Introduction
Pea crabs are small endosymbiotic crustaceans that colonise diverse marine invertebrates, being commonly found inside the mantle cavities of bivalves hosts such as cockles, clams, and mussels (e.g. Becker and Türkay, Reference Becker and Türkay2010, Reference Becker and Türkay2017; Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022). In European waters (Atlantic Ocean and Mediterranean Sea), five pea crabs species have been recorded, namely: Afropinnotheres monodi RB Manning, 1993; Nepinnotheres pinnotheres (Linnaeus, 1758); Pinnotheres bicristatus García Raso & Cuesta in Cuesta, García Raso, Abelló, Marco-Herrero, Silva & Drake 2019; Pinnotheres pectunculi Hesse, 1872; and Pinnotheres pisum (Linnaeus, 1767) (Becker, Reference Becker2010; Becker and Türkay, Reference Becker and Türkay2010, Reference Becker and Türkay2017; Subida et al., Reference Subida, Arias, Drake, García-Raso, Rodríguez and Cuesta2011; Marco-Herrero et al., Reference Marco-Herrero, Drake and Cuesta2017, Reference Marco-Herrero, Galimany, Abelló, Cuesta, Drake and Ramón2020; Cuesta et al., Reference Cuesta, García Raso, Abelló, Marco-Herrero, Silva and Drake2019; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a). Among these pea crabs, P. pisum is the pinnotherid species with the widest latitudinal and longitudinal distribution (Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a), being recorded along the Atlantic Ocean from the North Sea to the Gulf of Cádiz and the Canary Islands, and in the Mediterranean Sea from the Alboran Sea to the Marmara Sea (d'Udeken d'Acoz, Reference d'Udekem d'Acoz1999; Becker, Reference Becker2010; Becker and Türkay, Reference Becker and Türkay2017; Triay-Portella et al., Reference Triay-Portella, Perez-Miguel, González and Cuesta2018; González-Gordillo and Cuesta, Reference González-Gordillo and Cuesta2020).
Throughout its distributional range, P. pisum inhabits the mantle cavity of diverse bivalve species, including non-commercial bivalves (Acanthocardia echinata, Atrina pectinata, Clausinella fasciata, Dosinia lupinus, Gari fervensis, Mactra stultorum, and Pinna nobilis) and commercial bivalves exploited by professional fisheries and/or recreational harvesting activities (Cerastoderma edule, Cerastoderma glaucum, Chamelea gallina, Donax trunculus, Donax variegatus, Donax venustus, Donax vittatus, Ensis ensis, Ensis magnus, Modiolus modiolus, Mytilus edulis, Mytilus galloprovincialis, Ostrea edulis, Ruditapes decussatus, Spisula solida, and Venus verrucosa) (e.g. Haines et al., Reference Haines, Edmunds and Pewsey1994; Delongueville and Scaillet, Reference Delongueville and Scaillet2002; Becker, Reference Becker2010; Becker and Türkay, Reference Becker and Türkay2010, Reference Becker and Türkay2017; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020; de Gier and Becker, Reference de Gier and Becker2020; González-Gordillo and Cuesta, Reference González-Gordillo and Cuesta2020). Just like other pea crabs (Becker and Türkay, Reference Becker and Türkay2010), P. pisum usually has a facultative free-living stage in both sexes (males and hard females), whereas reproductive females (soft females) have a last obligate symbiotic stage inside their bivalve hosts that allows them reaching larger sizes (Becker, Reference Becker2010; Perez-Miguel et al., Reference Perez-Miguel, Cuesta, Navas, García Raso and Drake2018; González-Ortégon et al., Reference González-Ortegón, Perez-Miguel, Navas, Drake and Cuesta2021).
Bivalves play a key role in marine ecosystems and constitute important shellfish resources for coastal communities worldwide (e.g. Newell, Reference Newell2004), with several bivalve species being targeted by commercial fisheries and recreational harvesting activities (e.g. Gaspar et al., Reference Gaspar, Barracha, Carvalho, Vasconcelos and da Costa González2012). In Portugal, small-scale bivalve fisheries are traditional and relevant activities at cultural, social, and economic levels, due to fleet size, number of fishermen, and both volume and value of the catches (Gaspar et al., Reference Gaspar, Leitão, Santos, Sobral, Chícharo, Chícharo and Monteiro2002a; Oliveira et al., Reference Oliveira, Castilho, Cunha and Pereira2013; Rufino et al., Reference Rufino, Pereira, Pereira, Moura, Vasconcelos and Gaspar2017; Almeida et al., Reference Almeida, Gaspar, Castro and Rufino2021). In the last four decades, the Portuguese Institute for the Sea and Atmosphere (IPMA) performs annually bivalve dredge fishing surveys to assess the population status of the most important commercial bivalve species, including C. gallina, Donax semistriatus, D. trunculus, D. vittatus, and S. solida (with variable relevance depending on the fishing areas along mainland Portugal). These sampling campaigns cover an extensive coastal area and comprise hundreds of sampling stations within a substantial depth range. Besides gathering essential data for stock assessment and fishery management, these fishing surveys also provide an excellent opportunity to collect numerous samples of several species for diverse purposes (e.g. Gaspar et al., Reference Gaspar, Santos, Vasconcelos and Monteiro2002b; Cores et al., Reference Cores, Gaspar and Erzini2017; Rufino et al., Reference Rufino, Pereira, Batista and Gaspar2018; Vasconcelos et al., Reference Vasconcelos, Moura, Pereira, Pereira and Gaspar2018).
Due to pinnotherids life strategy and colonisation of bivalve hosts, depending on the infestation degree, pea crabs might induce negative impacts on artisanal fisheries, recreational harvesting and aquaculture activities (e.g. Perez-Miguel et al., Reference Perez-Miguel, Cuesta, Navas, García Raso and Drake2018; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020; Marco-Herrero et al., Reference Marco-Herrero, Galimany, Abelló, Cuesta, Drake and Ramón2020; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022). Indeed, pea crabs can injure bivalve gills and affect filtering efficiency, leading to slower growth, maturation and condition index of their bivalve hosts (Christensen and McDermott, Reference Christensen and McDermott1958; Sun et al., Reference Sun, Sun, Yuqi, Baowen and Weibo2006; Mena et al., Reference Mena, Salas-Moya and Wehrtmann2014; Yasuoka and Yusa, Reference Yasuoka and Yusa2017; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020). In addition, pea crab infestation of edible bivalve species can prompt consumer complaint and rejection, which might constitute a concern for their commercial exploitation (Trottier et al., Reference Trottier, Walker and Jeffs2012; Hutson and Cain, Reference Hutson, Cain, Lucas, Southgate and Tucker2019). In this context, the present study reports the spatial and depth distribution, occurrence and prevalence, infestation rate and intensity of the pea crab P. pisum colonising C. gallina, D. semistriatus, D. trunculus, D. vittatus, and S. solida along the south and southwest coasts of Portugal.
Materials and methods
Fishing surveys
Fishing surveys were performed on-board IPMA's research vessel ‘RV Diplodus’ in two fishing areas (south and southwest coasts) along mainland Portugal (Figure 1). In the south coast, surveys were conducted from the 6th to the 16th of July 2017 and covered all bivalve fishing grounds between Vila Real de Santo António and Olhos d’Água (37°9′40″N, 7°23′55″W to 37°4′59″N, 8°11′13″W) (Figure 1). The coast was subdivided into transects perpendicular to the shoreline (separated ½ nautical mile between each other) and were surveyed 266 sampling stations ranging between 3 and 15 m depth. In the southwest coast, surveys were performed from the 22nd to the 31st July 2017 and covered two main zones between Costa da Caparica and Sines (38°39′00″N, 09°15′29″W to 37°59′00″N, 08°51′02″W) (Figure 1). The coast was also subdivided into transects perpendicular to the shoreline (distanced 1 nautical mile apart) and were surveyed 187 sampling stations ranging between 3 and 25 m depth. Overall, the two fishing surveys required 17 days at sea (south: 9 days; southwest: 8 days).

Figure 1. Map showing the sampling stations for collecting bivalve species during the fishing surveys with mechanical dredges along the south and southwest coasts of Portugal.
Samples were collected with mechanical bivalve dredges similar to those used in commercial fisheries, towed at a constant speed of 1.5 knots for 5 min. All bivalve samples were kept in identified plastic bags, which were preserved in ice until being transported to the laboratory for species sorting and biological sampling.
Laboratory sampling
In the laboratory, bivalve species were sorted, identified and separated into commercial and bycatch species. Whenever available, were sampled 100 individuals of the main commercial bivalve species (C. gallina, D. semistriatus, D. trunculus, D. vittatus, and S. solida) per sampling station (otherwise if less than 100, all individuals of those species were sampled). Subsequently, shells of commercial bivalve species were opened using a scalpel and their mantle cavities were carefully examined for the existence of pea crabs, which were identified to the species level based on specialised literature (Becker and Türkay, Reference Becker and Türkay2010; Cuesta et al., Reference Cuesta, García Raso, Abelló, Marco-Herrero, Silva and Drake2019; Marco-Herrero et al., Reference Marco-Herrero, Galimany, Abelló, Cuesta, Drake and Ramón2020).
Commercial bivalves (with and without pinnotherids) were counted and measured for shell length (SL, maximum distance along the anterior–posterior axis) using a digital caliper (precision = 0.01 mm) and weighed for total weight (TW) on a top loading balance (precision = 0.01 g). Pea crabs were measured for carapace width (CW, maximum distance along the cephalothorax) and carapace length (CL, maximum distance across the cephalothorax) using a digital caliper (precision = 0.01 mm) and weighed for total weight (TW) on a high precision balance (precision = 0.0001 g).
Pea crabs multiple infestations (more than one pinnotherid per bivalve host) were also quantified (e.g. Seed, Reference Seed1969; Haines et al., Reference Haines, Edmunds and Pewsey1994; Perez-Miguel et al., Reference Perez-Miguel, Cuesta, Navas, García Raso and Drake2018; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022). Smaller and sexually undifferentiated pinnotherids were classified as juveniles. Pea crabs were sexed depending on their external morphological features: males (with gonopods) or females (with pleopods). In addition, depending on their abdomen characteristics, developmental and maturity stages, females were further classified as hard or soft females (non-ovigerous and ovigerous). Illustrative photographs of P. pisum juveniles, males and females (hard, soft non-ovigerous and soft ovigerous females) are presented in Figure 2.
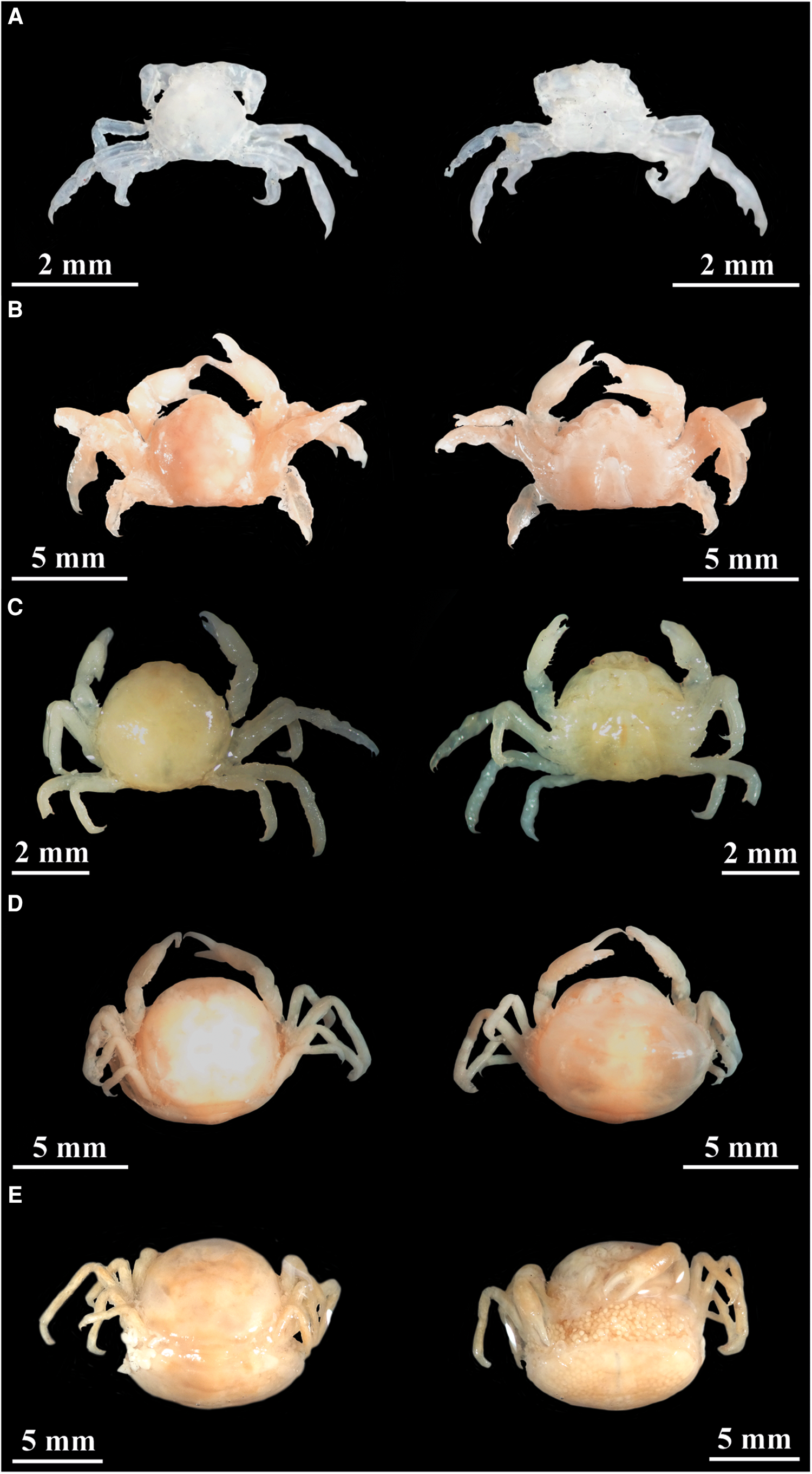
Figure 2. Illustrative photographs (dorsal and ventral views) of Pinnotheres pisum: (A) juvenile; (B) male; (C) hard female; (D) soft non-ovigerous female; (E) soft ovigerous female.
Data treatment and statistical analyses
Bivalve's colonisation by pinnotherids was assessed through the infestation rate (IR – proportion of bivalves hosting pea crabs, either single or multiple occurrences), prevalence rate (PR – proportion of pea crabs within all sampled bivalves) and infestation intensity (II – proportion of pea crabs single and multiple occurrences). In the absence of multiple infestations (i.e. more than one pea crab inside a single bivalve host), the IR is equivalent to the PR.
Pea crabs sex ratio, expressed as the proportion of females per male (females including hard and soft, non-ovigerous and ovigerous), was compared with parity (1M: 1F) using the chi-square (χ2) test. Pea crabs size (CW) was compared between locations (south and southwest coasts), sexes and female maturity stages through analysis of variance (ANOVA). Whenever ANOVA assumptions (data normality and homogeneity of variances) were not met, analyses were performed using the non-parametric Kruskal–Wallis test (K–W), followed by pairwise multiple comparisons to detect significant differences between groups.
In order to further analyse the relationships among diverse descriptors of pea crabs colonisation of commercial bivalve species (IR, PR, SL, CW, sex ratio, and depth) and detect eventual differences between fishing areas (south and southwest coasts), a principal component analysis (PCA) based on Pearson correlations was performed to improve the visualisation and interpretation of the dataset.
Relationships between bivalve host size (SL) and weight (TW), and pea crab size (CW) and weight (TW) were analysed through regression analysis, by fitting the linear function (Y = a + bX) to raw data and assessing the degree of association between measured morphometric variables with the correlation coefficient (r). In addition, aiming to examine the relative growth of pea crabs cephalothorax size and total weight during ontogeny, morphometric relationships were established through regression analysis between CW, CL and TW in juveniles, males and females (hard and soft females), by fitting the power function (Y = aXb) to raw data and assessing relative growth (isometry vs allometry) through the allometry coefficient (regression slope – b).
In relationships between linear variables (CW and CL), isometry growth occurs when b is not significantly different from 1, whereas in relationships between linear and ponderal variables (CW and TW) isometry occurs when b is not significantly different from 3, both reflecting similar growth rates between variables throughout ontogeny (Huxley and Teissier, Reference Huxley and Teissier1936; Mayrat, Reference Mayrat1970). Accordingly, a t-test (H0, b = 1 or 3; HA, b ≠ 1 or 3) was performed to confirm the isometric (b = 1 or 3) or allometric (negative allometry, b < 1 or 3; positive allometry, b > 1 or 3) relative growth between variables. All statistical analyses were performed following Sokal and Rohlf (Reference Sokal and Rohlf1987) and Zar (Reference Zar1996), with statistical significance level set for P < 0.05.
Results
Commercial bivalves
Commercial bivalve species included in this study (C. gallina, D. semistriatus, D. trunculus, D. vittatus, and S. solida) were collected in 371 sampling stations (south: 207 stations; southwest: 164 stations) at variable fishing depths ranging from 3 to 25 m depth (south: 6.2 ± 2.8 m; southwest: 10.1 ± 5.3 m) (Table 1). Overall, 33,370 individuals of commercial bivalves were analysed (south: 25,348 inds.; southwest: 8,022 inds.) belonging to the following species: S. solida (south: 8,892 inds.; southwest: 4,666 inds.), D. trunculus (south: 6,990 inds.; southwest: 1,706 inds.), C. gallina (south: 8,360 inds.; southwest: 132 inds.), D. vittatus (south: 47 inds.; southwest: 1,518 inds.), and D. semistriatus (south: 1,059 inds.) (Table 1).
Table 1. Descriptive statistics of host commercial bivalves (Chamelea gallina, Donax semistriatus, Donax trunculus, Donax vittatus, and Spisula solida) and pea crabs (Pinnotheres pisum) collected during bivalve dredge fishing surveys along the south and southwest coasts of Portugal

Ns, number of sampling stations where the bivalve species was collected; Nb, number of sampled bivalves; SLs, shell length of sampled bivalves; Ni, number of infested bivalves; SLi, mean shell length of infested bivalves; IR (%), infestation rate (%); Np, number of pea crabs; CW, mean carapace width of pea crabs; PR (%), prevalence rate (%); M, males; F, females; M:F, sex ratio; HF, hard females; SFnov, non-ovigerous soft females; SFov, ovigerous soft females; J, juveniles; M + HF, bivalve multiple infestation by one male and one hard female pea crab; M + SFov, bivalve multiple infestation by one male and one ovigerous soft female. Size data presented as mean ± SD and/or respective range (minimum and maximum).
Pea crabs
A total of 102 commercial bivalves hosted pea crabs, all belonging to the species P. pisum (south: 21 inds.; southwest: 81 inds.) (Table 1 and Figure 3). Most pea crabs colonised S. solida (south: 11 inds.; southwest: 67 inds.), distantly followed by D. vittatus (southwest: 11 inds.), D. trunculus (south: 5 inds.; southwest: 1 ind.), C. gallina (south: 3 inds.; southwest: 2 inds.), and D. semistriatus (south: 2 inds.) (Table 1 and Figure 3). Bivalve hosts comprised specimens with broad size and weight, ranging from 17.1 to 41.2 mm SL in C. gallina and from 1.1 g in D. vittatus to 16.5 g in S. solida. Overall, 106 P. pisum pea crabs (south: 23 inds.; southwest: 83 inds.) were detected inside bivalve shells, the vast majority as single occurrences (one pea crab per bivalve host) complemented by very few multiple occurrences (two pea crabs, one male and one female, per bivalve host) (Table 1).
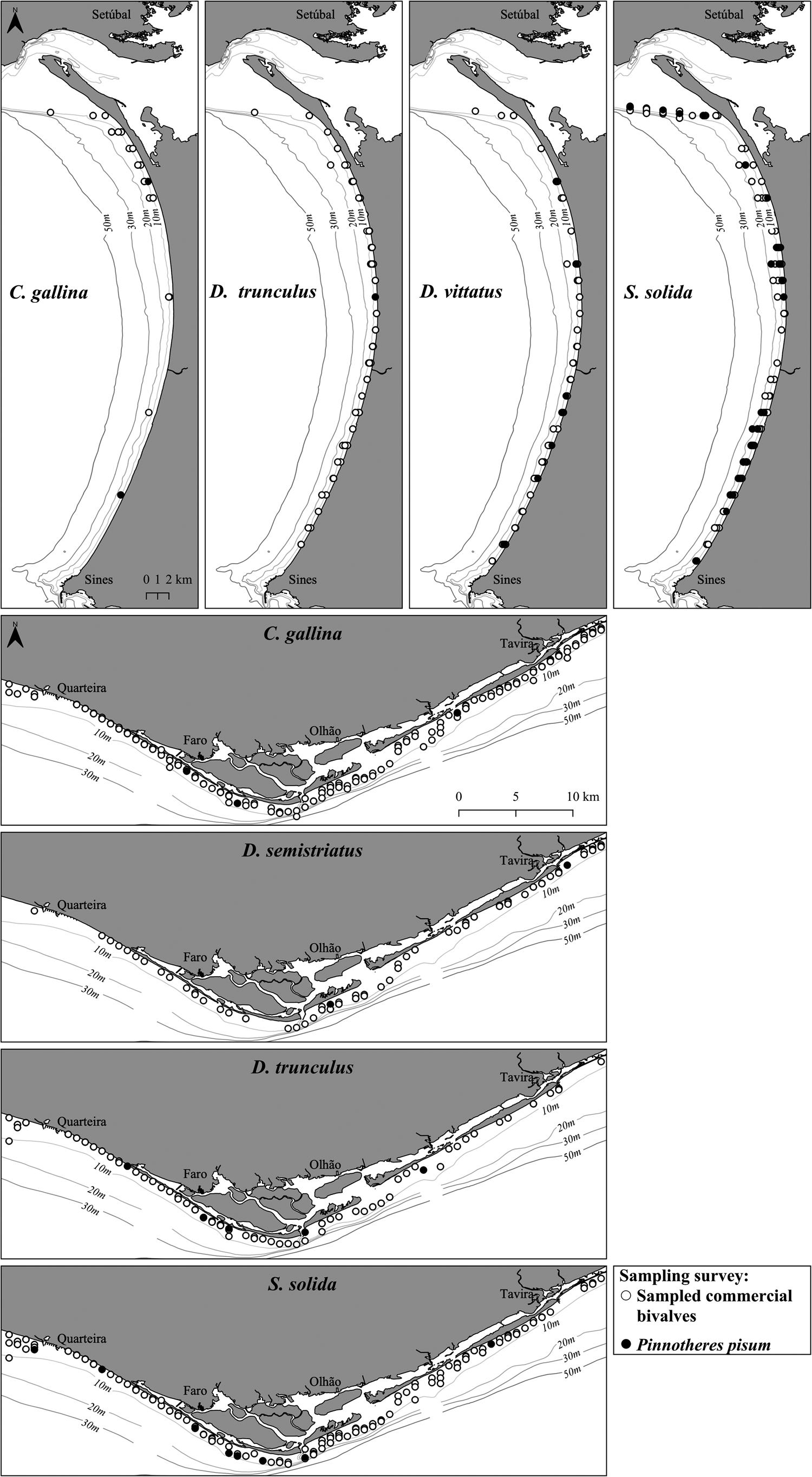
Figure 3. Spatial and depth distribution of Pinnotheres pisum colonising commercial bivalve species (Chamelea gallina, Donax semistriatus, Donax trunculus, Donax vittatus, and Spisula solida) along the south and southwest coasts of Portugal.
Pea crabs size and weight ranged from 1.3 to 13.5 mm CW and from 0.001 to 0.350 g TW. Mean carapace width (K–W: H = 0.194; P > 0.05) did not display significant differences between the south coast (4.3 ± 2.1 mm CW) and the southwest coast (4.9 ± 3.1 mm CW) (Table 1). Besides 13 juveniles of P. pisum (undistinguishable sex), 60 males and 33 females were recorded, corresponding to a highly unbalanced (χ2 = 7.269, P < 0.01) and male-biased sex ratio (1M: 0.6F). Among females, were identified 5 hard and 28 soft females, of which 3 non-ovigerous and 25 ovigerous females (Table 1). On average, males (3.5 ± 0.6 mm CW) were significantly smaller (K–W: H = 57.222, P < 0.001) than females (8.3 ± 2.8 mm CW). In addition, mean carapace width was also significantly different among female maturity stages (ANOVA: F = 13.290; P < 0.001), with hard females (4.3 ± 0.6 mm CW) being significantly smaller (Tukey test: q = 6.957; P < 0.001) than ovigerous soft females (9.4 ± 2.3 mm CW).
Pea crabs colonisation pattern
The colonisation pattern of commercial bivalves by P. pisum in terms of fishing depth and bivalve host size is presented in Figure 4. The number and relative frequency of pea crabs displayed a clear decreasing trend with increasing fishing depth (r = 0.974; P < 0.05), ranging from 39.6% at shallower depths (<10 m) to 14.2% in deeper sampling stations (≥20 m) (Figure 4A). Such differences in the occurrence and proportion of pea crabs at each depth interval were mainly due to the sampling effort and number of commercial bivalves caught at each depth interval, since the vast majority (N = 28,712; 86.0%) was collected at shallower depths (< 10 m) and only a minor fraction (N = 967; 2.9%) was collected at greater depths (≥20 m). The shallowest and deepest occurrences of P. pisum were recorded in D. trunculus at 3 m depth in the south coast and in S. solida at 25 m depth in the southwest coast, respectively. Pea crabs depth distribution was strongly influenced by the highly predominant bivalve host S. solida (comprising 81 P. pisum) mainly distributed at deeper bathymetrics (13.0 ± 4.9 m; range = 4.8–25.0 m). On the opposite, pea crabs colonising the hosts C. gallina (containing 6 P. pisum) and Donax spp. (hosting 19 P. pisum) occurred predominantly at intermediate (8.4 ± 3.5 m; range = 4.8–15.0 m) and shallower depths (6.9 ± 3.1 m; range = 3.0–12.0 m).

Figure 4. Number and frequency of Pinnotheres pisum colonising commercial bivalve species as a function of (A) sampling station depth (5 m depth intervals); (B) bivalve host size (10 mm SL size classes).
Although host bivalve species ranged from 17.1 to 41.2 mm SL, the vast majority of the pea crabs colonised intermediate-sized bivalve hosts belonging to the sizes classes 20–30 mm SL (52.8%) and 30–40 mm SL (41.5%), with only minor occurrence in smaller (<20 mm SL = 3.8%) and larger bivalves (≥40 mm SL = 1.9%) that were also scarcer in the overall samples (Figure 4B). Overall, host bivalve species displayed the following decreasing trend in mean shell length: S. solida (30.2 ± 6.4 mm SL), C. gallina (28.9 ± 5.9 mm SL) and D. semistriatus, D. trunculus, and D. vittatus (27.4 ± 4.6 mm SL). In addition, while smaller P. pisum males clearly prevailed in 20–30 mm SL bivalve hosts (38 males and 8 females), larger P. pisum females predominated in 30–40 mm SL bivalve hosts (23 females and 18 males).
Pea crabs infestation rate
Overall, the 102 bivalves hosting 106 P. pisum corresponded to an infestation rate of 0.31% (prevalence rate of 0.32% due to the multiple infestation of four bivalves colonised by two pea crabs). Although invariably low, the infestation rate was clearly higher in the southwest coast (IR = 1.01%) than in the south coast (IR = 0.08%) (Table 1). The spatial and depth variation in the infestation rate of the five commercial bivalve species by pea crabs along the south and southwest coasts of Portugal is showed in Figure 5. In the south coast, the infestation rate ranged from 0% in D. vittatus to 0.19% in D. semistriatus. In the southwest coast, the lowest value occurred in D. trunculus (IR = 0.06%), with highest infestation rates recorded in C. gallina (IR = 1.52%) closely followed by S. solida (IR = 1.44%) (Figure 5A). Regarding the bathymetric variation, the infestation rate displayed a clear growing trend with increasing fishing depth (r = 0.954; P < 0.05), which was particularly evident between <10 and ≥10 m depth, ranging from 0.14% at shallower depths (<10 m) to 1.55% in the deeper sampling stations (≥20 m) (Figure 5B).

Figure 5. Variation in the infestation rate of commercial bivalve species by Pinnotheres pisum as a function of (A) sampling station location (south and southwest coasts of Portugal); (B) sampling station depth (5 m depth intervals).
The PCA provided further insights into the relationships between host bivalves and guest pea crabs, with the two principal axes of the PCA accounting for 84.0% of the total variance in the dataset (PC1 = 58.9%; PC2 = 25.1%) (Figure 6). The PCA depicted a clear relationship between IR and PR by P. pisum in the host bivalves S. solida and C. gallina in the southwest coast, which was strongly related to fishing depth. The opposite trend was displayed in the south coast, where pea crabs occurrence was influenced by host bivalves caught at shallower depths. Furthermore, the PCA also highlighted that IR and PR are mostly related to the most frequent host bivalves inhabiting deeper fishing grounds (S. solida and C. gallina), whereas pea crab descriptors (CW and sex ratio) are mainly determined by host bivalve size (SL) (Figure 6).

Figure 6. Principal component analysis (PCA) biplot showing the relationship among diverse descriptors of Pinnotheres pisum colonisation of commercial bivalve species along the south and southwest coasts of Portugal. IR, infestation rate; PR, prevalence rate; SL, shell length; CW, carapace width.
Host bivalves – guest pea crabs relationships
The relationships established between bivalve hosts size and weight and colonising pea crabs size and weight are illustrated in Figure 7. In general, larger bivalves hosted larger P. pisum, as revealed by the highly significant correlation (r = 0.403; P < 0.001) and positive slope (b = 0.194) between bivalve shell length and pea crab carapace width (Figure 7A). Accordingly, following the same general trend, heavier bivalves also hosted heavier P. pisum, although with slightly lower correlation (r = 0.369; P < 0.001) and more gentle positive slope (b = 0.007) (Figure 7B).

Figure 7. Relationships between (A) bivalve host shell length and Pinnotheres pisum carapace width; (B) bivalve host total weight and Pinnotheres pisum total weight.
Pea crabs morphometric relationships and relative growth
The morphometric relationships established between pea crabs carapace width, carapace length, and total weight are displayed in Figure 8. Both relationships were highly significant (P < 0.001) and characterised by very high correlation coefficients between carapace width and length (r = 0.982) (Figure 8A) and between carapace width and total weight (r = 0.964) (Figure 8B). In both cases, negative allometric growth was recorded between variables, reflecting slower growth rates in both carapace length (b = 0.924) and total weight (b = 2.374) compared to carapace width throughout the ontogeny of P. pisum (Figures 8A and B).

Figure 8. Morphometric relationships of Pinnotheres pisum and relative growth between (A) carapace width and carapace length; (B) carapace width and total weight. J, juveniles; M, males; HF, hard females; SFnov, non-ovigerous soft females; SFov, ovigerous soft females.
In addition, the relative growth between pea crabs carapace width, carapace length and total weight as function of their sex, developmental and female maturity stages is compiled in Table 2. Overall, only isometric (b = 1 or 3) and negative allometric growth (b < 1 or 3) were recorded between variables. The whole specimens recorded negative allometries in both morphometric relationships (CL vs CW and TW vs CW), revealing a comparatively faster growth rate in carapace width than in carapace length and total weight during growth (i.e. gradual widening of P. pisum carapace throughout the species ontogeny) (Table 2).
Table 2. Descriptive statistics, morphometric relationships and relative growth between carapace width, carapace length and total weight of the pea crabs (Pinnotheres pisum) depending on their sex, developmental and female maturity stages

N, number of individuals; CW, mean carapace width (mm); CL, mean carapace length (mm); TW, mean total weight (g); Size and weight data presented as mean ± SD and respective range (minimum – maximum); r, correlation coefficient; b, allometry coefficient; SE, standard error; 95% CI, 95% confidence interval.
Superscript letters and asterisks denote statistical significance level (P-value): ns, P > 0.05 (not significant); *, P < 0.05 (significant); **, P < 0.01 (highly significant); ***, P < 0.001 (very highly significant). I = , isometry; A–, negative allometry.
However, different trends in relative growth were detected depending on P. pisum development (juveniles = isometries), sex (males = isometry or negative allometry; females = negative allometries), and female maturity stages (hard females = isometry; soft females = negative allometries) (Table 2). Isometric growth in both morphometric relationships only occurred in juveniles, reflecting a similar growth rate between CW, CL, and TW in the smallest pea crabs. The few other isometries also occurred in the tending smaller males (TW vs CW) and hard females (CL vs CW). On the opposite, the clearly larger soft females (and consequently the whole females) exhibited invariably negative allometries in both morphometric relationships, denoting a continuous broadening of the carapace towards more developed and mature females of P. pisum (Table 2).
Discussion
The pea crab P. pisum is among the five pinnotherid species previously reported for European waters (Becker, Reference Becker2010; Becker and Türkay, Reference Becker and Türkay2010, Reference Becker and Türkay2017; Subida et al., Reference Subida, Arias, Drake, García-Raso, Rodríguez and Cuesta2011; Marco-Herrero et al., Reference Marco-Herrero, Drake and Cuesta2017, Reference Marco-Herrero, Galimany, Abelló, Cuesta, Drake and Ramón2020; Cuesta et al., Reference Cuesta, García Raso, Abelló, Marco-Herrero, Silva and Drake2019; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a). This pea crab has been recorded at several localities along the NE Atlantic Ocean and across the Mediterranean Sea (Table 3), being the European pinnotherid species with broadest latitudinal distribution (Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a) and with widest range of confirmed bivalve host species (Becker and Türkay, Reference Becker and Türkay2017; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a; González-Gordillo and Cuesta, Reference González-Gordillo and Cuesta2020). In the present study, P. pisum occurred in C. gallina, D. semistriatus, D. trunculus, D. vittatus, and S. solida, corroborating previous colonisations of these commercial bivalve species in other geographical areas, such D. vittatus and S. solida in the North Sea (Becker and Türkay, Reference Becker and Türkay2017) and C. gallina and D. trunculus in the Mediterranean Sea (Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a) (Table 3). On the opposite, at least according to the present authors' best knowledge, this study reports the first record of P. pisum colonising the bivalve host D. semistriatus.
Table 3. Geographical distribution, bivalve host species and infestation rate of the pea crab Pinnotheres pisum throughout its distributional range in the Atlantic Ocean and Mediterranean Sea

Recent studies in Iberian waters reported very low infestation rates by the European native P. pisum but high infestation rates of the African pea crab A. monodi (e.g. Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a, Reference Perez-Miguel, González-Ortegón, Drake, Navas and Cuesta2019b; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022). During its northwards expansion along the Portuguese coast (Subida et al., Reference Subida, Arias, Drake, García-Raso, Rodríguez and Cuesta2011; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a, Reference Perez-Miguel, González-Ortegón, Drake, Navas and Cuesta2019b; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022), A. monodi colonised diverse bivalve species, including the commercially exploited C. gallina, D. trunculus, and S. solida. However, this pea crab was not recorded in the present study, absence that is probably related to inter-specific differences in preferential habitat, with P. pisum colonising preferentially subtidal bivalve hosts (Houghton, Reference Houghton1963; Seed, Reference Seed1969; Gam et al., Reference Gam, Bazaïri, Jensen and Montaudouin2008; Becker, Reference Becker2010; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a) and A. monodi preferring intertidal bivalve hosts (Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014; Perez-Miguel, Reference Perez-Miguel2018; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022).
High infestation rates by P. pisum have been recorded in C. edule from Spain (Bruzos et al., Reference Bruzos, Lafuente, Tubío, Díaz, Rey-Méndez, Fernández Casal, Lastres and Padín2020) and Morocco (Gam et al., Reference Gam, Bazaïri, Jensen and Montaudouin2008), as well as in M. edulis from England (Seed, Reference Seed1969; Haines et al., Reference Haines, Edmunds and Pewsey1994) and M. modiolus from the North Sea (Becker and Türkay, Reference Becker and Türkay2017) (Table 3). In the present study, infestation rates were much lower, ranging from 0.04% to 1.5% in C. gallina from the south and southwest coasts, respectively, more similar to values reported for C. gallina in the Mediterranean Sea (Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a) and for D. vittatus and S. solida in the North Sea (Becker and Türkay, Reference Becker and Türkay2017). Overall, differences in infestation rates by P. pisum seem intrinsically related to diverse factors, including the host bivalve species (e.g. higher infestation rates in C. edule, M. edulis, and M. modiolus compared to other bivalve species), prevailing environmental conditions (sheltered vs exposed sites), habitat location and depth (intertidal vs subtidal areas) (e.g. Houghton, Reference Houghton1963; Seed, Reference Seed1969, Reference Seed1971; Haines et al., Reference Haines, Edmunds and Pewsey1994; Becker, Reference Becker2010; Becker and Türkay, Reference Becker and Türkay2017; Perez-Miguel, Reference Perez-Miguel2018; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a; Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022).
Regarding the prevalence rate (i.e. multiple pea crabs colonising a single bivalve), the vast majority of infested bivalves contained a single P. pisum inside the shell (96.1%), with only four host bivalves (three S. solida and one C. gallina) presenting multiple infestations (3.9%). Higher prevalence rates of P. pisum were recorded in M. edulis from southwestern England, with 135 single infestations (87.7%), 17 double infestations (11.0%) and 2 triple infestations (1.3%) (Seed, Reference Seed1969). Likewise, in M. edulis from southern England, where most mussels hosted a single pea crab (range: 74.9–88.0%) distantly followed by two (range: 12.0–24.9%) or three (range: 0.1–0.3%) pea crabs inside a host mussel (Haines et al., Reference Haines, Edmunds and Pewsey1994), and in M. modiolus from Helgoland Trench, with 191 single infestations (75.2%) and 63 double infestations (24.8%) (Becker and Türkay, Reference Becker and Türkay2017).
Likewise the present study in southern and southwestern Portugal, where multiple infestations comprised only pairs of males and hard females or soft ovigerous females, in southwestern England all double infestations of M. edulis involved one male and one female P. pisum (Seed, Reference Seed1969), whereas in southern England the vast majority of double infestations also consisted of pea crabs couples (range: 85.2–90.2%) (Haines et al., Reference Haines, Edmunds and Pewsey1994). Indeed, although P. pisum tends to live alone inside bivalve hosts (just like other pea crab species), double infestations by male and female couples suggest mating encounters instead of casual co-occurrence by pure coincidence. Such recurrent and prevailing single infestations might be due to complementary strategies: firstly, pea crabs releasing a chemical cue that discourages conspecifics from entering their bivalve hosts, coupled with resident pea crabs showing aggressive behaviour towards invading conspecifics (e.g. Bell, Reference Bell1984; Haines et al., Reference Haines, Edmunds and Pewsey1994; Soong, Reference Soong1997; Takeda et al., Reference Takeda, Tamura and Washio1997; Drake et al., Reference Drake, Marco-Herrero, Subida, Arias and Cuesta2014); secondly, mature males swimming ability enable them to survive for some time outside bivalve hosts, whereas mature free-living females have never been reported in nature, further suggesting that males prompt mating encounters while females usually remain inside bivalve hosts (Haines et al., Reference Haines, Edmunds and Pewsey1994).
Although the number and relative frequency of pea crabs displayed an evident decreasing pattern with increasing depth of occurrence of commercial bivalve species, their infestation rate exhibited a clear growing trend with increasing fishing depth of host bivalves (graphically illustrated in Figure 6). In addition, the infestation rate was clearly higher in the southwest coast (IR = 0.31%) where fishing depth is higher (10.1 ± 5.3 m), than in the south coast (IR = 0.08%) where commercial bivalves occur and are exploited at lower bathymetrics (6.2 ± 2.8 m). These results corroborate previous studies that reported increasing P. pisum infestation rates increase with growing depth, namely from intertidal to subtidal sampling stations (Houghton, Reference Houghton1963; Seed, Reference Seed1969; Gam et al., Reference Gam, Bazaïri, Jensen and Montaudouin2008; Becker, Reference Becker2010; Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a). Moreover, higher infestations by P. pisum occurred in S. solida and C. gallina in the southwest coast and lower infestations occurred in D. trunculus in the south and southwest coasts, which is probably related to the preferential habitat of these bivalve species. In fact, while the former host species have a similar depth distribution (C. gallina: 3–10 m; S. solida: 3–14 m), D. trunculus occurs at much shallower depths (0–5 m, with higher densities at 3 m) (Gaspar et al., Reference Gaspar, Chícharo, Vasconcelos, García, Santos and Monteiro2002c; Anjos et al., Reference Anjos, Pereira, Vasconcelos, Joaquim, Matias, Erzini and Gaspar2018), which in intertidal areas implies a continuous unburial and reburial that certainly makes more difficult the colonisation of D. trunculus by P. pisum (Perez-Miguel et al., Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a).
In the present study, the size of P. pisum ranged from 1.3 to 13.5 mm CW, which is fairly similar to the ranges reported for this pea crab species inhabiting M. edulis in southern England (1.0–13.0 mm CW) (Haines et al., Reference Haines, Edmunds and Pewsey1994) and southwestern England (2.1–18.0 mm CW) (Atkins, Reference Atkins1926), as well as colonising both M. edulis and M. galloprovincialis from southwest England (1.0–15.0 mm CW) (Seed, Reference Seed1969). Moreover, a clear size dimorphism between sexes (males smaller than females) and female maturity stages (hard females smaller than ovigerous soft females) was detected in the present study, further corroborating previous studies of this pea crab species (Atkins, Reference Atkins1926; Seed, Reference Seed1969; Haines et al., Reference Haines, Edmunds and Pewsey1994). The space available inside the bivalve host shell apparently influences the distribution and size of colonising pea crabs (Haines et al., Reference Haines, Edmunds and Pewsey1994; Becker and Türkay, Reference Becker and Türkay2017). Similar to other studies that recorded larger pea crabs inhabiting larger bivalve hosts (e.g. Houghton, Reference Houghton1963; Seed, Reference Seed1969; Haines et al., Reference Haines, Edmunds and Pewsey1994), the correlations established in the present study between bivalve shell length and pea crab carapace width confirmed a general trend for larger bivalves hosting larger P. pisum. Consequently, just like previously reported by Haines et al. (Reference Haines, Edmunds and Pewsey1994), due to P. pisum size dimorphism between sexes, smaller bivalves were mainly colonised by male pea crabs (smaller males prevailed in 20–30 mm bivalves), whereas larger bivalves were mostly inhabited by female pea crabs (larger females predominated in 30–40 mm bivalves).
The overall population of P. pisum exhibited negative allometries between morphometric variables, indicative of slower growth rate in both carapace length and total weight compared to carapace width (b = 0.924 and b = 2.374, respectively). However, while the morphometric relationships between carapace width and total weight displayed an isometry in males (b = 0.905) and a negative allometry in females (b = 1.924), the relative growth between carapace width and carapace length was hypoallometric in both sexes (b = 0.905 and b = 0.818, respectively), reflecting a progressive widening of pea crabs carapace throughout ontogeny. Just for comparison purposes, isometries in males (b = 2.979) and hard females (b = 2.519) and negative allometries in soft females (b = 2.597) between carapace width and total weight, were also recorded in P. bicristatus colonising Anomia ephippium along the Atlantic and Mediterranean coasts of Andalusia (Spain) (Cuesta et al., Reference Cuesta, García Raso, Abelló, Marco-Herrero, Silva and Drake2019). Moreover, such morphometric relationships also detected differential growth between female maturity stages, with hard females being isometric (b = 0.868) and soft females being hypoallometric (b = 0.652), with this gradual enlargement of the carapace during maturation probably allowing soft females to carry more eggs at the subsequent ovigerous stage. Similarly to the present study, morphometric relationships between carapace width and carapace length of A. monodi colonising M. galloprovincialis in southern Portugal also revealed a negative allometry in males (b = 0.961), isometric growth in hard females (b = 0.985) and another hypoallometry in non-ovigerous soft females (b = 0.805) (Santos et al., Reference Santos, Vasconcelos, Pereira, Piló, Carvalho and Gaspar2022).
Previous studies reported that bivalves infestation by pea crabs can injure their gills and impair filtering efficiency, which might lead to reduced growth, reproductive maturation and condition index (e.g. Stauber, Reference Stauber1945; Christensen and McDermott, Reference Christensen and McDermott1958; Sun et al., Reference Sun, Sun, Yuqi, Baowen and Weibo2006; Mena et al., Reference Mena, Salas-Moya and Wehrtmann2014; Yasuoka and Yusa, Reference Yasuoka and Yusa2017; Perez-Miguel et al., Reference Perez-Miguel, Cuesta, Navas, García Raso and Drake2018, Reference Perez-Miguel, Drake, García Raso, Mamán Menéndez, Navas and Cuesta2019a; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020). Just for instance, populations of M. edulis with a high prevalence of P. pisum in southwest England exhibited gill damages, with infested mussels showing lower tissue weight than uninfected mussels of comparable size (Seed, Reference Seed1969). In fact, more recently, P. pisum has been considered a constant pest in mussels and oysters in European coasts (Becker, Reference Becker2010). The severity of pea crabs harmful effects on their hosts depends on diverse factors, such as the prevalence, intensity and duration of the infestation, as well as on the relative size of pea crabs and bivalve hosts (e.g. Sun et al., Reference Sun, Sun, Yuqi, Baowen and Weibo2006; Trottier et al., Reference Trottier, Walker and Jeffs2012; Mena et al., Reference Mena, Salas-Moya and Wehrtmann2014; Perez-Miguel et al., Reference Perez-Miguel, Cuesta, Navas, García Raso and Drake2018; Cuesta et al., Reference Cuesta, Perez-Miguel, González-Ortegón, Roque and Drake2020). Although this study did not aim to assess host bivalves condition index, the fairly low infestation rates of P. pisum recorded in all commercial bivalves do not constitute a health concern for these highly valued species and fishery-exploited resources in the south and southwest coasts of Portugal. Overall, the present study provided further information and valuable insights on bivalves' colonisation by P. pisum, which should be periodically monitored in the mid- and long-term under a climate change scenario, in order to follow its evolution, eventual harmful effects on hosts' condition index and, therefore, on commercial bivalve populations.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgements
The authors thank Professor Chris Hauton (editor of JMBA UK) and two anonymous reviewers for their useful comments and detailed suggestions that greatly improved this article.
Author's contributions
L. Nicolau: Data curation, formal analysis, writing, review and editing. P. Vasconcelos: conceptualisation, data curation, formal analysis, writing, review and editing. F. Pereira: conceptualisation, fishing surveys and laboratory sampling, methodology, investigation, data curation, formal analysis, writing, review and editing. A.N. Carvalho: conceptualisation, fishing surveys and laboratory sampling, data curation, formal analysis, writing, review and editing. D. Piló: conceptualisation, fishing surveys and laboratory sampling, data curation, formal analysis, writing, review and editing. M.B. Gaspar: funding acquisition, supervision and coordination.
Financial support
The fishing surveys were supported by the project ‘Estudo Integrado dos Bancos Naturais de Moluscos Bivalves no Golfo de Cádis para a sua Gestão Sustentável e Conservação dos Habitats Associados – VENUS’ (POCTEP – Spain-Portugal Cooperation Programme – Interreg V-A), co-financed by the European Regional Development Fund (ERDF 2014–2020), and by the project ‘Sistema Nacional de Monitorização de Moluscos Bivalves – Algarve (SNMB – Sul)’, funded by the Fisheries Operational Programme (MAR 2020) and co-financed by the European Maritime and Fisheries Fund (EMFF 2014–2020). The first author Lídia Nicolau received a research grant (Ref: IPMA-2019-054A-BI) awarded by IPMA within the framework of the project ‘Contributo para a Gestão Sustentada da Pequena Pesca e da Apanha – PESCAPANHA’, funded by the Fisheries Operational Programme (MAR 2020) and co-financed by the European Maritime and Fisheries Fund (EMFF 2014–2020).
Competing interest
None.
Ethical standards
Not applicable.













