Introduction
Exposure to early life adversity (ELA), including experiences of abuse and neglect, is a potent risk factor for impaired psychosocial functioning. The implications of ELA are wide-reaching, increasing the risk of delinquency (Ford et al., Reference Ford, Elhai, Connor and Frueh2010; Turner et al., Reference Turner, Shattuck, Finkelhor and Hamby2016), behavioral problems (Choi et al., Reference Choi, Wang and Jackson2019; Schroeder et al., Reference Schroeder, Slopen and Mittal2020), social dysfunction (McCrory et al., Reference McCrory, Foulkes and Viding2022; Salzinger et al., Reference Salzinger, Feldman, Hammer and Rosario1993), and psychopathology (Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010; Kessler et al., Reference Kessler, McLaughlin, Green, Gruber, Sampson, Zaslavsky, Aguilar-Gaxiola, Alhamzawi, Alonso, Angermeyer, Benjet, Bromet, Chatterji, de Girolamo, Demyttenaere, Fayyad, Florescu, Gal, Gureje and Williams2010; McLaughlin et al., Reference McLaughlin, Greif Green, Gruber, Sampson, Zaslavsky and Kessler2012) throughout the lifespan. ELA is hypothesized to disrupt healthy functioning in part by sensitizing neural circuitry to motivationally salient and threat-relevant cues (Callaghan & Tottenham, Reference Callaghan and Tottenham2016; McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016), resulting in heightened vigilance for potential threat (McLaughlin & Lambert, Reference McLaughlin and Lambert2017; Nusslock & Miller, Reference Nusslock and Miller2016; Silvers et al., Reference Silvers, Goff, Gabard-Durnam, Gee, Fareri, Caldera and Tottenham2017). This sensitization may result in a tendency to overestimate threat in ambiguous situations (Chen & Matthews, Reference Chen and Matthews2001, Reference Chen and Matthews2003; Lange et al., Reference Lange, Goossens, Bakker, Michielse, van Winkel, Lissek, Leibold, Marcelis, Wichers, van Os, van Amelsvoort and Schruers2019; McLaughlin et al., Reference McLaughlin, DeCross, Jovanovic and Tottenham2019). Critically, in samples not selected for ELA exposure, the tendency to assume threat in the face of ambiguity has been linked to psychosocial challenges (Carleton, Reference Carleton2016; Chen & Lovibond, Reference Chen and Lovibond2020; Dodge, Reference Dodge2006; Hirsch et al., Reference Hirsch, Meeten, Krahé and Reeder2016; Taghavi et al., Reference Taghavi, Moradi, Neshat-Doost, Yule and Dalgleish2000), whereas positive evaluations of ambiguity have been shown to mitigate risk for psychosocial challenges following ELA (Lange et al., Reference Lange, Goossens, Bakker, Michielse, van Winkel, Lissek, Leibold, Marcelis, Wichers, van Os, van Amelsvoort and Schruers2019; Troller-Renfree et al., Reference Troller-Renfree, McDermott, Nelson, Zeanah and Fox2015, Reference Troller-Renfree, McLaughlin, Sheridan, Nelson, Zeanah and Fox2017; VanTieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017). Although an extensive literature has demonstrated ELA-based differences in neural responses to objectively negative cues (Doretto & Scivoletto, Reference Doretto and Scivoletto2018; Herzberg & Gunnar, Reference Herzberg and Gunnar2020; Saarinen et al., Reference Saarinen, Keltikangas-Järvinen, Jääskeläinen, Huhtaniska, Pudas, Tovar-Perdomo, Penttilä, Miettunen and Lieslehto2021; da Silva Ferreira et al., Reference da Silva Ferreira, Crippa and de Lima Osório2014), few studies have examined neural responses to ambiguous stimuli as a function of adversity exposure. Notably, work in clinical populations suggests that responses to ambiguous stimuli are more predictive of psychosocial health than responses to explicitly threatening stimuli (Lissek et al., Reference Lissek, Rabin, Heller, Lukenbaugh, Geraci, Pine and Grillon2010). While understudied, examining how ELA shapes responses to ambiguity stands to transform our understanding of how early experiences contribute to psychopathology and to eventually identify modifiable protective factors in vulnerable populations.
Prior behavioral studies suggest that ELA is associated with developmental differences in processing ambiguity. For instance, Bick et al. (Reference Bick, Luyster, Fox, Zeanah and Nelson2017) found that children with a history of institutional orphanage care differed from age-matched comparisons in recognition accuracy for ambiguous, but not unambiguous, facial expressions. Further research has demonstrated that ELA-exposed youth tend to interpret ambiguity as threatening more often than age-matched peers (Dodge et al., Reference Dodge, Bates and Pettit1990) (although see Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017, which finds the opposite). For instance, Chen and Matthews (Reference Chen and Matthews2001, Reference Chen and Matthews2003) demonstrated that low SES youth were more likely than high SES youth to interpret ambiguous scenarios as threatening, whereas no group differences were observed when presented with explicitly threatening scenarios. Similarly, Pollak and Kistler (Reference Pollak and Kistler2002) found that, relative to comparison youth, abused children overidentified anger in morphed facial expressions. At present, little is known about how ELA exposure impacts ambiguity processing in adulthood, limiting our understanding of how enduring the effects of ELA are on interpretations of ambiguity.
Although several studies suggest that, on average, ELA-exposed individuals overestimate threat when evaluating ambiguity, there is marked heterogeneity amongst ELA-exposed individuals. Such individual differences may be particularly important for psychosocial functioning in ELA-exposed individuals. For example, VanTieghem et al. (Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017) found that the tendency to evaluate ambiguous facial expressions positively mitigated risk for internalizing symptoms in previously institutionalized, but not comparison, youth, suggesting that this behavioral phenotype may be a uniquely protective factor for ELA-exposed populations. Ambiguous stimuli may thus be useful for probing hypersensitivity to potential threats (Neta et al., Reference Neta, Cantelon, Haga, Mahoney, Taylor and Davis2017; Pollak & Kistler, Reference Pollak and Kistler2002) and more broadly indexing individual differences relevant to psychosocial functioning (Lissek et al., Reference Lissek, Rabin, Heller, Lukenbaugh, Geraci, Pine and Grillon2010; Neta & Brock, Reference Neta and Brock2021; Petro et al., Reference Petro, Tottenham and Neta2021; Puccetti et al., Reference Puccetti, Villano, Stamatis, Torrez, Neta, Timpano and Heller2020; Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017). Identifying such individual differences may be particularly important during the transition from adolescence to adulthood, a developmental stage characterized by heightened risk for psychiatric disorders (Arnett et al., Reference Arnett, Žukauskienė and Sugimura2014), especially among individuals exposed to ELA (van der Vegt et al., Reference van der Vegt, van der Ende, Ferdinand, Verhulst and Tiemeier2009).
The tendency to appraise ambiguity as threatening may stem in part from difficulty distinguishing between ambiguous and threatening stimuli. At the neural level, this may manifest in the brain representing ambiguous and threatening information similarly (Lecei & van Winkel, Reference Lecei and van Winkel2020), particularly within affective neural circuitry responsive to valenced stimuli. Although this possibility has received theoretical support (Lecei & van Winkel, Reference Lecei and van Winkel2020), prior empirical work examining neural discrimination among affective cues following ELA has largely relied on univariate analyses to capture average BOLD responses to affective stimuli (Green et al., Reference Green, Goff, Gee, Gabard-Durnam, Flannery, Telzer, Humphreys, Louie and Tottenham2016; Saarinen et al., Reference Saarinen, Keltikangas-Järvinen, Jääskeläinen, Huhtaniska, Pudas, Tovar-Perdomo, Penttilä, Miettunen and Lieslehto2021; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011; van Harmelen et al., Reference van Harmelen, van Tol, Demenescu, van der Wee, Veltman, Aleman, van Buchem, Spinhoven, Penninx and Elzinga2013). While useful, univariate approaches rely on averaging brain activity over several units into a single index of activity and thus cannot capture more detailed, distributed patterns of representations of ambiguity — or, crucially, how similar these distributed patterns are to representations of threat. Multivariate tools offer a unique opportunity to examine more detailed, distributed patterns of neural activity. Such methods are particularly sensitive to subtle variations in social and affective stimuli (Weaverdyck et al., Reference Weaverdyck, Lieberman and Parkinson2020). For example, representational similarity analysis (RSA) is a technique that assesses representational overlap between stimulus types (e.g., ambiguous versus threatening) based on voxelwise, distributed patterns of neural activity (Dimsdale-Zucker & Ranganath, Reference Dimsdale-Zucker and Ranganath2018; Kriegeskorte, Reference Kriegeskorte2008). Using RSA, recent studies in community samples demonstrate that multivariate representations within the amygdala track subtle variations in perceived trustworthiness of ambiguous social stimuli (FeldmanHall et al., Reference FeldmanHall, Dunsmoor, Tompary, Hunter, Todorov and Phelps2018; Tashjian et al., Reference Tashjian, Guassi Moreira and Galván2019). RSA may similarly elucidate whether similarity between multivariate representations of ambiguity and threat differs as a function of adversity history.
In this study, we used RSA to investigate similarity in neural representations of ambiguous and threatening images as a function of ELA history. We chose to focus on the transition from adolescence to adulthood (i.e., “emerging adulthood”) (Arnett et al., Reference Arnett, Žukauskienė and Sugimura2014) based on prior work suggesting that individual differences in responses to ambiguity are particularly important for shaping wellbeing during this period of development (Bardi et al., Reference Bardi, Guerra and Ramdeny2009; Silvers & Peris, Reference Silvers and Peris2023). This developmental stage is characterized by a number of ambiguous challenges (e.g., moving to a new and unfamiliar city for college or a first job, choosing a career path, living independently for the first time). The uncertainties associated with emerging adulthood are thought to be especially stressful during the earliest stages of this transitional period, when these novel stressors are the most unfamiliar and ambiguous (Bardi et al., 2009). For this reason, we recruited freshmen college students in order to capture this initial transition period in which ambiguity is thought to be most closely linked to wellbeing (Bardi et al., 2009). Our decision to focus on this developmental stage was additionally motivated by work demonstrating heightened risk for psychopathology during the transition to adulthood (Arnett et al., Reference Arnett, Žukauskienė and Sugimura2014), especially within populations with a history of caregiving adversity (van der Vegt et al., Reference van der Vegt, van der Ende, Ferdinand, Verhulst and Tiemeier2009).
A sample of 41 emerging adults with varying levels of ELA exposure underwent fMRI while viewing ambiguous, threatening, and nonthreatening images. Outside of the scanner, participants rated the images. We hypothesized that individuals with higher ELA would demonstrate greater sensitivity to threat, indicated by greater similarity (i.e., less differentiation) in their representations of ambiguous and threatening images. We expected this pattern to be specific to ambiguity and threat — that is, we did not hypothesize ELA-based differences in representational overlap between ambiguous and nonthreatening, or between threatening and nonthreatening, images. These hypotheses were tested in four a priori regions of interest, selected based on (1) their sensitivity to motivationally salient stimuli, especially ambiguous and potentially threatening signals, and (2) research demonstrating ELA-related differences in function within these regions: the amygdala (Fareri & Tottenham, Reference Fareri and Tottenham2016; FeldmanHall et al., Reference FeldmanHall, Dunsmoor, Tompary, Hunter, Todorov and Phelps2018; Tashjian et al., Reference Tashjian, Guassi Moreira and Galván2019; Xu et al., Reference Xu, Peng, Luo and Gong2021), nucleus accumbens (Fareri & Tottenham, Reference Fareri and Tottenham2016; Gee et al., Reference Gee, Bath, Johnson, Meyer, Murty, van den Bos and Hartley2018; Ray et al., Reference Ray, Russ, Walker and McDannald2020; Xu et al., Reference Xu, Peng, Luo and Gong2021), anterior insula (Hein & Monk, Reference Hein and Monk2017; Menon & Uddin, Reference Menon and Uddin2010; Tanovic et al., Reference Tanovic, Gee and Joormann2018; Xu et al., Reference Xu, Peng, Luo and Gong2021), and ventromedial prefrontal cortex (vmPFC) (Chavez & Heatherton, Reference Chavez and Heatherton2015; Cohodes et al., Reference Cohodes, Kitt, Baskin-Sommers and Gee2021; Hart et al., Reference Hart, Lim, Mehta, Simmons, Mirza and Rubia2018; Xu et al., Reference Xu, Peng, Luo and Gong2021).
Given that ELA is associated with impairments across a broad range of domains, we also assessed global functioning, a construct that encapsulates mental health and other features of psychosocial functioning (Pirkis et al., Reference Pirkis, Burgess, Kirk, Dodson, Coombs and Williamson2005; Wing et al., Reference Wing, Beevor, Curtis, Park, Hadden and Burns1998). We hypothesized that individuals exposed to greater levels of ELA would be more likely to appraise the ambiguous images negatively and would exhibit worse global functioning. While prior work has demonstrated robust effects of ELA on emotional outcomes, it has also revealed marked heterogeneity among ELA-exposed groups (Callaghan et al., Reference Callaghan, Gee, Gabard-Durnam, Telzer, Humphreys, Goff, Shapiro, Flannery, Lumian, Fareri, Caldera and Tottenham2019; Gee, Reference Gee2021; Lange et al., Reference Lange, Goossens, Bakker, Michielse, van Winkel, Lissek, Leibold, Marcelis, Wichers, van Os, van Amelsvoort and Schruers2019; Silvers et al., Reference Silvers, Goff, Gabard-Durnam, Gee, Fareri, Caldera and Tottenham2017; Stevens et al., 2021). Thus, we also tested whether individual differences in behavioral responses to ambiguity processing moderated links between ELA and global functioning. Based on prior work, we hypothesized that positive evaluations of ambiguity would be associated with better global functioning in high ELA individuals (Lange et al., Reference Lange, Goossens, Bakker, Michielse, van Winkel, Lissek, Leibold, Marcelis, Wichers, van Os, van Amelsvoort and Schruers2019; Troller-Renfree et al., Reference Troller-Renfree, McDermott, Nelson, Zeanah and Fox2015, Reference Troller-Renfree, McLaughlin, Sheridan, Nelson, Zeanah and Fox2017; Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017). Lastly, prior work demonstrates that taking longer to evaluate ambiguity is associated with more positive evaluations — potentially reflecting an adaptive regulatory process (Neta et al., Reference Neta, Harp, Tong, Clinchard, Brown, Gross and Uusberg2022; Neta & Tong, Reference Neta and Tong2016). We sought to replicate this finding and determine whether ELA moderates links between time spent evaluating ambiguity and global functioning, which ostensibly encompasses aspects of self-regulation.
Methods and materials
Data and code availability
All data, task scripts, and analysis scripts for this study are available on GitHub (https://github.com/nsaragosaharris/earlylifeadversity_ambiguity_study).
Participants
We recruited participants via flyers and online recruitment. An a priori, planned sample size of 40 was selected based on prior neuroimaging studies that used similar multivariate modeling techniques to the planned analyses (Dimsdale-Zucker & Ranganath, Reference Dimsdale-Zucker and Ranganath2018; FeldmanHall et al., Reference FeldmanHall, Dunsmoor, Tompary, Hunter, Todorov and Phelps2018; Stolier & Freeman, Reference Stolier and Freeman2016). In total, 41 participants completed the neuroimaging and post-scan behavioral tasks (N = 29 females, age = 18 to 19 years old,
![]() $\overline{X}$
age = 18.34, SDage = 0.48). Three participants did not complete the global functioning questionnaire (see Questionnaires). Sample demographics and summary statistics for questionnaire data are included in the Supplement (Supplemental Table 1; Supplemental Figure 2
). Participants provided written consent. All study procedures were completed in accordance with the University of California Los Angeles Institutional Review Board (IRB# 19-001000).
$\overline{X}$
age = 18.34, SDage = 0.48). Three participants did not complete the global functioning questionnaire (see Questionnaires). Sample demographics and summary statistics for questionnaire data are included in the Supplement (Supplemental Table 1; Supplemental Figure 2
). Participants provided written consent. All study procedures were completed in accordance with the University of California Los Angeles Institutional Review Board (IRB# 19-001000).
Participants completed questionnaires at an initial lab session and subsequently underwent fMRI testing within two weeks of their lab session. Immediately after fMRI testing, participants completed a behavioral task. Participants were compensated for participation.
Participant inclusion criteria
Data were collected as part of a larger study investigating mental health in individuals transitioning from adolescence to adulthood. Eligibility for inclusion in the study was based on the following criteria and assessed via brief in-person interview and an MRI screening form: (1) individuals in their freshman year of college who were at least 18 years old; (2) no medical or psychiatric conditions contraindicating study participation (e.g., psychosis); (3) no current use of a psychiatric medication; (4) no current treatment for anxiety or depression; (5) no presence of metal in the body; (6) no current report of pregnancy; (7) no pressing mental health concern requiring immediate follow up (e.g. psychosis); and (8) no fear of enclosed spaces (claustrophobia).
Questionnaires
Early life adversity
Early life adversity (ELA) was measured using the Childhood Trauma Questionnaire Short Form (CTQ-SF) (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003), a 28-item scale that assays experiences of emotional abuse, emotional neglect, physical abuse, physical neglect, and sexual abuse before age fourteen. The CTQ-SF has been validated in clinical and non-clinical samples and corresponds well to therapists’ interview-based ratings of abuse and neglect (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003). For each item, participants rated on a scale of 1 to 5 (1 = never true, 5 = very often true) how much they agreed with various statements (e.g., “I believe that I was physically abused”). Responses were totaled across subtypes of abuse and neglect, with higher scores indicating greater experiences of childhood trauma. Total scores were log-transformed and then z-scored to meet the assumptions of the planned statistical tests (i.e., normality).
Global functioning
Global functioning was measured by the self-rated version of the Health of the Nation Outcomes Scale for Children and Adolescents (HoNOSCA-SR) (Gowers et al., Reference Gowers, Levine, Bailey-Rogers, Shore and Burhouse2002), a 13-item measure based on the Health of the Nation Outcomes Scale (Wing et al., Reference Wing, Beevor, Curtis, Park, Hadden and Burns1998) that assesses symptoms and functioning across four domains: behavioral problems (aggressive/antisocial, overactivity/attention, self-harm, substance misuse), impairment (scholastic/language skills, physical disability), symptomatic problems (hallucinations and delusions, non-organic somatic symptoms, emotional and related symptoms), and social problems (peer relationships, self-care and independence, family life and relationships, poor school attendance) (Pirkis et al., Reference Pirkis, Burgess, Kirk, Dodson, Coombs and Williamson2005). The HoNOSCA-SR has been validated (Pirkis et al., Reference Pirkis, Burgess, Kirk, Dodson, Coombs and Williamson2005) and correlates with a number of other mental health scales (Gowers et al., Reference Gowers, Levine, Bailey-Rogers, Shore and Burhouse2002). For each item on the HoNOSCA, participants used a 5-point Likert scale to indicate the degree to which they were affected by a given symptom or experience (e.g., “Have you been troubled by your disruptive behavior, physical or verbal aggression?”) in the last two weeks (0 = “Not at all”, 1 = “Insignificantly”, 2 = “Mild but definitely”, 3 = “Moderately”, 4 = “Severely”). Responses across domains were totaled, with higher scores indicating poorer functioning.
fMRI task and analyses
fMRI paradigm
The fMRI task used an event-related design coded in PsychoPy2 (Peirce et al., Reference Peirce, Gray, Simpson, MacAskill, Höchenberger, Sogo, Kastman and Lindeløv2019). Participants viewed a set of faces with 99 unique actors from the racially diverse affective expression dataset (Conley et al., Reference Conley, Dellarco, Rubien-Thomas, Cohen, Cervera, Tottenham and Casey2018) while undergoing fMRI scanning. Actors in the selected images were 22% Asian, 32% Black/African American, 19% Hispanic or Latinx, and 26% White. 51% were female. Each of the 99 actors had three unique facial expression images (angry, happy, and surprised). Based on prior work, angry, happy, and surprised faces were considered the threatening, nonthreatening, and ambiguous stimuli, respectively (Neta et al., Reference Neta, Cantelon, Haga, Mahoney, Taylor and Davis2017; Pine et al., Reference Pine, Mogg, Bradley, Montgomery, Monk, McClure, Guyer, Ernst, Charney and Kaufman2005; Pollak & Kistler, Reference Pollak and Kistler2002; Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017). In addition to the emotional expressions, participants viewed a blurred image on 27 trials, which was created by superimposing all of the 297 face images from the task and served as an attentional control. Participants viewed a single image per trial. Within each run (three total), participants viewed 33 threatening (angry), 33 nonthreatening (happy), and 33 ambiguous (surprised) faces in addition to 9 blurred images (a composite of all face images), for a total of 108 trials per run and 324 total trials (Figure 1A). Every actor was shown three times (once per run), each time with a different facial expression (threatening, nonthreatening, or ambiguous). Participants were instructed to press the button box only when they saw the blurred image (attention check trials). Each stimulus was presented for 500 ms. Between trials, there was a jittered fixation cross. Jitter times were created in OptSeq2 (https://surfer.nmr.mgh.harvard.edu/optseq/; mean length = 3 s, range = 1.5 to 8 s). Each run lasted 6 minutes and 36 s in total. Images within each of the three runs were shown in a randomized order and the order of runs was counterbalanced across participants.
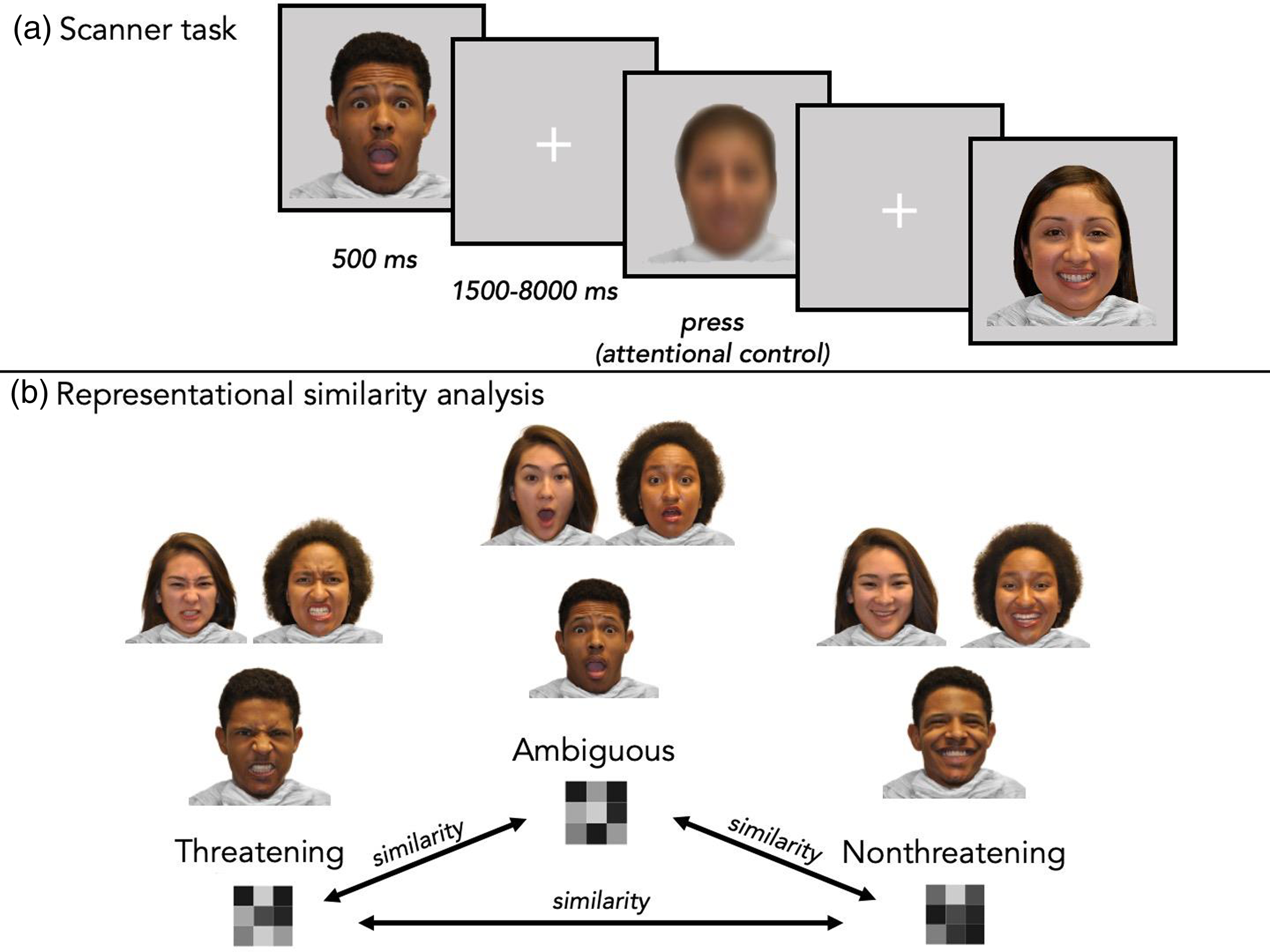
Figure 1. During the MRI task (a), participants passively viewed threatening, nonthreatening, and ambiguous faces. Catch trials included a blurred image and required a button box response. In representational similarity analyses (b), each expression type was modeled to create three multivoxel, vectorized patterns (within participant, run, and ROI). Pairwise correlations (indicated by arrows) were computed to index relative similarity between patterns of responses.
fMRI acquisition
Data were acquired on a 3T Siemens Magnetom Prisma scanner using a 32-channel head coil. Functional data were acquired with 2.0 × 2.0 × 2.0 mm voxel size, 2.0 mm slice thickness, 60 interleaved slices, 2.0 mm slice thickness, 1000m repetition time (TR), 37 ms echo time, 60° flip angle, 208 mm field of view, and 6x multiband acceleration, and using Autoalign for slice positioning and alignment. Structural images were acquired using a high-resolution MPRAGE sequence (voxel size = 0.8 × 0.8 × 0.8 mm; TR = 2400 ms, echo time = 2.22 ms, field of view = 256 mm, slice thickness = 0.8 mm, 208 slices).
fMRI preprocessing
Preprocessing of fMRI data was carried out in FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) using FEAT (FMRI Expert Analysis Tool) Version 6.00. Boundary based registration (Greve & Fischl, Reference Greve and Fischl2009) was used to register participants’ functional data to their high-resolution structural images (i.e., to native space). FLIRT (FMRIB’s Linear Image Registration Tool) (Jenkinson et al., Reference Jenkinson, Bannister, Brady and Smith2002; Jenkinson & Smith, Reference Jenkinson and Smith2001) and FNIRT nonlinear registration (Andersson et al., Reference Andersson, Jenkinson and Smith2007) were used to register high-resolution structural images to standard space (MNI 2.0 × 2.0 × 2.0 mm stereotaxic space) with 12 degrees of freedom. Preprocessing included motion correction using MCFLIRT (Jenkinson et al., Reference Jenkinson, Bannister, Brady and Smith2002) using 24 standard and extended regressors, non-brain extraction using BET (Brain Extraction Tool) (Smith, Reference Smith2002), grand-mean intensity normalization, and a 100 s high-pass temporal filter (Gaussian-weighted least-squares straight line fitting, with sigma = 50 s). Based on similar multivariate pattern analysis and RSA work (Glenn et al., Reference Glenn, Fox, Pine, Peters and Michalska2020; Harry et al., Reference Harry, Williams, Davis and Kim2013; Jin et al., Reference Jin, Zelano, Gottfried and Mohanty2015; Lee et al., Reference Lee, Perino, McElwain and Telzer2020; Liang et al., Reference Liang, Liu, Xu, Zhang, Li, Wang and Wang2017; Tashjian et al., Reference Tashjian, Guassi Moreira and Galván2019) and current recommendations (Dimsdale-Zucker & Ranganath, Reference Dimsdale-Zucker and Ranganath2018; Misaki et al., Reference Misaki, Luh and Bandettini2013; Weaverdyck et al., Reference Weaverdyck, Lieberman and Parkinson2020), in order to maintain fine-grained spatial details across voxels for RSA, we did not apply smoothing prior to multivariate analyses. Analyses were carried out using FILM (FMRIB’s Improved Linear Model) prewhitening with local autocorrelation correction (Woolrich et al., Reference Woolrich, Ripley, Brady and Smith2001). Plots of temporal signal-to-noise ratios within these minimally preprocessed, unmodeled BOLD data by region are provided in the supplement (Supplemental Figure 5 ).
First level modeling
BOLD response patterns were modeled separately by trial expression type (threatening, nonthreatening, or ambiguous) in FSL using first level (i.e., within participant, within run) models, each of which included a regressor for each expression type. Blurred face trials (attention checks) were modeled but not further analyzed. Between-trial fixation crosses served as implicit baseline (i.e., were not explicitly modeled). This resulted in three general linear models (GLMs) per participant (one per run), each of which included BOLD estimates for threatening, nonthreatening, and ambiguous trials. Temporal derivatives for all regressors were included as covariates. Regressors were modeled using a double-gamma hemodynamic response function. To account for head motion, individual volumes with a framewise displacement greater than 0.9 mm were included as regressors (spike regressors created using ‘fsl_motion_outliers’). Motion regressors and their derivatives were included as regressors of no interest.
Regions of interest
Four regions of interest (ROIs; amygdala, nucleus accumbens, anterior insula, and vmPFC) were selected a priori based on (1) their hypothesized role in responding to motivationally salient stimuli, especially ambiguous and potentially threatening signals (Tanovic et al., Reference Tanovic, Gee and Joormann2018), and (2) research demonstrating ELA-related functional differences within these regions (Fareri & Tottenham, Reference Fareri and Tottenham2016). An additional region, V1, was tested as a control region expected to respond to the affective visual stimuli (Kragel et al., Reference Kragel, Reddan, LaBar and Wager2019) but not expected to differ in functional activity based on ELA. The amygdala and nucleus accumbens were defined based on FSL’s Harvard-Oxford atlas and were thresholded in MNI space using Harvard-Oxford’s probabilistic masks, which specify the probability that a given voxel falls within the specified brain region. The amygdala was thresholded at p = 0.50 and the nucleus accumbens was thresholded at p = 0.25 based on prior work (Guassi Moreira et al., Reference Guassi Moreira, Méndez Leal, Waizman, Saragosa-Harris, Ninova and Silvers2021; Tashjian et al., Reference Tashjian, Guassi Moreira and Galván2019) and visual inspection of anatomical alignment. V1 was defined based on FSL’s Juelich atlas (Amunts et al., Reference Amunts, Malikovic, Mohlberg, Schormann and Zilles2000) and thresholded at p = 0.75. The anterior insula and vmPFC were defined using anatomical masks from a relevant ROI-based meta-analysis (Xu et al., Reference Xu, Peng, Luo and Gong2021). This meta-analysis examined neural responses to the dimensional emotions and valence portrayed in facial expressions (including angry, happy, and surprised faces). In the Xu et al. (Reference Xu, Peng, Luo and Gong2021) meta-analysis, the anterior insula and vmPFC were anatomically defined in MNI space using the automated anatomical labeling template (Tzourio-Mazoyer et al., Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002), and these ROIs were shared with our research team. All masks were originally defined in MNI space and transformed into participant-specific native space prior to multivariate (RSA) analyses. Because masks were participant-specific and in native space, there was variability in ROI size. In cases in which an ROI-based statistical estimate was significant, we conducted sensitivity analyses in which we controlled for the number of voxels within the ROI to ensure that differences in ROI size across participants did not affect statistical estimates.
Representational similarity analysis
The function ‘NiftiMasker’ in the Python package ‘nilearn’ (Abraham et al., Reference Abraham, Pedregosa, Eickenberg, Gervais, Mueller, Kossaifi, Gramfort, Thirion and Varoquaux2014) was used to extract vectors of voxel-level coefficients within each ROI. All vectors were participant-specific, run-specific, ROI-specific, and condition-specific: Each vector corresponded to a regressor of interest (ambiguous, threatening, or nonthreatening) from the aforementioned GLMs for a given ROI per run (e.g., run 1 ambiguous vector, run 1 threatening vector, run 1 nonthreatening vector). These vectors were used to compute three pairwise Pearson correlations (ambiguous/threatening, ambiguous/nonthreatening, threatening/nonthreatening) for each run. Next, these correlations were averaged across runs, resulting in three correlations per ROI for a given participant (Figure 1B). Fisher’s r-to-z transformation was then applied to the averaged Pearson correlation values (Dimsdale-Zucker & Ranganath, Reference Dimsdale-Zucker and Ranganath2018). These z-transformed values represent similarity in patterns of representations between (1) ambiguous and threatening, (2) ambiguous and nonthreatening, and (3) threatening and nonthreatening facial expressions within a given ROI, with greater values indicating relatively greater similarity in voxelwise patterns of activation. Parallel analyses were run in a control region (V1). We hypothesized that individuals with higher ELA would demonstrate greater similarity (“overlap”) in their representations of ambiguous and threatening images within the four ROIs (but not V1), and did not expect to see ELA-based differences in representational overlap between ambiguous and nonthreatening, or between threatening and nonthreatening, images. While hypotheses centered around RSA, in the interest of contributing to data sharing efforts, we report univariate methods and results in the supplement.
Post-scan categorization task
Paradigm
Participants completed a surprise, post-scan task in which they were shown a subset of images seen in the fMRI task. After completing six practice trials, participants were shown 200 images (100 ambiguous) over ten blocks (ten ambiguous, five threatening, and five nonthreatening faces per block). More ambiguous faces (N = 100) were shown because this was the primary condition of interest for this study. On each trial, participants pressed a button to indicate whether the person in the image “feels good” or “feels bad” (Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017) by pressing a button on the keyboard (1 or 0, counterbalanced across participants). Each face was presented for 500 ms, followed by a screen with text requesting their response, which lasted for 1500 ms regardless of when they responded (Supplemental Figure 1 ). Early responses (during the initial 500 ms presentation screen) were accepted and included in analyses. If participants made more than one response, their final appraisal was used in analysis to minimize the possibility of analyzing responses made in error. A 200 ms fixation cross was included between trials. Block order was randomized and faces within each block task were shown in a randomized order. In between blocks, there was a ten second fixation screen.
Behavioral data analysis
To index an individual’s propensity to interpret ambiguous faces negatively (“negativity bias”), we computed the percent of ambiguous trials in which participants selected the “feels bad” option. Based on prior work examining responses to ambiguity (Neta & Tong, Reference Neta and Tong2016), we also computed average response times (RTs) by expression type (threatening, nonthreatening, ambiguous), as well as RT differences by expression type.
Sensitivity analyses of post-scan behavioral task. One participant demonstrated a decline in performance partway through the post-scan task, which resulted in five blocks in which this participant had low accuracy on angry trials due to repeated button presses. To account for these blocks in which this participant appeared not to be engaging with the task, we conducted sensitivity analyses for all statistical models involving data from the post-scan task. In these analyses, we excluded this participant’s response data from the five blocks in which their accuracy on angry and happy trials was less than 80% (N = 100 usable trials). Unless otherwise stated, all reported results from behavioral analyses remained after excluding this participant’s low-accuracy blocks.
Results
ELA, global functioning, and post-scan task behavior
ELA and global functioning
As hypothesized, individuals with greater self-reported ELA reported poorer global functioning (i.e., higher HoNOS scores; β = 0.32, 95% CI [0.02, 0.63], t (35) = 2.19, p = 0.04).
Post-scan task behavior
We conducted one-tailed t-tests to ensure that accuracy was significantly above chance performance (50%) on the threatening and nonthreatening (i.e., unambiguous) trials in the post-scan task. These analyses were conducted in order to verify that participants understood the task instructions. Results confirmed that participants correctly rated angry facial expressions negatively (mean accuracy = 0.93; t (40) = 30.6; p < 0.001) and happy facial expressions positively (mean accuracy = 0.96; t (40) = 71.5; p < 0.001; Supplemental Figure 3 ). This pattern of high accuracy and agreement in ratings also verified that the valences of these two types of images were indeed unambiguous. In line with prior work (Neta et al., Reference Neta, Norris and Whalen2009), participants took longer on average to evaluate surprised faces than angry (t (40) = 8.23, mean difference = 0.069 ms, 95% CI [0.05, 0.09], p < 0.001) or happy (t (40) = 9.85, mean difference = 0.097 ms; 95% CI [0.08, 0.12], p < 0.001) faces, supporting the idea that that surprised facial expressions are more ambiguously valenced.
We next tested whether ELA or global functioning related to negativity biases (i.e., the percent of ambiguous trials categorized negatively). After controlling for global functioning, individuals with greater ELA scores demonstrated a greater negativity bias (categorized a greater number of ambiguous faces negatively; β = 0.34, 95% CI [0.002, 0.68], t (34) = 2.04, p = 0.049). After excluding one participant’s low-accuracy blocks in a sensitivity analysis (see Sensitivity analyses of post-scan behavioral task for description), this association was trending (β = 0.34, 95% CI [−0.005, 0.68], t (34) = 2.00, p = 0.05). Based on this sensitivity analysis and given that, on their own, neither ELA nor global functioning was associated with negativity biases (Supplemental Tables 3 and 4 ), we caution against strong interpretation of this finding. ELA and negativity biases did not interact to predict psychosocial functioning (Supplemental Table 5 ).
We next examined the relationship between reaction time (RT) and evaluations of the stimuli. Between-subject average RTs to ambiguous images did not predict negativity biases (Supplemental Table 2 ). However, in line with prior work (Neta & Tong, Reference Neta and Tong2016), longer within-subject, trial-level RTs predicted more positive ratings of ambiguous stimuli in a multilevel model (OR = 1.95; 95% CI [1.44, 2.65], z = 4.29, p < 0.01).
Based on prior work suggesting that taking time to evaluate ambiguity may engage emotion regulation processes (Neta et al., Reference Neta, Harp, Tong, Clinchard, Brown, Gross and Uusberg2022), we sought to determine whether ELA moderated association between time spent evaluating ambiguous (relative to unambiguous) stimuli and global functioning. We observed an interaction between RT differences and ELA exposure, such that taking more time on average to evaluate ambiguous, relative to threatening (b = 5.88, 95% CI [0.81, 10.95], t (33) = 2.36, p = 0.02; Figure 2A) and nonthreatening (b = 6.86, 95% CI [0.73, 12.99], t (33) = 2.28, p = 0.03; Figure 2B), images was associated with better global functioning, specifically in individuals with lower ELA scores.

Figure 2. ELA interacted with reaction time to ambiguous cues to predict global functioning. For individuals exposed to lower levels of ELA, taking more time on average to evaluate ambiguous, relative to threatening (a) and nonthreatening (b) images was associated with better global functioning. The simple slopes for the association between ambiguous vs. threatening reaction difference and global functioning (a) are b = 4.36 and b = −13.28 for high and low ELA, respectively. The simple slopes for the association between ambiguous vs. nonthreatening reaction difference and global functioning (b) are b = 7.58 and b = −13.01 for high and low ELA, respectively. ELA was measured continuously, but is plotted categorically, at high (z = 1.5, in green) and low (z = −1.5, in gray) levels for visualization purposes only. Shaded regions represent 95% confidence intervals.
ELA and similarity in neural representations of nonthreatening, threatening, and ambiguous stimuli
Using RSA, we tested our hypothesis that individuals with higher self-reported ELA would demonstrate greater similarity (“overlap”) in representations of ambiguous and threatening stimuli within the regions of interest. We expected this association to be specific to ambiguous/threatening overlap — that is, we hypothesized no association between ELA and ambiguous/nonthreatening overlap or threatening/nonthreatening overlap.
Representational overlap: ambiguous and threatening stimuli
As hypothesized, individuals exposed to higher levels of ELA demonstrated greater similarity (“overlap”) in multivariate representations of ambiguous and threatening stimuli bilaterally within the amygdala, nucleus accumbens, anterior insula, and vmPFC but not within the control region, V1 (Table 1; Figure 3). All associations remained significant after controlling for the number of voxels within a participant’s ROI and after adjusting for multiple comparisons across different ROIs by using false discovery rate (FDR)-corrected q values (Table 1; Benjamini & Hochberg, Reference Benjamini and Hochberg1995).

Figure 3. As hypothesized, individuals exposed to higher levels of ELA demonstrated greater representational overlap between ambiguous and threatening stimuli bilaterally within the nucleus accumbens, amygdala, anterior insula, and vmPFC, but not within V1.
Table 1. ELA was positively associated with greater similarity in multivariate representations of ambiguous and threatening stimuli in regions of interest. The same pattern was not evident in V1, the control region tested. Table includes the standardized beta coefficients and test statistics from a linear regression with z-scored log-transformed CTQ scores as the predictor and Fisher-z-transformed ambiguous/threatening RSA values as the outcome. Table includes false discovery rate (FDR)-corrected q values that adjust for multiple comparisons across different regions. Column on the right includes the same statistics after participant-specific ROI size was added as a covariate in the model.

Representational overlap: ambiguous and nonthreatening stimuli
We next tested whether ELA was associated with representational overlap between ambiguous and nonthreatening stimuli. Contrary to our hypothesis, we found that ELA was positively associated with greater similarity in multivariate representations of ambiguous and nonthreatening stimuli in the right amygdala (β = 0.03; 95% CI [0.01, 0.06]; t (38) = 2.56, p = 0.01), even after controlling for participant-specific number of voxels within the region (β = 0.03, 95% CI [0.01, 0.06], t (37) = 2.51, p = 0.02). However, this was no longer significant following FDR-corrected adjustments for multiple comparisons across different ROIs (FDR-adjusted q = 0.14; Supplemental Table 7 ). Although a similar pattern was observed in the right anterior insula, this association did not reach significance (β = 0.03; 95% CI [0.00, 0.06]; t (38) = 1.96, p = 0.06). This association was not evident within any of the other regions tested (Supplemental Table 7 ).
Representational overlap: threatening and nonthreatening stimuli
We next tested whether ELA was associated with representational overlap between threatening and nonthreatening stimuli. Contrary to our hypothesis, we found that individuals with higher ELA scores evidenced greater similarity in representations of threatening and nonthreatening stimuli within the right anterior insula (β = 0.03, 95% CI [0.003, 0.06], t (38) = 2.23, p = 0.03), even after controlling for the number of voxels within the region (β = 0.04, 95% CI [0.01, 0.06], t (37) = 2.51, p = 0.02). However, this was no longer significant following FDR-corrected adjustments for multiple comparisons across different ROIs (FDR-adjusted q = 0.22; Supplemental Table 9 ). This association was not evident in the left anterior insula or within any of the other regions tested (Supplemental Table 9 ).
Associations between brain, behavior, and global functioning
Representational overlap between ambiguous and threatening stimuli and global functioning
We hypothesized that greater representational overlap between ambiguous and threatening stimuli would be associated with poorer global functioning. However, we did not observe any significant associations between global functioning and representational similarity between ambiguous and threatening stimuli (Supplemental Table 11 ).
Representational overlap between ambiguous and threatening stimuli and post-scan task behavior
We hypothesized that greater representational similarity between ambiguous and threatening stimuli would predict greater negativity biases in the post-scan task, but we did not observe this hypothesized association (Supplemental Table 12 ).
Discussion
Exposure to ELA impacts the development of threat-sensitive neural circuitry (Fareri & Tottenham, Reference Fareri and Tottenham2016; Herzberg & Gunnar, Reference Herzberg and Gunnar2020). Altered functioning within these networks may underlie hypersensitivity to potential threat in the face of ambiguity, potentiating risk for impaired psychosocial functioning (Lecei & van Winkel, Reference Lecei and van Winkel2020; Nusslock & Miller, Reference Nusslock and Miller2016). Leveraging RSA to characterize multivariate representations of affective stimuli, we found that emerging adults exposed to ELA demonstrated greater similarity (“overlap”) in their representations of ambiguous and threatening images within affective and threat-sensitive circuitry. Notably, rather than a general effect in which individuals with a history of ELA simply exhibited general impairments in differentiating among affective cues, we found that ELA specifically related to attenuated discrimination between ambiguity and threat. These results were not evident in the tested control region (V1), suggesting specificity of the effect to threat-sensitive affective circuitry commonly found to be affected by ELA exposure (Cohodes et al., Reference Cohodes, Kitt, Baskin-Sommers and Gee2021; Fareri & Tottenham, Reference Fareri and Tottenham2016; Hein & Monk, Reference Hein and Monk2017).
These results provide support for Lecei and van Winkel’s (Reference Lecei and van Winkel2020) theoretical model, which stipulates that ELA results in impaired pattern separation (i.e., impaired differentiation, or greater similarity) of emotional information, specifically in the presence of negative or ambiguous stimuli. In turn, this impairment is hypothesized to result in increased fear generalization, threat anticipation, and psychopathological symptoms. Our behavioral findings suggest that individual differences in processing ambiguity relate to global functioning, and that this association varies as a function of ELA exposure. Crucially, we examined these processes in individuals experiencing the transition from adolescence to adulthood. During this developmental stage, risk for psychopathology is heightened (Solmi et al., Reference Solmi, Radua, Olivola, Croce, Soardo, Salazar de Pablo, Il Shin, Kirkbride, Jones, Kim, Kim, Carvalho, Seeman, Correll and Fusar-Poli2022) — especially within populations with a history of caregiving adversity (van der Vegt et al., Reference van der Vegt, van der Ende, Ferdinand, Verhulst and Tiemeier2009) — and responses to ambiguity are believed to have an increased effect on mental health (Bardi et al., Reference Bardi, Guerra and Ramdeny2009; Silvers & Peris, Reference Silvers and Peris2023). Results from the current study have implications both for basic models regarding how ELA shapes neural representations of threat and ambiguity, as well as for the role that ambiguity processing may play in psychosocial functioning following early life adversity.
Effects of ELA on representations of threat and ambiguity
The tendency to represent ambiguity similarly to threat following ELA may reflect an adaptive, learned response stemming from childhood experiences (Lecei & van Winkel, Reference Lecei and van Winkel2020). When repeatedly faced with threatening experiences, it is rational to infer threat when presented with an ambiguous scenario (Dunsmoor & Paz, Reference Dunsmoor and Paz2015). Furthermore, having a low threshold for threat detection is an adaptive response that serves to protect an individual living in a high-threat environment from further harm (Boyce & Ellis, Reference Boyce and Ellis2005; Chaby et al., Reference Chaby, Sheriff, Hirrlinger and Braithwaite2015; Pollak & Kistler, Reference Pollak and Kistler2002). The observed pattern of results, in which ELA-exposed individuals demonstrate impaired neural differentiation between ambiguous and threatening social cues, could stem from hypersensitive threat detection mechanisms. Notably, associations with ELA were only robustly observed when comparing neural representations of ambiguous and threatening cues, suggesting that ELA-exposed individuals do not simply exhibit general impairments in differentiating among affective cues. This specificity in observed results dovetails with existing theoretical models of hypersensitivity to threat (McLaughlin & Lambert, Reference McLaughlin and Lambert2017), especially in the face of ambiguity (Lecei & van Winkel, Reference Lecei and van Winkel2020), following early life adversity. However, given that representational similarity did not predict subsequent appraisals of the ambiguous stimuli, it is possible that while rapid, initial responses to ambiguity are highly similar to responses to threat in ELA-exposed individuals, top-down compensatory mechanisms regulate responses in the decision-making phase. The role of potential regulatory mechanisms is especially important to consider given that participants were from a sample of college students with relatively healthy psychosocial functioning. Further research on how initial representations of ambiguity and regulatory processes interact to shape behavior is warranted.
Ambiguity processing and psychosocial functioning after ELA exposure
Contrary to our hypothesis, we did not observe a robust association between ELA and negativity biases in interpretations of ambiguity. Replicating prior work (Neta & Tong, Reference Neta and Tong2016), we found that taking longer to evaluate ambiguous, relative to unambiguous, stimuli predicted subsequent positive appraisals, lending support to the idea that positive evaluations of ambiguity may require top-down regulatory mechanisms (Neta et al., Reference Neta, Harp, Tong, Clinchard, Brown, Gross and Uusberg2022; Neta & Tong, Reference Neta and Tong2016). Based on research linking responses to ambiguous stimuli to psychosocial outcomes (Lissek et al., Reference Lissek, Rabin, Heller, Lukenbaugh, Geraci, Pine and Grillon2010; Williams et al., Reference Williams, Kemp, Felmingham, Liddell, Palmer and Bryant2007), we tested whether behavioral responses to ambiguity related to global functioning, and whether this differed as a function of ELA exposure. In line with the notion that slower responses to ambiguity reflect regulatory processes, taking longer to evaluate ambiguous relative to unambiguous images was associated with better global functioning, specifically in individuals with lower ELA levels. Research suggests that more deliberative and regulated responses are more advantageous in predictable environments (Kidd et al., Reference Kidd, Palmeri and Aslin2013). In line with this reasoning, the observed interaction suggests that reliance on slower and ostensibly more calculated evaluations of ambiguity are associated with better functioning in individuals with low exposure to adversity. Investigation in a larger sample is needed to understand how this effect differs at various levels of ELA exposure and whether these group differences are driven by variations in self-regulation or other relevant mechanisms.
Strengths and limitations
The current study offers novel insights into the associations between ELA and neural processing of emotional information by leveraging multivariate pattern analyses. Our analytic approach enabled us to examine distributed patterns of brain activity within affective circuitry and, crucially, test similarity between multivariate representations of ambiguity and threat. This study design also allowed us to demonstrate specificity in our findings in that we provide evidence that these effects primarily pertain to ambiguity and threat, and within putatively affective, threat-sensitive circuitry. That said, given the limited sample size, the results from this study should be treated as provisional. Future studies replicating the current findings in a larger longitudinal sample could provide greater clarity into how ELA shapes representations of ambiguity.
Prior research suggests that negative responses to ambiguity may reflect hypersensitivity in the affective processes that govern rapid threat detection (Chen & Lovibond, Reference Chen and Lovibond2016; Grupe & Nitschke, Reference Grupe and Nitschke2013; Mathews et al., Reference Mathews, Mackintosh and Fulcher1997). Based on this work, we designed a task to probe rapid, uninstructed representations of ambiguity while participants were in the scanner. To this end, we asked participants to simply view the images while in the scanner and measured explicit categorizations of the images during the post-scan task. An important next step for future research is to characterize neural representations during the interpretation stage in which participants explicitly evaluate the valence of ambiguous stimuli. Moreover, based on similar prior work (Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer, Caldera, Louie, Shapiro, Bolger and Tottenham2017), and to avoid biasing responses, participants rated whether the person in the image “feels good” or “feels bad”. As a result, we did not capture explicit ratings of threat. Future work may benefit from a more precise measurement of the extent to which participants interpret the images as threatening.
Lastly, we measured ELA by incorporating retrospective reports of abuse and neglect into a broader summary measure. Examining ELA continuously via CTQ scores enabled us to demonstrate a linear relationship between severity of adversity history and the degree of similarity in representations of ambiguity and threat. While we did not have adequate power to investigate how different dimensions of experiences (e.g., threat or unpredictability in the caregiving environment) may differentially shape representations of ambiguity, this is an important avenue for future research.
Conclusion
Exposure to ELA, including experiences of abuse and neglect, is estimated to account for between 30% to 45% of psychopathologies worldwide (Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010; Kessler et al., Reference Kessler, McLaughlin, Green, Gruber, Sampson, Zaslavsky, Aguilar-Gaxiola, Alhamzawi, Alonso, Angermeyer, Benjet, Bromet, Chatterji, de Girolamo, Demyttenaere, Fayyad, Florescu, Gal, Gureje and Williams2010; McLaughlin et al., Reference McLaughlin, Greif Green, Gruber, Sampson, Zaslavsky and Kessler2012). Using multivariate pattern analysis, we provide novel insight into how ELA shapes threat-sensitive neural circuitry, evidencing reduced neural differentiation between ambiguous and threatening cues in ELA-exposed individuals, and link behavioral responses to ambiguity to psychosocial wellbeing during the transition to adulthood. Interventions that target responses to ambiguity may be particularly powerful in mitigating the detrimental effects of adverse early experiences.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579424000683.
Data and code availability
All data, task scripts, and analysis code for this study are available on GitHub (https://github.com/nsaragosaharris/earlylifeadversity_ambiguity_study).
Acknowledgements
This work was supported by the UCLA Academic Senate funds (JAS) and Semel Institute funds (TSP). During study design, data collection, and manuscript preparation, NMSH was supported by a Eugene V. Cota-Robles Fellowship Grant, a National Science Foundation Graduate Research Fellowship Program Grant (DGE-1650604), an Edwin Pauley Fellowship, a Graduate Summer Research Mentorship Program Grant, and National Institute of Child Health and Human Development T32 Predoctoral Training Program Grant. JFGM was supported by a National Science Foundation Graduate Research Fellowship (Fellow ID: 2016220797) and a National Institute of Child Health and Human Development T32 Predoctoral Training Program Grant during study design, data collection, and manuscript preparation. We thank our participants for taking part in this study and thank Maria Calderón, Elizabeth Campbell, Joyce Forster, Breanna Gomez, Casandra Gomez Alvarado, Ambar Hernandez, and Aayush Patel for help with recruitment, data collection, and data management.
Competing interests
The authors report no conflicts of interest.







