernardia) woodland which has been speculated to form a barrier to lovebird gene exchange (Dodman Reference Dodman1995b; Dodman et al. Reference Dodman, Katanekwa, Apsinwall and Stjernstedt2000). Although this 45-km barrier (Warburton Reference Warburton2003) has reduced over the past 30 years and now includes scattered patches of fully grown mopane, there is little surface water within it (Warburton and Perrin Reference Warburton and Perrin2005b; CGP 2022, personal observation). The stands of mopane around the Machile, Sichifulo, Ngwezi, and Nanzhila rivers are considered the species’ strongholds (Leonard Reference Leonard2005). All the mopane that hosts the Black-cheeked Lovebird is associated with alluvial clay deposits of an ancient wetland, possibly an earlier course of the Kafue River, thus forming an isolated stand (Bingham Reference Bingham1994; Leonard Reference Leonard2005) and explaining the lovebird’s restricted range (Warburton Reference Warburton2003). The southern portion of this range is dominated by seasonal rivers that include the Machile, Sichifulo, Ngwezi, and Simatanga (Dodman Reference Dodman1995b; Warburton Reference Warburton2003), flowing into the Kasaya River, a major tributary of the Zambezi. The north is dominated by the Nanzhila River, a major tributary of the Kafue, fed by its seasonal tributaries (Ashley and Murphy Reference Ashley and Murphy2012).
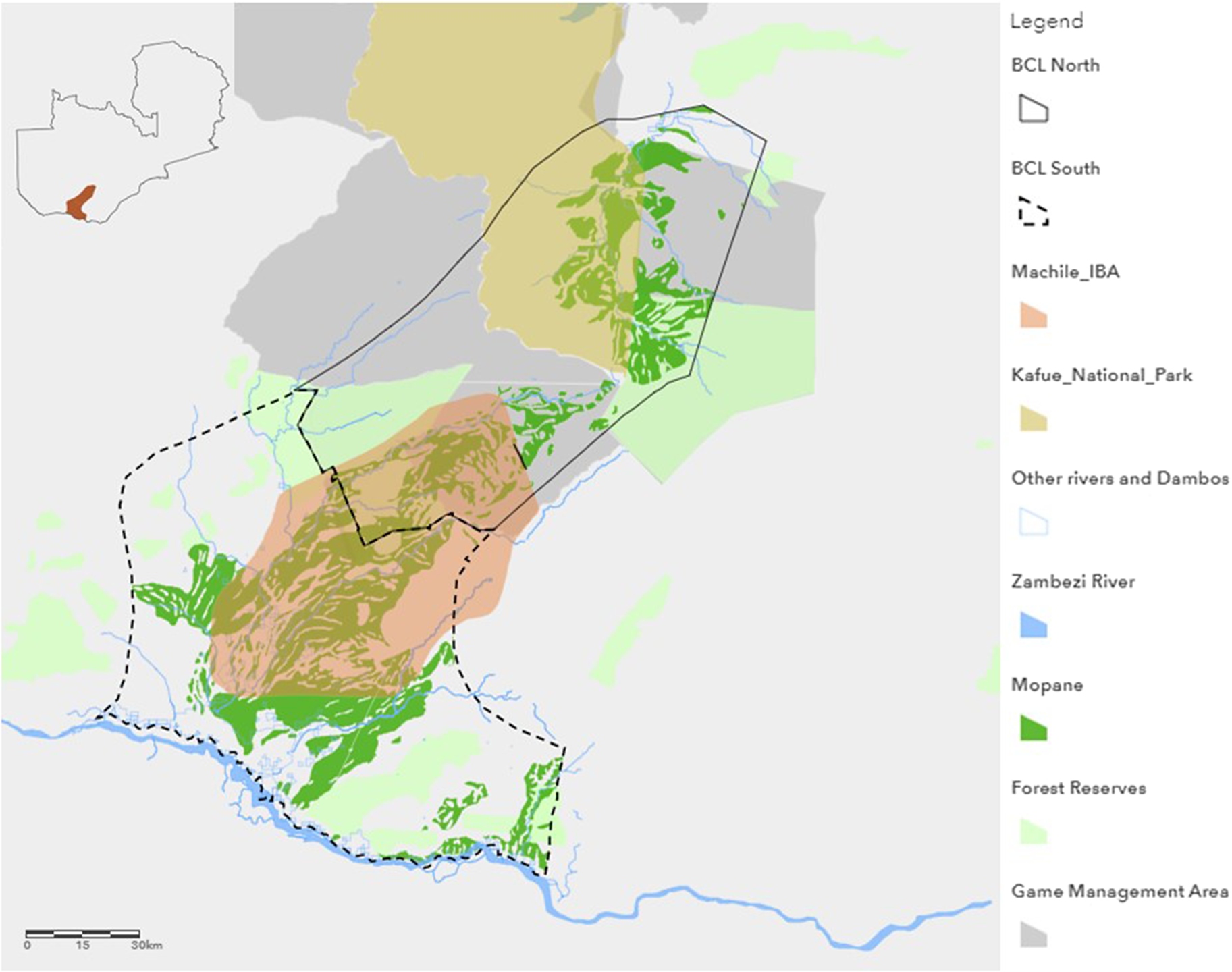
Figure 1. Range and study area of Black-cheeked Lovebird (BCL) Agapornis nigrigenis as documented by BirdLife International (2024).
Within the species’ range there are three designations of protected area and one area of informal conservation status. The KNP is Zambia’s largest protected area (Ashley and Murphy Reference Ashley and Murphy2012; ZAWA 2004), currently under a co-management agreement with the Zambian government and African Parks. Three game management areas (GMAs) serve as buffer zones between the national park and unprotected land, forming prime legal hunting areas (ZAWA 2010). Fifteen forest reserves are intended to conserve the headwaters of major streams and rivers, although these are largely encroached by both settlements and agricultural fields (Shitima Reference Shitima2005). Informal conservation status is bestowed by Machile Important Bird and Biodiversity Area (IBA), notable as a stronghold of the Black-cheeked Lovebird (Leonard Reference Leonard2005). No settlements are present in the national park, but they exist in the park’s GMAs (Leonard Reference Leonard2005). The human population within the species’ range is estimated to be about 55,000, with the majority being semi-nomadic cattle-herders, fishermen, and small-scale crop farmers (Zambia Vulnerability Assessment Committee 2015). For the purpose of this study, the species’ range is divided into a northern and southern region based on area management type, with the north falling within state-protected areas under the Zambian Wildlife Act of 2015 and the south forming predominantly communal or customary owned land.
Pool location and monitoring
Data were collected over a period of 30 months; pool monitoring was undertaken between December 2018 and December 2020, while searches for other pools continued for an extra six months (May–October 2021) to ensure wider coverage of the species’ range and to compensate for fieldwork lost to COVID-19 travel restrictions on CGP between May and December 2020 (pool monitoring by community members – see below – continued in this period). Transects covering multiple habitat types including mopane woodland were undertaken to survey the lovebirds using distance sampling (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Armitage and Colton2005). An initial 70 transects were pre-selected in ArcGIS Pro using the Generate Transects Along Lines tool (ESRI 2019).
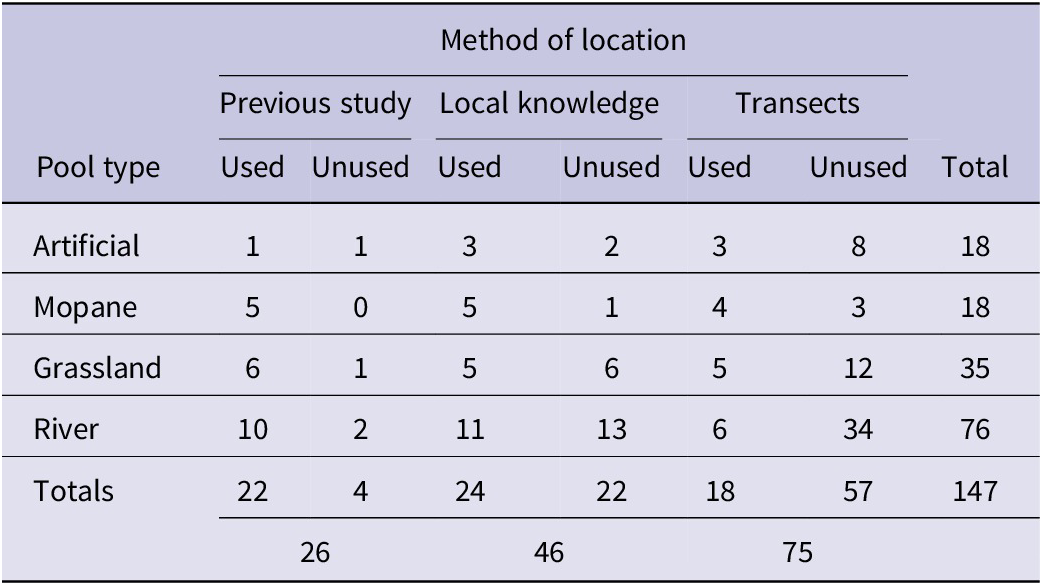
To locate pools, a combination of methods was used. A desk review collected the coordinates of all the pools used by lovebirds during earlier studies (Warburton Reference Warburton2003). Pools were also located by interviewing local inhabitants, and along transects. Transects were run systematically across the study area from east to west but, to increase the likelihood of finding pools, some transects followed footpaths and streams (Mirzaei and Bonyad Reference Mirzaei and Bonyad2016). Along transects we constantly attempted to record lovebird presence and numbers at pools. Every pool encountered, whether classified as used or unused by Black-cheeked Lovebirds, was georeferenced, given a project identification number, and categorised as mopane, river, grassland or artificial, following Warburton (Reference Warburton2003). Table 1 summarises the number of pools by type, the methods used to locate them, and the number of pools found by each method.
Table 1. Summary of pool numbers by type and in relation to the search methods used
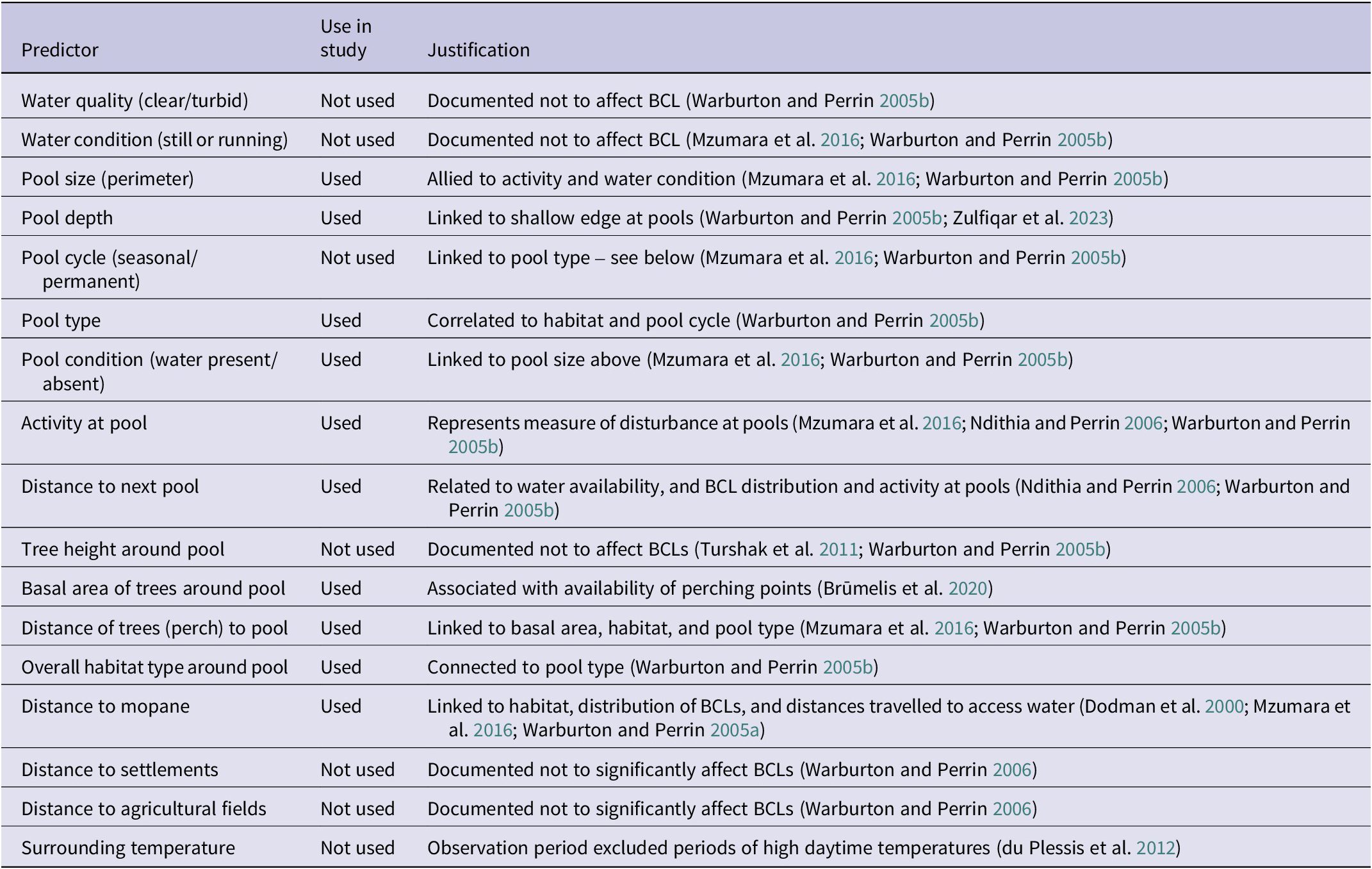
Data were collected on: (i) vegetation type (mopane – dominated by mopane trees; non-mopane – dominated by other species); (ii) tree diameter at breast height (DBH) of the five largest trees (mopane and non-mopane) within 50 m of the pool (DBH presented here as average basal area in m²); (iii) human activities (presence of people or livestock or their tracks) up to 50 m from the pool edge; (iv) distance to mopane (in km, measured as linear distance to the nearest edge of mopane woodlands as defined by the Zambian forests shapefile); (v) distance to the nearest known pool (in km, measured as linear distance to the nearest edge of surface water as defined by the World ESA surface shapefile); (vi) pool condition (water present or not); (vii) pool size gauged as perimeter length (in m) of the polygon around the water’s edge, measured using a calibrated rope laid by the designated monitor. Human activity was classified as “no activity evident” or “activity evident” (domestic use and livestock use seen or inferred to be present on each visit). Table 2 shows a full range of parameters considered likely to have an influence on the drinking patterns of Black-cheeked Lovebirds. The distance from each pool to the edge of the species’ range as defined by BirdLife International was determined using ArcGIS.
Table 2. Range of pool parameters/predictors likely to influence drinking patterns of the Black-cheeked Lovebird (BCL) Agapornis nigrigenis
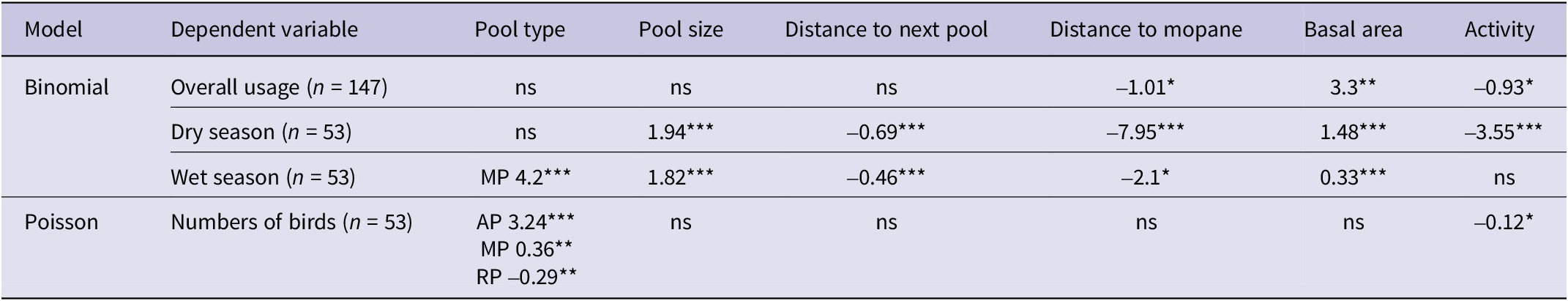
Lovebird usage (presence at/around the pool) and the number of lovebirds observed were also recorded. A pool was classified as used following the confirmation of lovebird presence when the pool was initially located or during the monitoring period. Monitored pools were visited between 24 and 72 times depending on when the pool was initially identified, while those not monitored were visited 2–3 times prior to being classified as used or unused. Of the 64 total pools classified as used, 43 had confirmed lovebird presence 81–100% of the time while the 83 pools classified as unused had no lovebirds on all visits. This strongly suggests that pools used by lovebirds tended to be visited on most days. By extension, it also suggests that it will have been relatively rare for us to have missed lovebirds on the 2–3 visits to pools coded as unused under this study. Figure 2 shows the proportions of lovebirds present (used) and absent (unused) in all the 147 pools included in the analysis in this study.
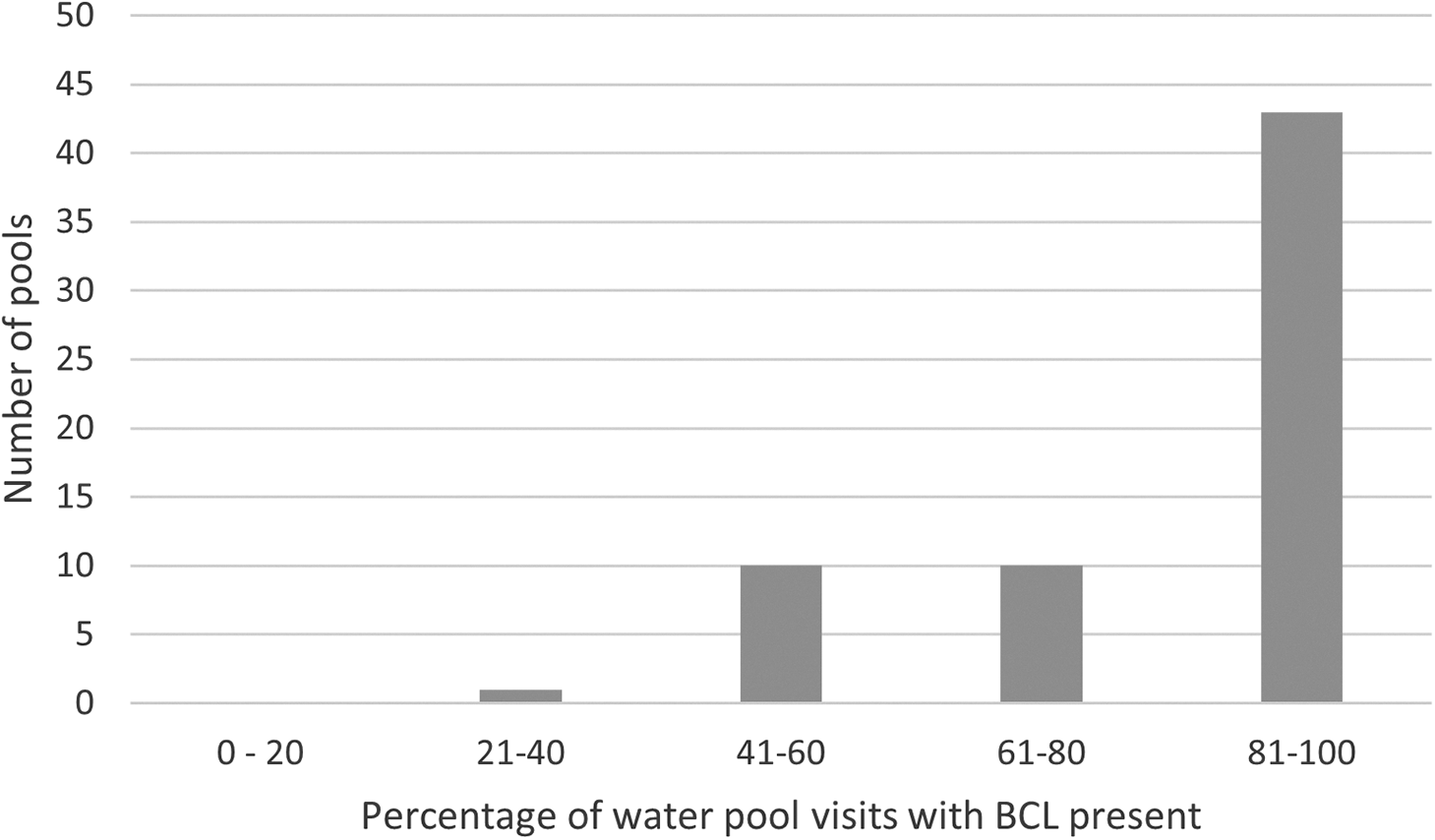
Figure 2. Proportion of pool monitoring visits with Black-cheeked Lovebird Agapornis nigrigenis present.
Local community members living close to the pools were engaged to assist with twice-monthly monitoring of pools across the species’ entire range, a task that was too expensive and logistically unfeasible for a single researcher. A total of 147 pools were located (Table 1), of which 53 were monitored from December 2018 to December 2020 by 13 observers who were paid for their services. The monitored pools were selected based on their proximity (within 2 km) to community members willing to be part of the monitoring effort. Based on the Wilcoxon rank sum test, there was no significant difference in pool size (w = 2,679; P = 0.45) and distance to mopane (w = 2,474; P = 0.95) between the monitored and unmonitored pools.
The monitors were trained to make twice-monthly observations at each pool for 10 minutes in either the morning (05h30–10h00) or late afternoon (16h00–18h00), and to record human activity, pool condition, pool size, and the total number of lovebirds present during the monitoring period. This was carried out at both used and unused pools with the same effort. The choice of observation times was based on peak time of lovebird activity at pools observed and documented during the drinking behaviour study by Warburton and Perrin (Reference Warburton and Perrin2005b). Monitors were advised to arrive at a pool 15 minutes earlier than known site-specific lovebird arrival times to record other parameters before sitting quietly at least 50 m from the pool to avoid affecting lovebird behaviour. Weekly telephone contact was maintained with the monitors to remind them of upcoming monitoring visits and to obtain news of any changes at the pools. A total of 1,144 visits were made during the wet season (November–April) and 1,212 in the dry season (May–October) over a period of 54 fortnights from December 2018 to December 2020. Worth noting is a six-month monitoring gap between the first group of pools located in December 2018 and the second group in May–June 2019 as fieldwork could not be undertaken during the peak of the rainy season when most of the study area was inaccessible.
Data analysis
All analyses were performed using R version 4.1.2 (RStudio Team 2023).
Distribution of pools
To identify possible patterns in the distribution of used vs unused pools, and to obtain insights on pool availability, a chi-squared test was used to check for an association between the proportion of used and unused pools in the north (protected) and south (communal areas) as well as any variation across the different pool types. Furthermore, a paired t-test was used to detect any significant differences in the mean distance of used vs unused pools from the edge of the species’ range.
Overall patterns of pool usage
To identify the variables most strongly associated with overall pool usage by lovebirds, binomial generalised linear modelling (GLM) (Zhao and Cen Reference Zhao and Cen2013) was performed on the complete data set of 147 pools, with presence/absence of lovebirds at pools as the response and predictors selected from candidate covariates in Table 2. All numeric predictors were scaled prior to inclusion in the model, and model selection was based on minimum Akaike information criterion (AIC), adjusted for small sample sizes, on ΔAICc (difference between the AICc of a given model and the lowest AICc model in the set) and on Akaike weight (Burnham and Anderson Reference Burnham and Anderson2002; Symonds and Moussali Reference Symonds and Moussali2011). A model was considered to be well supported if it had ΔAICc <2, and to be strongly supported as the most plausible model in the set if it had a weight (wᵢ) > 0.03 (Banner et al. Reference Banner and Higgs2017). Additional binomial GLM was undertaken to factor in seasonal variations across the study area with the data set split into wet and dry season.
Variation in numbers of birds at pools
To test whether the number of birds drinking at a pool varied between morning and afternoon as well as between wet and dry season, a pairwise Wilcoxon rank sum test (Mangiafico Reference Mangiafico2016) with continuity correction was used to compare the number of drinking birds and monitoring time. Furthermore, Poisson GLM was used to assess the factors influencing the number of birds drinking at each pool during the monitoring period (Zhao and Cen Reference Zhao and Cen2013) based on the list of predictors in Table 2. To evaluate the consistency of pool usage, coefficients of variation (CV) were calculated for both seasons across the monitoring period and Spearman’s rank correlation (Schober et al. Reference Schober, Boer and Schwarte2018) used to measure the association between the mean number of birds in each season and the season’s CV.
Results
Distribution of pools
Of the 147 pools located during the study, 64 (43%) were confirmed to be used by Black-cheeked Lovebirds (Figure 3). Of the 53 monitored pools, 27 were used by lovebirds during the monitoring period, while 14 pools used in the earlier study were found to be no longer available owing to land-use change; all available unused pools from the earlier study remained unused and all available used pools were still used by lovebirds. There was no significant difference (χ² = 0.16, df = 1, P = 0.67) between the proportions of used and unused pools in the northern and southern portions of the species’ range. Pools used by lovebirds tended to be closer to the centre of the species’ range, as indicated by the significant difference (SD) between the mean distance from the edge of the range of used (x̄ = 22.56 km; SD = 9.41) vs unused (x̄ = 15.46; SD = 10.9) pools (t = −4.13, df = 145, P <0.001). Of the 64 used pools, river pools accounted for 41%, grassland 26%, mopane 22%, and artificial 11%.
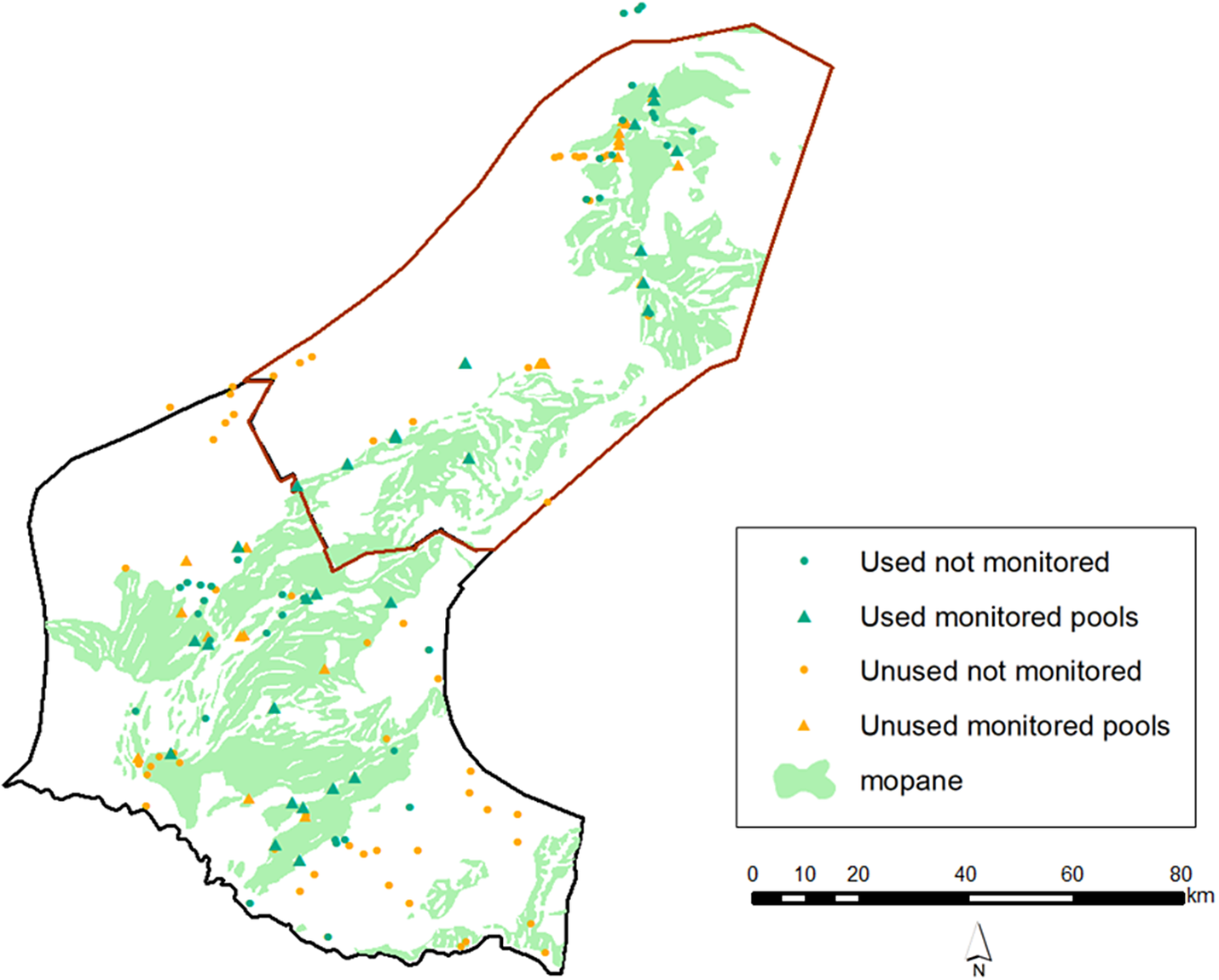
Figure 3. Distribution of pools across the study area; the red border represents the northern region while the black line represents the south.
Overall patterns of pool usage
There were significant differences in the proportions of pools used across the four pool types (χ² = 11.8, df = 3, P = 0.008) (Table 3). From the GLM results, used pools tended to be close to mopane, have a high basal area of surrounding trees, and lack human activity (Table 4). In the dry season, however, pool usage was influenced by distance to mopane, the basal area of surrounding trees, human activity, distance to nearest pool, and pool size, while pool type was not significant (Table 4). In the wet season, key parameters influencing lovebird usage of pools were almost the same but with pool type replacing human activity.
Table 3. Overview of characteristics of used and unused pools
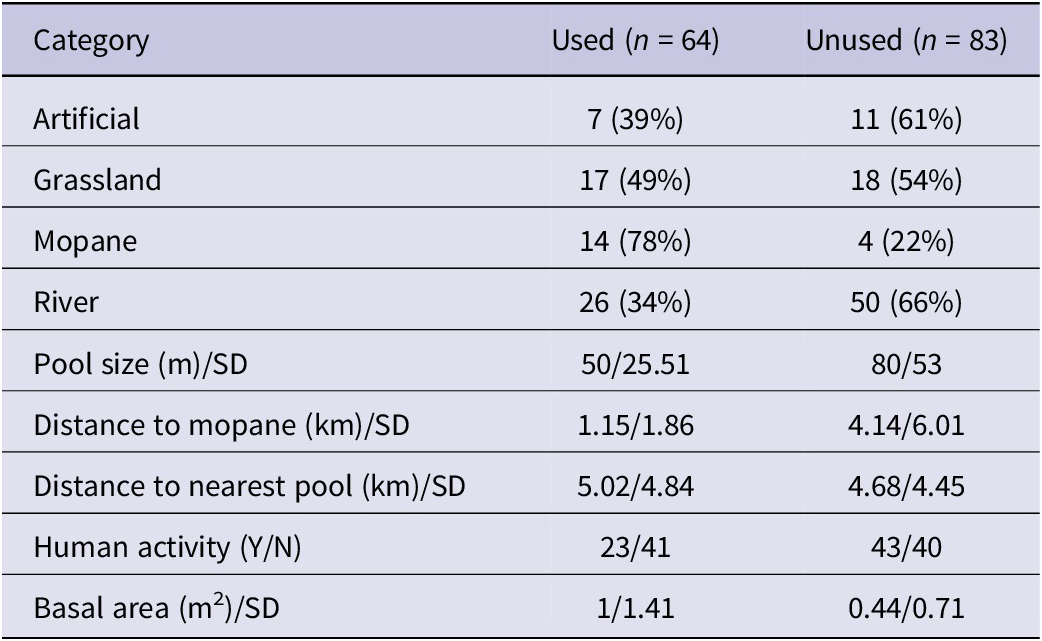
Table 4. Most supported models from logistic regression for overall and seasonal usage, and numbers of Black-cheeked Lovebirds Agapornis nigrigenis using pools from Poisson regression. Figures given are coefficients for each predictor. *P <0.05; **P <0.01; ***P <0.001; ns = not significant
AP = artificial pool, MP = mopane pool, RP = river pool (reference level used = artificial pool). No activity present = reference level on human activity
Variation in lovebird numbers at pools
Numbers of birds were influenced only by pool type and were higher where human activity was lower. Pool monitors paid a total of 837 visits to used pools, of which 412 were in the morning. Time of day (morning or afternoon) did not significantly affect the number of birds recorded (χ² = 0.75, df = 1, P = 0.39).
Lovebird numbers at monitored pools varied with pool type and season (wet/dry). The correlation between season and pool type in the dry season (rₛ = −0.73, n = 53, P <0.001) was stronger than that in the wet season (rₛ = −0.48, n = 53, P = 0.01), suggesting that pool type has a more substantial and significant impact on the number of birds visiting during the dry season compared with the wet season (an average of 1,920 in the dry season and 2,064 in the wet season). Artificial pools recorded their highest number of lovebirds in May (just after the rains) and October (just before the rains). Mopane pools on the other hand exhibited trends aligning with their documented cycle, with a constant number of birds from January to June and a drop until November as these pools tended to dry out completely after the wet season. Grassland pools hosted their highest numbers in June and March (Figure 4), with their lowest numbers coinciding with the periods of flooding (February) and desiccation (October). The number of birds drinking at river pools was consistent throughout the year, with a slight drop in May and August.

Figure 4. Variation in the average number of Black-cheeked Lovebirds Agapornis nigrigenis recorded per month across all pool types. The wet season runs from November to May while the dry season is from June to October.
Discussion
Black-cheeked Lovebirds used only 64 of the 147 pools located in this study, with used pools tending to be located close to the centre of the species’ range. A usage rate of 78% for mopane pools is striking, as it underlines the significance of mopane woodland to the survival of the species (Moura et al. Reference Moura, Maquia, Rija, Ribeiro, Ribeiro-Barros and Bitz2017), as well as the need for concerted action to manage such pools for the benefit of the lovebirds. Notable from this and earlier studies is that mopane and grassland pools dry out and become unusable by the lovebirds during the peak of the dry season (Mzumara et al. Reference Mzumara, Perrin and Downs2016; Warburton and Perrin Reference Warburton and Perrin2005b; CGP 2021, personal observation).
Of the 28 confirmed used pools georeferenced as part of a lovebird drinking study in the 2000s (Warburton Reference Warburton2003), only 22 were still used during this study while the six available unused pools remained unused. Such a reduction in the number of pools appears to reflect a reduction in rainfall and land-use change over the last 20 years (Musonda et al. Reference Musonda, Jing, Nyasulu and Mumo2021), resulting in several modifications within riverine ecosystems (IPCC 2022). This aligns with evidence across south-western Zambia where reductions in rainfall have been observed since 1978 and are projected to continue past the year 2050 (Libanda and Ngonga Reference Libanda and Ngonga2018; Musonda et al. Reference Musonda, Jing, Nyasulu and Mumo2021). Overall, the results of our study provide evidence of additional conditions that need to be met to ensure the long-term survival of Zambia’s endemic arid-country parrot.
The probability of a pool being used by Black-cheeked Lovebirds increased with greater proximity to mopane, a larger basal area of adjacent trees, and an absence of human activity. Being dependent on mopane for nesting and roosting cavities as well as general cover, the species presumably saves energy and reduces predation risk by foraging and drinking close to mopane (Devereux et al. Reference Devereux, Whittingham, Fernández-Juricic, Vickery and Krebs2005; Molokwu et al. Reference Molokwu, Nilsson, Ottosson and Olsson2010; Mzumara et al. Reference Mzumara, Martin, Tripathi, Phiri and Amar2019). Visits to distant and isolated sites by other bird species inhabiting arid areas, for example in the dry season when water is scarce, are only undertaken in large numbers that may lower the individual chances of predation (Devereux et al. Reference Devereux, Whittingham, Fernández-Juricic, Vickery and Krebs2005; Lima et al. Reference Lima and Dill1990). Variations in numbers drinking at pools probably reflected variations in the lovebird’s abundance within its range (Warburton and Perrin Reference Warburton and Perrin2005b; Phiri et al. in prep.). Arid-habitat species minimise their energetic expenditure to maintain a water balance (Jong Reference Jong1976; Smit and McKechnie Reference Smit and McKechnie2015), so using pools close to mopane where the species roosts and nests (Warburton and Perrin Reference Warburton and Perrin2005a) must prevent large energy outflows (Bryant Reference Bryant1997).
At pools the lovebirds initially gather in nearby bushes or trees before descending collectively to drink (Dodman et al. Reference Dodman, Katanekwa, Apsinwall and Stjernstedt2000; Warburton and Perrin Reference Warburton and Perrin2005b). They avoid pools that lack perches within 20 m of the water’s edge (Warburton and Perrin Reference Warburton and Perrin2005b; CGP 2021, personal observation), and in our study basal area of nearest trees served as a proxy for the quantity of available perches and cover near pools (Brumelis et al. 2020). Nevertheless, although our results confirm the strong association between lovebirds and mopane, some used pools were surrounded not by pure mopane but by mixtures of mopane and other tree species or by other species alone, including Combretum imberbe and Diospyros mespiliformis (Dodman et al. Reference Dodman, Katanekwa, Apsinwall and Stjernstedt2000; CGP 2019, personal observation). Overall, fringing vegetation at water points is known to be more critical for small and mid-sized forest birds, whose vulnerability to predation increases when they access drinking sites with too distant or no vegetation (Votto et al. Reference Votto, Schlesinger, Dyer, Caron and Davis2022).
Lovebirds are extremely cautious drinkers that avoid drinking from a pool during periods of loud and obvious human activity (Mzumara et al. Reference Mzumara, Perrin and Downs2016; Warburton and Perrin Reference Warburton and Perrin2005b; CGP 2021, personal observation). In the flood months (February–April) water is abundant in most of the shallow wells within villages and livestock enclosures (Lewanika Reference Lewanika, Bernard, Mosepele and Ramberg2003; Thole and Dodman Reference Thole and Dodman1997), reducing the need for human activity at natural pools. In the dry season, when smaller pools dry out, most pools, particularly the larger ones, saw an increase in human activity, coinciding with a steady increase in the number of lovebirds visiting river and artificial pools.
Other factors known to influence the numbers of lovebirds using pools include local lovebird abundance, pool physical characteristics, and breeding regime (Mzumara et al. Reference Mzumara, Perrin and Downs2016; Warburton and Perrin Reference Warburton and Perrin2005b, Reference Warburton and Perrin2005d). In density surface models developed for the species (Phiri et al. in prep), mopane cover was a significant determinant of local abundance, which in turn will likely influence bird usage of pools. Our study also confirms that the physical features of pools play a significant role in determining their usage by lovebirds, aligning with previous findings related to desert birds (Fisher et al. Reference Fisher, Lindgren and Dawson1972).
Mopane and grassland pools dried out by October, the former refilling after a heavy downpour, the latter refilling according to the flooding regimes of the adjacent river (Naidoo et al. Reference Naidoo, Brennan, Shapiro, Beytell, Aschenborn and Du Preez2020). Consequently, these two pool types showed the most variation in the number of birds across the monitoring period. By contrast river pools, which form in major channels along seasonally dry riverbeds (Warburton and Perrin Reference Warburton and Perrin2005b) and last until the onset of the rains, generally hosted a constant number of birds, with a slight increase in the dry season. Similarly, artificial pools recorded the highest number of birds between October and December, when water is extremely scarce in the study area. Greater dependence on artificial pools has been reported previously in the lovebirds’ southern rather than in their northern subpopulation (Warburton and Perrin 2000b). The north is dominated by the Nanzhila River and its inundated floodplains (Ashley and Murphy Reference Ashley and Murphy2012), which have water all year round. In the south, locals dig small dams or maintain dams left after road maintenance activities for their livestock and gardens; we found that nearly 40% of all artificial pools located in the south were used by lovebirds.
River pools and artificial pools evidently play a pivotal role in maintaining lovebird populations throughout the annual cycle. Potential management interventions to improve the long-term survival prospects of the Black-cheeked Lovebird include simply enhancing the capacity of artificial pools to meet the needs of the lovebirds, by ensuring that each pool’s characteristics meet the criteria of the best-fitting model. However, the creation of small, shallow-sided, undisturbed pools in or near mopane woodland with larger trees within 50 m must also be considered. This highlights the wider need to review existing best practices of water resource provisioning for wildlife species, to devise interventions that will extend the water retention of mopane pools beyond the wet season. Water provision programmes are prominent in many key biodiversity areas in southern Africa in response to increases in water stress (Selebatso et al. Reference Selebatso, Maude and Fynn2018). High-profile sites with such programmes include the Central Kalahari Game Reserve, and Hwange and Kruger National Parks, where diesel engines are used to pump water into pools, evidently promoting the presence and abundance of certain bird species, particularly seedeaters (Abdu et al. Reference Abdu, Lee and Cunningham2018; Kamanda et al. Reference Kamanda, Ndiweni, Imbayarwo-Chikosi, Muvengwi and Musakwa2008). Information from these and similar initiatives can help to identify locations within the mopane woodlands where pools can be excavated and/or sustained by pumps in the dry season. Additional water sources would benefit other wildlife populations also, by expanding animal distributions and increasing productivity, survival rates, and fitness (Rosenstock et al. Reference Rosenstock, Ballard and Devos1999). Such interventions, however, need to be carefully planned to ensure compatibility with long-term landscape management and community welfare objectives as well as the natural mosaic of spatio-temporal variability in surface water (Redfern et al. Reference Redfern, Grant, Gaylard and Getz2005; Smit Reference Smit2013).
Acknowledgements
We thank the Loro Parque Foundation, A. G. Leventis Foundation, and African Bird Club (ABC) for financial support, BirdWatch Zambia for the administrative support to manage the ABC grant, and the Department of National Parks and Wildlife for the research permits. We also thank three referees for their insightful comments on the submitted draft of this paper. Special appreciation goes to the 13 pool monitors in Ngwezi, Machile, Mulanga, and Kafue National Parks, whose commitment and contribution to this work was invaluable. We dedicate this paper to Simaata Mutelo and Brian Zuze, water pool monitors and guardians of the Black-cheeked Lovebirds who passed away before the completion of this study.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000261.










