Introduction
Quantum computing hardware and software have made enormous strides over the last years (Gill et al., Reference Gill, Kumar, Singh, Singh, Kaur, Usman and Buyya2022). Questions around quantum computing’s impact on research and society have changed from “if” to “when/how”. The 2020s have been described as the “quantum decade” (Sieger et al., Reference Sieger2023), and the first production solutions that drive scientific and business value are expected to become available over the next years. Thus, a cross-industry race has begun to secure quantum talent, build quantum skills, map real-world problems to quantum algorithms, capture quantum application intellectual property (IP), and prepare for quantum advantages. Certain types of applications gathered research interest right from the start; for instance, simulating nature through enhanced chemistry and physics calculations (Daley et al., Reference Daley, Bloch, Kokail, Flannigan, Pearson, Troyer and Zoller2022) and solving finance problems (Herman et al., 2022).
In healthcare and life sciences, the initial focus was on biochemical and computational biology problems (Emani et al., Reference Emani, Warrell, Anticevic, Bekiranov, Gandal, McConnell, Sapiro, Aán, Baker, Bastiani, Murray, Sotiropoulos, Taylor, Senthil, Lehner, Gerstein and Harrow2021; Outeiral et al., Reference Outeiral, Strahm, Shi, Morris, Benjamin and Deane2021; Fedorov and Gelfand, Reference Fedorov and Gelfand2021; Marchetti et al., Reference Marchetti, Nifosì, Martelli, Da Pozzo, Cappello, Banterle, Trincavelli, Martini and D’Elia2022; Cordier et al., Reference Cordier, Sawaya, Guerreschi and McWeeney2022; Baiardi et al., Reference Baiardi, Christandl and Reiher2022). Recently, the possibilities of quantum computing have increasingly sparked research interest in other fields as well. This is evidenced by clinical and medical proof-of-concept studies, which have seen a remarkable growth over the last years in conjunction with the exploration of use cases in healthcare (Flöther et al., Reference Flöther, Murphy, Murtha and Sow2022a), medicine (Maniscalco et al., Reference Maniscalco, Borrelli, Cavalcanti, Foti, Glos, Goldsmith, Knecht, Korhonen, Malmi, Nykänen, Rossi, Saarinen, Sokolov, Walter Talarico, Westergren, Zimborás and García-Pérez2022), and life sciences (Flöther et al., Reference Flöther, Moose, Tavernelli, Fraser and Pureswaran2022b).
Defined by the characteristics of the algorithms and the types of problems for which the algorithms are used, three primary quantum algorithm application categories can generally be distinguished:
-
1. Simulating nature – including chemistry, materials science, and physics
-
2. Processing data with complex structure – including artificial intelligence / machine learning (AI/ML), factoring, and ranking
-
3. Search and optimization – including pricing, risk analysis, and sampling
Note that a given quantum algorithm may be part of more than one category. For example, the variational quantum eigensolver (VQE) algorithm (Tilly et al., Reference Tilly, Chen, Cao, Picozzi, Setia, Li, Grant, Wossnig, Rungger, Booth and Tennyson2022) has been applied to strongly correlated systems in chemistry (“Simulating nature”) as well as finding the optimal configuration of nonquantum systems (“Search and optimization”).
A common misconception about quantum computing is that the hardware and software are very similar to their classical counterparts. This is not the case. In fact, quantum algorithms leverage the principles of quantum mechanics, including quantum entanglement, interference, and superposition, in order to tackle problems in novel ways. For a classical computer, the computational power is closely related to the number of transistors. On the other hand, for a quantum computer the number of quantum basis states that can be explored and manipulated in a calculation doubles with the addition of each qubit, thus growing exponentially. This exponential growth underlies the power of quantum algorithms and enables a range of use case-dependent benefits, which may include one or more of:
-
Accuracy (e.g., of an AI/ML model)
-
Calculation speed
-
Energy efficiency
-
Input data requirements (quality, volume)
Results
The studies are grouped into three main use case areas:
-
1. Genomics and clinical research
-
2. Diagnostics
-
3. Treatments and interventions
The connection strengths between the use case areas and the algorithm application categories are illustrated in Figure 1; these were assigned based on the number of proof-of-concept studies associated with each category as well as the applicability of each category to problems typical for a given use case area. It is evident that the category “Processing data with complex structure” is particularly relevant to health and medicine; most of the proof-of-concept studies in this review are based on quantum AI/ML methods.

Figure 1. Three key quantum computing use case areas in health and medicine linked to quantum algorithm application categories. The wider the connecting line, the more applicable the category.
In the context of quantum AI/ML, variational quantum circuits (VQCs) are sometimes considered to be building blocks of quantum neural networks (QNNs) (Wu et al., Reference Wu, Tao and Li2022), that is, neural networks where parameterized quantum circuits are introduced in the hidden layers. In other instances, a VQC is treated as a synonym for a QNN (along with a parameterized quantum circuit and quantum circuit learning) (Schuld et al., Reference Schuld, Sweke and Meyer2021). In this review, no hard distinction is made.
An overview of the explored use cases is given in Figure 2 and a list of the studies and their approaches is provided in Table 1. For many of the proof-of-concept use cases outlined, the quantum approaches are already competitive with the classical benchmarks; while many studies have considered downsized versions of the problems, there is generally no reason to suppose that these benefits will not carry over to more realistic problem variants. Moreover, although an entire “quantum algorithm zoo” exists (Quantum Algorithm Zoo, 2022), the algorithms are all based on a limited number of core primitives. Therefore, notwithstanding the particular characteristics of a given problem, such as the data structure, success of applying one algorithm/primitive in a certain field likely bodes well for uses of that algorithm/primitive in other fields. In the following, each study will now be discussed.

Figure 2. Health and medicine quantum computing use cases that have been investigated in proof-of-concept studies.
Table 1. Overview of quantum algorithms applied in clinical and medical proof-of-concept studies grouped by algorithm application category
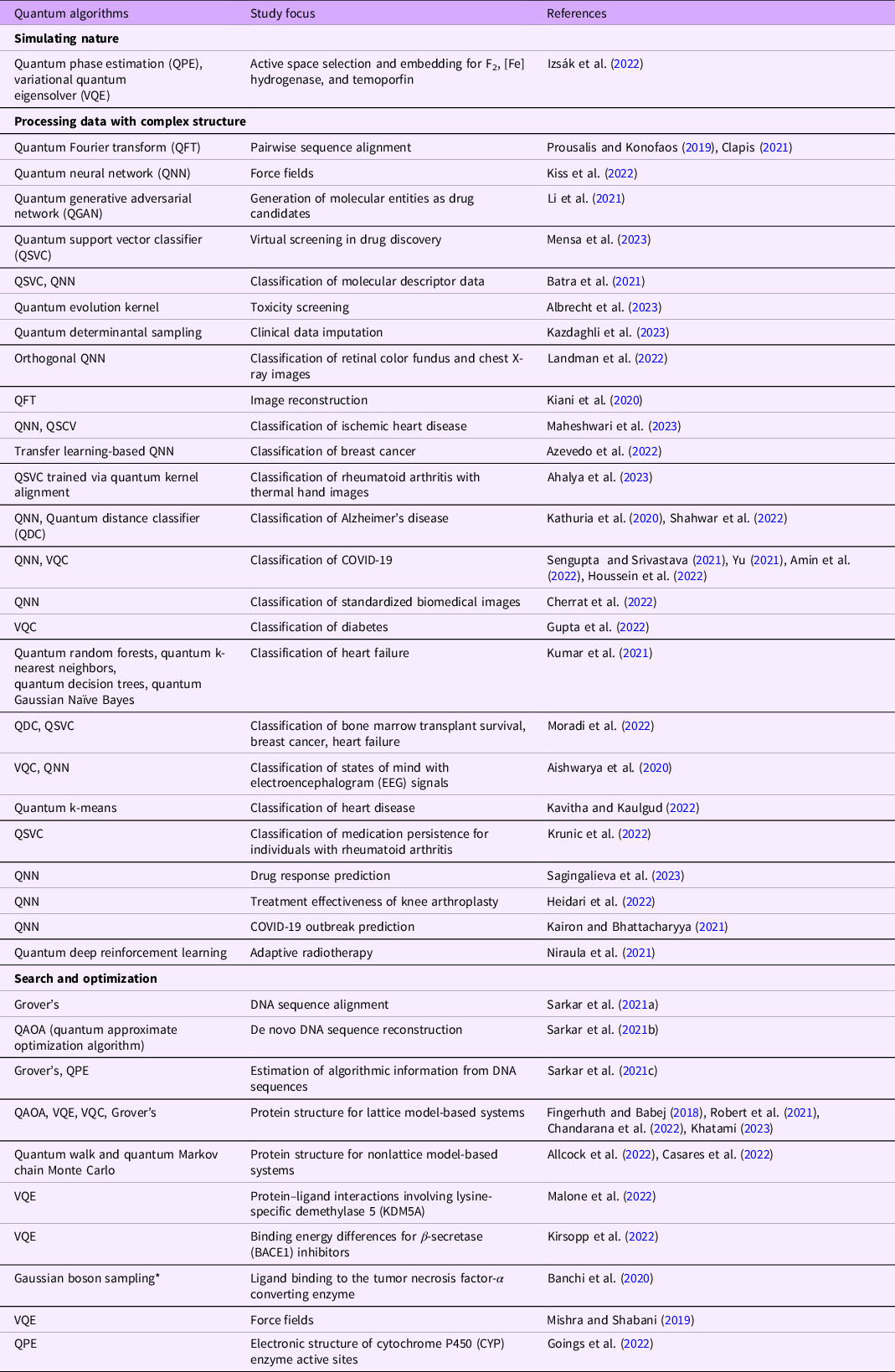
* Gaussian boson sampling is a nonuniversal quantum computational method.
Genomics and clinical research
How can we truly understand an individual at the most granular level? Clearly, genomics is crucial. Over the past decades, we have seen milestones such as the sequencing of the human genome as well as genome-wide association studies (GWAS). It has now become clear, however, that the function and workings of the human genome are much more complex than imagined. The correlations between genomes and outcomes are convoluted and there are, for instance, generally not one-to-one links between genes and diseases. Moreover, pattern problems in the study of haplotypes (groups of genes that are inherited together) and single nucleotide polymorphisms (genomic variations at single base positions between people) quickly become very complicated, reaching nondeterministic polynomial-time (NP) hardness (Lippert et al., Reference Lippert, Schwartz, Lancia and Istrail2002).
As a result, there is great interest to adapt the quantum techniques that have already been developed for problems such as string search and matching, for instance, based on Grover’s algorithm (Niroula and Yunseong, 2021), to genomic problems. Many experiments have focused on better understanding genomic patterns, leveraging algorithms from the “Processing data with complex structure” and “Search and optimization” categories. For example, DNA sequence alignment was explored with Grover’s algorithm (Sarkar et al., Reference Sarkar, Al-Ars, Almudever and Bertels2021) and the quantum Fourier transform (QFT) was applied to pairwise sequence alignment (Prousalis and Konofaos, Reference Prousalis and Konofaos2019; Clapis, 2021). De novo DNA sequence reconstruction was carried out through a framework involving the quantum approximate optimization algorithm (QAOA) (Sarkar et al., Reference Sarkar, Al-Ars, Almudever and Bertels2021). Once (genomic) sequences have been obtained, it is then of great interest to analyze the algorithmic information in them; this was explored using Grover’s algorithm and phase estimation (Sarkar et al., Reference Sarkar, Al-Ars, Almudever and Bertels2021). Note that many of these early advances in better understanding genomic strings and sequences may of course also be applied to related problems in omics in due course, for instance, involving RNA sequences. Likewise, the pattern and information encoding perspective can also be complemented with deeper insights at the molecular level through the application of quantum algorithms from the “Simulating nature” and “Search and optimization” categories.
In addition to genomics, there are diverse fields of clinical research in biology and biochemistry that seem poised to benefit from future quantum advantages. The discovery, and ultimately development, of new molecular entities and drugs is of central importance here. While millions of compounds have already been considered in the literature, the total number of possible carbon-based compounds whose molecular masses are similar to those of living systems is around 10^60. Given the large fraction of this gargantuan chemical space that has not yet been explored, the significant potential for future breakthroughs is clear (Dobson, Reference Dobson2004). Multiple overviews about quantum opportunities in the drug discovery space have been published (Cao et al., Reference Cao, Romero and Aspuru-Guzik2018; Li et al., Reference Li, Topaloglu and Ghosh2021; Blunt et al., Reference Blunt, Camps, Crawford, Izsák, Leontica, Mirani, Moylett, Scivier, Sünderhauf, Schopf, Taylor and Holzmann2022; Santagati et al., Reference Santagati, Aspuru-Guzik, Babbush, Degroote, Gonzalez, Kyoseva, Moll, Oppel, Parrish, Rubin, Streif, Tautermann, Weiss, Wiebe and Utschig-Utschig2023). “Simulating nature” algorithm applications play a prominent role, but the other two categories have also been investigated in this context. A general theme is to reduce the need for lengthy and expensive experiments through better simulations of biology, thus creating in silico laboratories. The biological molecules and systems that can be modeled with quantum computing today are still relatively small, but these are expected to continually scale as quantum hardware and software further mature.
Protein folding and design have gained much attention in recent years through both classical (Callaway, Reference Callaway2022; Lin et al., Reference Lin, Akin, Rao, Hie, Zhu, Lu, Smetanin, Verkuil, Kabeli, Shmueli, Dos Santos Costa, Fazel-Zarandi, Sercu, Candido and Rives2022) and quantum advances. For instance, lattice model-based systems were explored using variational quantum algorithms, including QAOA (Fingerhuth and Babej, Reference Fingerhuth and Babej2018), VQE (Robert et al., Reference Robert, Barkoutsos and Woerner2021), and other variational techniques (Chandarana et al., 2022), as well as Grover’s algorithm (Khatami, Reference Khatami, Mendes, Wiebe, Kim and Ben-Tal2023). For the VQE adaptation (Robert et al., Reference Robert, Barkoutsos and Woerner2021), it was even shown that the number of physical qubits required scales only as the square of the number of amino acids (but without a convergence guarantee), putting structures with 100+ amino acids within reach as quantum hardware develops over the next years (Gambetta, Reference Gambetta2022). Generalization to nonlattice models was investigated through quantum walk and quantum Markov chain Monte Carlo methods (Allcock et al., Reference Allcock, Vangone, Meyder, Adaszewski, Strahm, Hsieh and Zhang2022; Casares et al., Reference Casares, Campos and Martin-Delgado2022). Given the classical advances, it is likely that quantum methods will be particularly advantageous for those problem variations that are most challenging for classical methods. For example, this includes trying to predict the structures of proteins with unnatural amino acids (where classical machine learning struggles due to a lack of training data) or trying to understand conformations and behavior in dynamic settings, such as when proteins interact with water molecules, ligands, and other proteins. For protein–ligand interactions, symmetry-adapted perturbation theory (SAPT) was combined with VQE in benchmarks for systems containing the human cancer-relevant protein lysine-specific demethylase 5 (KDM5A) (Malone et al., Reference Malone, Parrish, Welden, Fox, Degroote, Kyoseva, Moll, Santagati and Streif2022), and VQE was also extended through density matrix embedding theory (DMET) in order to calculate the binding energy differences for β-secretase (BACE1) inhibitors (Kirsopp et al., Reference Kirsopp, Di Paola, Manrique, Krompiec, Greene-Diniz, Guba, Meyder, Wolf, Strahm and Muñoz Ramo2022). In addition, docking was investigated through Gaussian boson sampling (Banchi et al., Reference Banchi, Fingerhuth, Babej, Ing and Arrazola2020) (a restricted form of quantum computing (Hamilton et al., Reference Hamilton, Kruse, Sansoni, Barkhofen, Silberhorn and Jex2017)) by predicting ligand binding to the tumor necrosis factor-α converting enzyme, which is connected with immune system diseases and cancer.
A variety of further applications to help accelerate the drug discovery process has been explored. These include estimating force fields, accurate calculations of which are crucial for scaling molecular dynamics techniques, through QNNs (Kiss et al., Reference Kiss, Tacchino, Vallecorsa and Tavernelli2022) and VQE (Mishra and Shabani, Reference Mishra and Shabani2019). In addition, QPE and VQE methods were studied for the active space, the limited number of orbitals that are of primary interest and treated fully quantum mechanically, of (strongly correlated) chemical systems; F2, [Fe] hydrogenase, and the photosensitizer temoporfin were considered (Izsák et al., Reference Izsák, Riplinger, Blunt, de Souza, Holzmann, Crawford, Camps, Neese and Schopf2022). Other studies focused on estimating the quantum (and classical) resources to compute the electronic structure of cytochrome P450 enzymes (CYPs) via QPE (Goings et al., Reference Goings, White, Lee, Tautermann, Degroote, Gidney, Shiozaki, Babbush and Rubin2022) and applying quantum generative adversarial networks (QGANs) to create new drug candidates (Li et al., Reference Li, Topaloglu and Ghosh2021). Quantum machine learning, specifically quantum support vector classifiers (QSVCs) that enhance calculation of the kernel, also yielded promising results compared with classical state-of-the-art methods for virtual screening in drug discovery (Mensa et al., Reference Mensa, Sahin, Tacchino, Kl Barkoutsos and Tavernelli2023). In another investigation, cheminformatic molecular descriptor data sets for COVID-19, as well as whole-cell screening sets for plague and Mycobacterium tuberculosis, were compressed and then classified using QSVCs and QNN-like methods (Batra et al., Reference Batra, Zorn, Foil, Minerali, Gawriljuk, Lane and Ekins2021). Finally, absorption, distribution, metabolism, excretion, and toxicity (ADMET) studies may be enhanced, as was demonstrated in a toxicity screening experiment where a quantum graph machine learning algorithm (quantum evolution kernel) was applied to a biochemistry data set with information about 286 molecules and their effects on mice (Albrecht et al., Reference Albrecht, Dalyac, Leclerc, Ortiz-Gutiérrez, Thabet, D'Arcangelo, Cline J.R., Elfving, Lassablière, Silvério, Ximenez, Henry, Signoles and Henriet2023).
Diagnostics
Only when it is possible to accurately assess an individual’s health status and potential future development in fine detail can tailored treatments and interventions be properly assigned. As such, quantum computing may enable the move away from (late) diagnoses focused on single diseases toward a regime where a continually updated health status can be determined for each individual; this will only be possible by building on new insights from the previous use case area, “Genomics and clinical research.”
Quantum AI/ML algorithms are particularly relevant for diagnostic applications. Not medical care but health-related behaviors, socioeconomic factors, and environmental aspects are now believed to contribute up to 90% to health outcomes (Hood et al., Reference Hood, Gennuso, Swain and Catlin2016). Hence, it is imperative to understand the quickly growing and increasingly heterogeneous health-relevant data that are becoming available, particularly real-world data (RWD) such as information from electronic health records (EHRs), claims, disease registries, and fitness trackers (Real-World Evidence, 2022). The many potentially pertinent variables lead to high-dimensional feature spaces and interactions between the variables result in complex interdependencies, correlations, and patterns; quantum AI/ML algorithms can penetrate such data structures in ways that are beyond the means of purely classical methods. Furthermore, “Processing data with complex structure” quantum algorithms can even help with enhancing clinical data. For example, quantum determinantal sampling circuits based on Clifford loaders were used to impute a synthetic data set as well as the Medical Information Mart for Intensive Care (MIMIC-III) data set, which contains diagnostic and procedural information for 7,214 patients (Kazdaghli et al., Reference Kazdaghli, Kerenidis, Kieckbusch and Teare2023).
Analyzing and getting actionable insights from medical images is a field that has significantly grown in importance over the last years and decades. As such, a broad array of quantum applications is being explored in this space, including the enhancement of processing steps such as image edge detection, segmentation, and classification (Elaraby et al., Reference Elaraby2022). In classifying retinal color fundus and chest X-ray images, orthogonal QNNs were investigated, and quantum circuits were also used to accelerate the training of classical neural networks (Landman et al., Reference Landman, Mathur, Li, Strahm, Kazdaghli, Prakash and Kerenidis2022). Based on computed tomography (CT) and positron emission tomography (PET) data, QFT-based algorithms were developed for enhancing image reconstruction (Kiani et al., Reference Kiani, Villanyi and Lloyd2020). QNNs and QSCVs were applied to EHRs to classify ischemic heart disease (Maheshwari et al., Reference Maheshwari, Ullah, Osorio Marulanda, García-Olea Jurado, Gonzalez, Ormaetxe Merodio and Garcia-Zapirain2023), while transfer learning-based QNNs were explored in the context of classifying breast cancer (Azevedo et al., Reference Azevedo, Silva and Dutra2022). Rheumatoid arthritis was detected by classifying thermal hand images with QSVCs trained via quantum kernel alignment (Ahalya et al., Reference Ahalya, Snekhalatha and Dhanraj2023). Alzheimer’s disease was classified with MRI images using QNNs (Shahwar et al., Reference Shahwar, Zafar, Almogren, Zafar, Rehman, Shafiq and Hamam2022), and COVID-19 was classified with QNNs using chest X-ray (Houssein et al., Reference Houssein, Abohashima, Elhoseny and Mohamed2022) as well as CT lung images (Sengupta and Srivastava, Reference Sengupta and Srivastava2021; Amin et al., Reference Amin, Sharif, Gul, Kadry and Chakraborty2022). Finally, quantum transformers were explored to achieve more efficient neural network architectures for classifying standardized biomedical images; the quantum architectures only required thousands of parameters, compared with millions for the best classical approaches (Cherrat et al., Reference Cherrat, Kerenidis, Mathur, Landman, Strahm and Yvonna Li2022).
Next to images, diseases and disease risks have also been classified and predicted in early studies of supervised quantum AI/ML. Copy number variations (CNVs), differences in the number of repetitions of a genomic section between individuals, in neuronal single-cell samples from healthy individuals and those with Alzheimer’s disease were used as features; building on the efficiency with which quantum computers can evaluate inner products, this allowed quantum distance classifiers (QDCs) to predict whether a given sample is from a healthy or a sick individual (Kathuria et al., Reference Kathuria, Ratan, McConnell and Bekiranov2020). COVID-19 was diagnosed through VQCs based on features such as temperature (fever), fatigue, muscle pain, and coughing (Yu, Reference Yu2021). VQCs were also employed to predict diabetes (Gupta et al., Reference Gupta, Varshney, Sharma, Pachauri and Verma2022). A diversity of methods – quantum random forests, quantum k-nearest neighbors, quantum decision trees, and quantum Gaussian Naïve Bayes – was studied for the purpose of classifying heart failure (Kumar et al., Reference Kumar, Koul, Sisodia, Shafi, Verma, Gheisari, Davoodi and Khosravi2021). Conversely, QDCs and QSVCs were applied to assess multiple conditions in the same study, namely bone marrow transplant survival, breast cancer, and heart failure (Moradi et al., Reference Moradi, Brandner, Spielvogel, Krajnc, Hillmich, Wille, Drexler and Papp2022). Moreover, VQCs as well as QNNs were even used to predict states of mind based on electroencephalogram (EEG) signals from individuals who responded toward a product with a like/dislike in a neuromarketing experiment (Aishwarya et al., Reference Aishwarya, Abeer, Sathish and Subramanya2020). Finally, in the same way that unsupervised learning is a younger discipline than supervised learning for classical ML, the field of unsupervised quantum AI/ML is younger than supervised quantum AI/ML. Still, even here there is already early medical work underway – the quantum k-means algorithm was used for clustering individuals based on their demographic and laboratory measurement data and predicting heart disease (Kavitha and Narasimha, 2022).
Treatments and interventions
The applications outlined in the previous two use case areas, “Genomics and clinical research” as well as “Diagnostics,” form the foundation for tailored treatments and interventions. As for diagnostics, quantum AI/ML algorithms lend themselves particularly well to treatment and intervention use cases.
Next to knowing an individual’s health status and disease risks, it is essential to understand likely adherence, engagement, and behavior in order to achieve optimal outcomes (Dentzer, Reference Dentzer2013). RWD again plays a central role here. Based on EHRs, for instance, the medication persistence of individuals with rheumatoid arthritis was predicted with QSVCs and a general framework to help assess empirical quantum advantage potential was introduced (Krunic et al., Reference Krunic, Flöther, Seegan, Earnest-Noble and Shehab2022). Another essential research topic on the road to precision medicine is treatment effectiveness. In one study, drug response was predicted by deriving IC50 values (the drug concentrations where the response is half of the maximum) using QNNs (Sagingalieva et al., Reference Sagingalieva, Kordzanganeh, Kenbayev, Kosichkina, Tomashuk and Melnikov2023). In addition, for the purpose of forecasting knee arthroplasty QNNs were applied to clinico-demographic data from 170 individuals that were treated over two years. The results were encouraging, but the study also noted that further validation using unstructured RWD is needed (Heidari et al., Reference Heidari, Olgiati, Meloni, Pirovano, Noorani, Slevin and Azamfirei2022). Optimal measures at the population level require better models too, for example, regarding outbreak prediction and disease spread dynamics. Using a COVID-19 time series data set with confirmed cases, number of deaths, and number of recovered individuals, different types of QNNs (including continuous-variable ones) were applied for this purpose (Kairon and Bhattacharyya, Reference Kairon and Bhattacharyya2021).
As quantum techniques continue to mature and proliferate, there is hope that they can accelerate the discovery process itself as well as enable progress for some of the thorniest medical treatment and intervention problems. Precision oncology is a case in point. Currently, only a third of individuals respond to drug-based cancer therapies (Spilker, Reference Spilker2022). One key challenge is the need to make sense of terabytes and terabytes of relevant data for an individual with cancer. Work has already begun on leveraging quantum algorithms for the purpose of getting actionable insights from such data and ultimately tailoring cancer treatments to the level of the individual (Abbott, Reference Abbott2021). One of the early applications showing promise is adaptive radiotherapy, as was demonstrated by modeling the clinical decisions as quantum states and applying quantum deep reinforcement learning to an institutional data set based on 67 stage III nonsmall cell lung cancer patients (Niraula et al., Reference Niraula, Jamaluddin, Matuszak, Haken R.K. and Naqa2021). Yet another research frontier concerns the intersection of quantum algorithms with single-cell technologies with the aim to enhance the development of cell-centric therapeutics (Basu et al., Reference Basu, Born, Bose, Capponi, Chalkia, Chan, Doga, Goldsmith, Gujarati, Guzman-Saenz, Iliopoulos, Jones, Knecht, Madan, Maniscalco, Mariella, Morrone, Najafi, Pati, Platt, Anna Rapsomaniki, Ray, Rhrissorrakrai, Shehab, Tavernelli, Tolunay, Utro, Woerner, Zhuk, Garcia and Parida2023).
Conclusion and perspective
Ever since the beginnings of medicine thousands of years ago, medicine has continually incorporated new ideas, knowledge, and methods to become more effective. Quantum computing is very young but, as the only known computational model that has exponential speedups compared with traditional approaches (National Academies of Sciences, Engineering, and Medicine, 2019), poised to become a mighty tool in healthcare and medicine with the power to make previously intractable problems now solvable. Despite its youth, quantum computing has already achieved a number of general successes, as summarized in Table 2.
Table 2. General achievements of quantum computing in healthcare, medicine, and life sciences as well as other fields

For quantum computing to become this powerful enabler for health and medicine and for a wide range of quantum-enhanced solutions to go into production, however, a wide range of technical and ethical challenges must still be overcome (Figure 3). First, quantum hardware and software need to continue improving, including more efficient algorithms, decreased error rates, and increased qubit numbers. Second, there are various challenges around making quantum computing practical for medicine which are similar to those in digital health efforts. These include data accessibility (without which even quantum computing cannot wield its power), model explainability (essential for obtaining the support of clinicians, medical practitioners, and individuals), and patient privacy (critical for developing the long-term trust of individuals in the technology). Third, new challenges specific to quantum computing have appeared. Examples are data security, replicability, and skill development, which will now be discussed in turn.
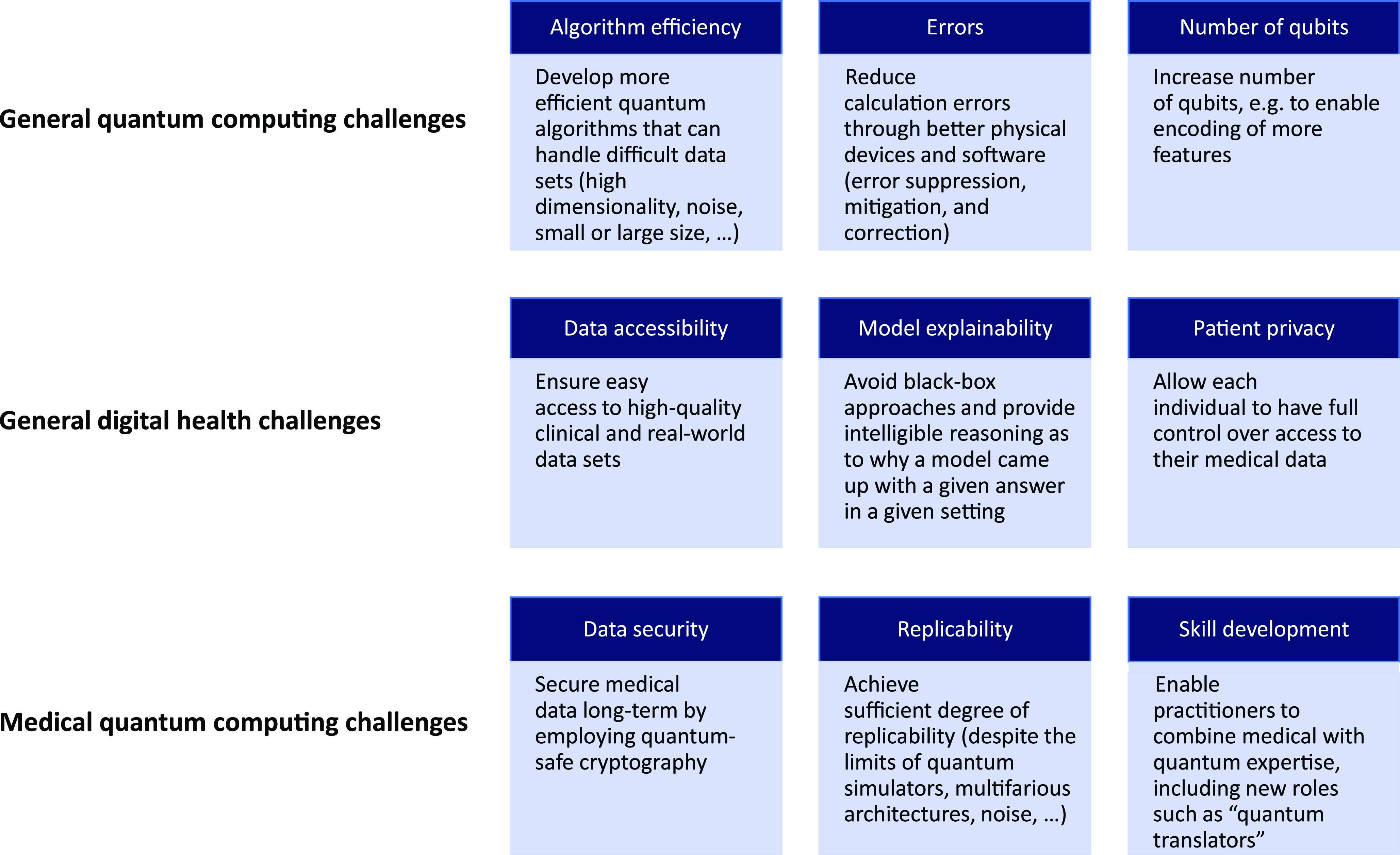
Figure 3. Examples of technical and ethical challenges that must be addressed for quantum computing to become transformative in health and medicine.
Some quantum algorithms, specifically Shor’s and Grover’s algorithm, are able to solve the mathematically hard problems at the heart of current cryptography significantly faster than classical methods. All data that are not encrypted with quantum-safe protocols are thus already at risk due to the possibility of “harvest now, decrypt later” attacks (Harishankar et al., Reference Harishankar, Schaefer, Osborne, Muppidi and Rjaibi2023); given the sensitivity and long security time value of medical data, this problem is exacerbated. As a result, cross-industry quantum-safe standards are already being developed (NIST Announces First Four Quantum-Resistant Cryptographic Algorithms, 2022) and will soon be implemented (Migrating to Post-Quantum Cryptography, 2022). Furthermore, replicability, required in order to achieve clinical approvals and individual acceptance, is a challenge for quantum computers. Quantum computers, by their very nature, are designed to go beyond traditional means and address classically intractable problems; for many problems, however, new (quantum) solutions cannot be efficiently verified. Replicability is further complicated by the probabilistic nature of quantum computing, the multifarious architectures, the presence of noise, and the (still) limited access to quantum hardware. Hence, methodologies and frameworks to secure regulatory approvals and general support will need to be developed, as has been done for classical AI/ML (Benjamens et al., Reference Benjamens, Dhunnoo and Meskó2020). Finally, there is fierce competition for quantum talent, particularly practitioners who combine quantum skills with medical expertise. As a result, talent development needs to be extended, including the introduction of new roles such as “quantum translators” (Mohr et al., Reference Mohr, Peltz, Zemmel and Zesko2022).
The development of medicine-focused quantum computing collaborations and consortia is critical with regard to addressing many of these challenges. Such ecosystems are beginning to emerge (Zinner et al., Reference Zinner, Dahlhausen, Boehme, Ehlers, Bieske and Fehring2021a, Reference Zinner, Dahlhausen, Boehme, Ehlers, Bieske and Fehring2021b; Major investment for developing Denmark’s first fully functional quantum computer, Reference Malone, Parrish, Welden, Fox, Degroote, Kyoseva, Moll, Santagati and Streif2022; Cleveland Clinic and IBM Begin Installation of IBM Quantum System One, Reference Cordier, Sawaya, Guerreschi and McWeeney2022; uptownBasel opens first quantum computer hub for commercial use in Switzerland, Reference Wu, Tao and Li2022) to help practitioners tackle problems with a quantum state of mind. In healthcare, there has been much discussion about the journey towards precision medicine and the quadruple aim (better health, lower costs, enhanced patient experiences, and improved healthcare practitioner work lives) (Bodenheimer and Christine, Reference Bodenheimer and Christine2014). While a range of technical and ethical challenges remain, quantum computing is poised to become a key enabler for advancing towards the holy grail: keeping people healthy through proactive medical care and guidance at the level of an individual. All of this will take time and effort, but the significant rewards along the road toward quantum-enhanced health and medicine make it a highly worthwhile journey to start sooner rather than later.
Methods
The literature search was conducted primarily through the Google Scholar and PubMed platforms. The logical operators OR and AND were combined with search terms such as the following: AI, algorithm, application, artificial intelligence, biology, chemistry, clinical, clinical research, diagnosis, diagnostics, drug, genomics, health, intervention, machine learning, medicine, ML, nature, optimization, QML, quantum, quantum AI, quantum artificial intelligence, quantum computing, quantum machine learning, search, simulation, and treatment.
Studies were only included if the work explored quantum computing algorithms for applications within, or closely related to, health and medicine. Moreover, the focus of this review is on the quantum circuit model and gate-based quantum computers. Gaussian boson sampling is briefly touched on, but other nonuniversal approaches, such as quantum annealing, were excluded.
Acknowledgments
The author would like to thank Travis L. Scholten for helpful discussions.
Competing interests
The author declares no conflicts of interest.









Comments
No accompanying comment.